Cholinergic/Adrenergic drugs

On this page
🎯 The Autonomic Arsenal: Mastering Cholinergic and Adrenergic Pharmacology
Your autonomic nervous system orchestrates every heartbeat, breath, and stress response through two chemical messengers: acetylcholine and norepinephrine. This lesson equips you to manipulate these pathways with precision, exploring how cholinergic and adrenergic drugs activate or block specific receptors to treat conditions from glaucoma to hypertension. You'll master the mechanisms behind direct agonists, enzyme inhibitors, and selective antagonists, building a framework to predict therapeutic effects and adverse reactions. By understanding receptor subtypes and signaling cascades, you'll transform pharmacology from memorization into strategic clinical reasoning.
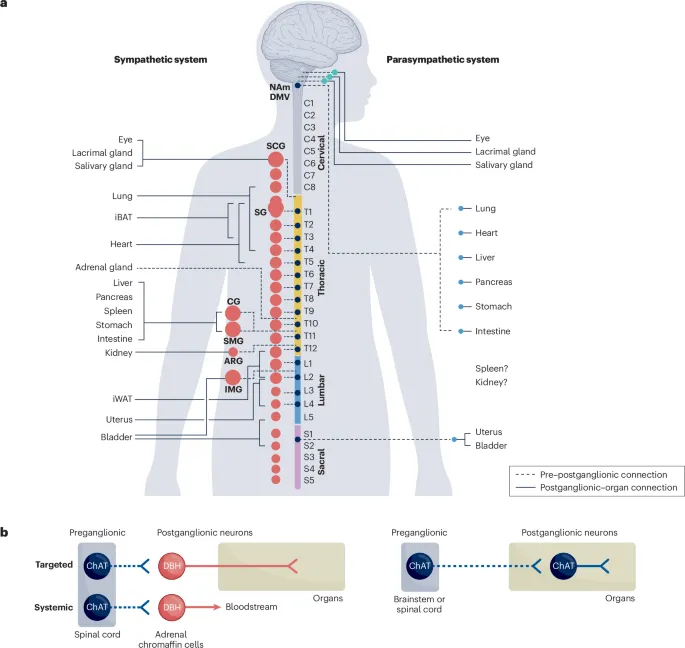
📌 Remember: SLUDGE-BBB for cholinergic excess - Salivation, Lacrimation, Urination, Defecation, GI upset, Emesis, plus Bradycardia, Bronchospasm, Bronchorrhea
The autonomic nervous system operates through two primary neurotransmitters: acetylcholine (ACh) and norepinephrine, each activating distinct receptor families with >95% selectivity when properly targeted. Cholinergic drugs modulate acetylcholine pathways affecting muscarinic (G-protein coupled) and nicotinic (ligand-gated ion channel) receptors, while adrenergic agents target α1, α2, β1, β2, and β3 receptor subtypes.
-
Cholinergic System Architecture
- Preganglionic neurons: 100% nicotinic ACh receptors
- Postganglionic parasympathetic: muscarinic ACh receptors
- Neuromuscular junction: nicotinic ACh receptors
- Onset time: 30-60 seconds for IV administration
- Duration: 15-30 minutes for most direct agonists
- Therapeutic window: 2-4x baseline ACh levels
-
Adrenergic System Architecture
- Postganglionic sympathetic: α and β adrenergic receptors
- Adrenal medulla: 80% epinephrine, 20% norepinephrine
- Receptor distribution varies by organ: β1 cardiac > β2 pulmonary > α1 vascular
- α1 effects: vasoconstriction, mydriasis, urinary retention
- β1 effects: ↑ heart rate, ↑ contractility, ↑ conduction
- β2 effects: bronchodilation, vasodilation, ↑ glucose
| Receptor Type | Location | Primary Effects | Therapeutic Targets | Clinical Significance |
|---|---|---|---|---|
| Muscarinic M1 | CNS, Gastric | ↑ Cognition, ↑ Acid | Alzheimer's, PUD | 60% CNS ACh activity |
| Muscarinic M2 | Cardiac | ↓ HR, ↓ Contractility | Bradycardia | 40% cardiac parasympathetic |
| Muscarinic M3 | Smooth Muscle | Bronchoconstriction, ↑ Secretions | Asthma, COPD | 85% glandular activity |
| Nicotinic Nm | NMJ | Muscle Contraction | Anesthesia, Paralysis | 100% voluntary movement |
| Nicotinic Nn | Ganglia | Autonomic Transmission | HTN, Smoking | 90% ganglionic transmission |
💡 Master This: Cholinergic crisis presents with DUMBBELSS (Diarrhea, Urination, Miosis, Bronchospasm, Bradycardia, Emesis, Lacrimation, Salivation, Sweating) and requires atropine 2-4mg IV plus pralidoxime 1-2g IV for organophosphate poisoning
The therapeutic index for autonomic drugs varies dramatically: atropine TI = 100-200, succinylcholine TI = 3-5, and epinephrine TI = 10-20. Understanding receptor selectivity and tissue distribution patterns enables precise clinical interventions while minimizing adverse effects through targeted pharmacological approaches.
Connect these foundational autonomic principles through cholinergic receptor subtypes to understand how muscarinic and nicotinic pathways create distinct therapeutic opportunities.
🎯 The Autonomic Arsenal: Mastering Cholinergic and Adrenergic Pharmacology
🔑 Cholinergic Command Center: Receptor Subtypes and Signaling Mastery
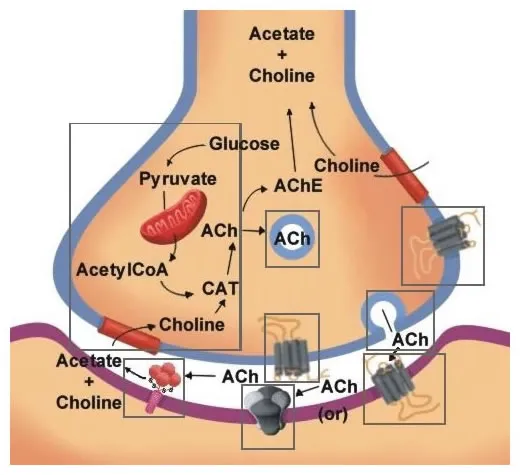
📌 Remember: M-QIGS for muscarinic receptor coupling - M1/M3/M5 use Gq (↑ IP3/DAG), M2/M4 use Gi (↓ cAMP, ↑ K+ channels)
-
Muscarinic Receptor Architecture
- M1 receptors: CNS dominance (65% cortical ACh activity)
- Location: Cerebral cortex, hippocampus, gastric parietal cells
- Function: ↑ cognition, ↑ gastric acid secretion
- Clinical relevance: Alzheimer's target, peptic ulcer disease
- M2 receptors: Cardiac control (40% parasympathetic cardiac effects)
- Location: Atria, SA/AV nodes, smooth muscle
- Function: ↓ heart rate, ↓ contractility, ↓ AV conduction
- Clinical relevance: Bradycardia management, heart block
- M3 receptors: Secretory powerhouse (85% glandular activity)
- Location: Exocrine glands, bronchial smooth muscle, bladder
- Function: ↑ secretions, bronchoconstriction, bladder contraction
- Clinical relevance: Asthma, urinary retention, dry mouth
- M1 receptors: CNS dominance (65% cortical ACh activity)
-
Nicotinic Receptor Architecture
- Neuronal type (Nn): Ganglionic transmission (90% autonomic relay)
- Composition: α3β4 subunits primarily
- Location: All autonomic ganglia, adrenal medulla
- Function: Fast synaptic transmission (2-5 milliseconds)
- Clinical relevance: Hypertension, smoking cessation
- Muscle type (Nm): Neuromuscular junction (100% voluntary movement)
- Composition: α1β1δε (adult) or α1β1δγ (fetal)
- Location: Motor endplate exclusively
- Function: Muscle contraction initiation
- Clinical relevance: Anesthesia, paralysis, myasthenia gravis
- Neuronal type (Nn): Ganglionic transmission (90% autonomic relay)
| Receptor | Subtype | G-Protein | Primary Location | Key Function | Onset Time | Clinical Drug Target |
|---|---|---|---|---|---|---|
| M1 | Gq/11 | ↑ IP3/DAG | CNS, Stomach | Cognition, Acid | 2-5 sec | Alzheimer's, PUD |
| M2 | Gi/o | ↓ cAMP | Heart | ↓ HR, ↓ Contractility | 1-3 sec | Bradycardia |
| M3 | Gq/11 | ↑ IP3/DAG | Glands, Airways | Secretion, Constriction | 2-4 sec | Asthma, Xerostomia |
| Nn | N/A | Na+/Ca2+ | Ganglia | Autonomic Relay | 2-5 msec | Hypertension |
| Nm | N/A | Na+/Ca2+ | NMJ | Muscle Contraction | 0.5-1 msec | Anesthesia |
💡 Master This: Physostigmine crosses the blood-brain barrier (unlike neostigmine) and reverses CNS anticholinergic toxicity from atropine, scopolamine, or tricyclic antidepressants with 2-5mg IV dosing
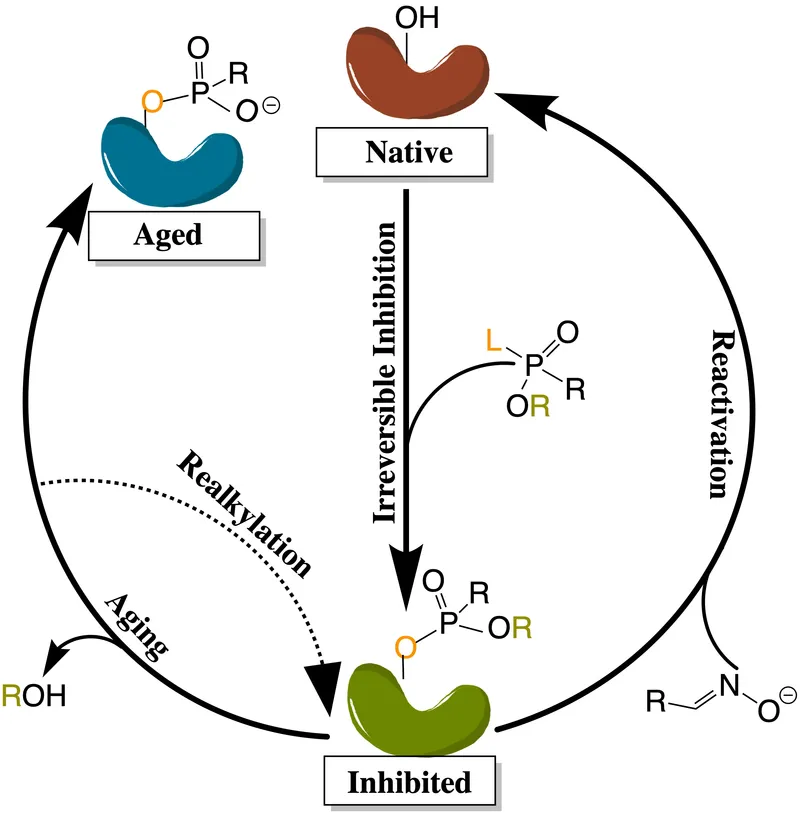
The acetylcholinesterase enzyme terminates ACh signaling with turnover rates >10,000 molecules/second, making it a critical therapeutic target. Reversible inhibitors (physostigmine, neostigmine) have half-lives of 1-4 hours, while irreversible organophosphate inhibitors require enzyme regeneration over 7-14 days.
⚠️ Warning: Organophosphate poisoning creates irreversible AChE inhibition requiring pralidoxime within 24-48 hours before enzyme aging occurs-delayed treatment reduces efficacy by >80%
Connect cholinergic receptor mastery through direct and indirect agonist mechanisms to understand how different drugs achieve parasympathetic stimulation.
🔑 Cholinergic Command Center: Receptor Subtypes and Signaling Mastery
⚡ Cholinergic Agonist Arsenal: Direct Strikes and Indirect Amplification
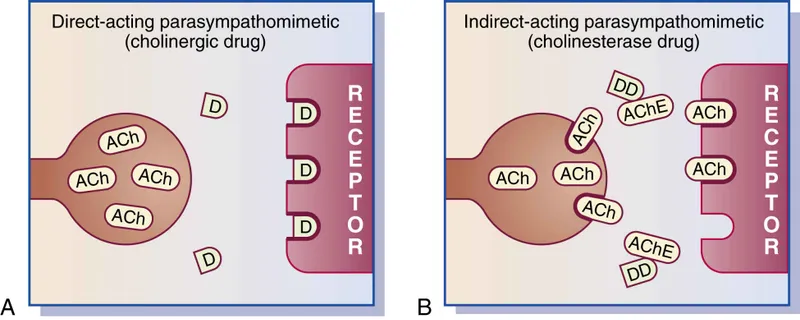
📌 Remember: DIRECT-PIMB for direct cholinergic agonists - Pilocarpine, Isoarecoline, Methacholine, Bethanechol (plus carbachol)
-
Direct Cholinergic Agonists
- Bethanechol: Selective M3 agonist (90% muscarinic selectivity)
- Clinical use: Postoperative urinary retention, neurogenic bladder
- Dosing: 25-50mg PO TID or 2.5-5mg SC
- Onset: 30-90 minutes PO, 5-15 minutes SC
- Duration: 1-6 hours depending on route
- Pilocarpine: Non-selective muscarinic agonist
- Clinical use: Glaucoma (1-4% ophthalmic drops), xerostomia
- Mechanism: ↓ intraocular pressure through ciliary muscle contraction
- Efficacy: 20-30% IOP reduction within 30 minutes
- Methacholine: Bronchial challenge testing
- Use: Asthma diagnosis through bronchial hyperresponsivity
- Threshold: PC20 <8mg/mL indicates positive test
- Sensitivity: >95% for asthma detection
- Bethanechol: Selective M3 agonist (90% muscarinic selectivity)
-
Indirect Cholinergic Agonists (AChE Inhibitors)
- Neostigmine: Perioperative standard (quaternary ammonium)
- Clinical use: Neuromuscular blockade reversal
- Dosing: 0.04-0.07mg/kg IV (max 5mg)
- Onset: 2-5 minutes, Peak: 10-30 minutes
- Duration: 30-60 minutes
- Limitation: Does not cross blood-brain barrier
- Physostigmine: CNS penetration (tertiary amine)
- Clinical use: Anticholinergic toxicity reversal
- Dosing: 1-2mg IV (adults), 0.02mg/kg (pediatric)
- Onset: 2-5 minutes, Duration: 30-60 minutes
- Advantage: Crosses blood-brain barrier
- Pyridostigmine: Myasthenia gravis maintenance
- Dosing: 60-120mg PO q4-6h
- Half-life: 3-4 hours (longer than neostigmine)
- Advantage: Better oral bioavailability (10-20% vs 1-2%)
- Neostigmine: Perioperative standard (quaternary ammonium)
| Drug | Mechanism | Selectivity | BBB Penetration | Primary Use | Onset (IV) | Duration |
|---|---|---|---|---|---|---|
| Bethanechol | Direct M3 | Muscarinic | No | Urinary Retention | 5-15 min | 1-6 hrs |
| Pilocarpine | Direct | Muscarinic | Limited | Glaucoma | 30 min | 4-8 hrs |
| Neostigmine | Indirect AChE | Non-selective | No | NMB Reversal | 2-5 min | 30-60 min |
| Physostigmine | Indirect AChE | Non-selective | Yes | Anticholinergic OD | 2-5 min | 30-60 min |
| Pyridostigmine | Indirect AChE | Non-selective | No | Myasthenia Gravis | 15-30 min | 3-6 hrs |
💡 Master This: Sugammadex (16mg/kg) provides faster neuromuscular recovery (2-3 minutes) than neostigmine (10-30 minutes) for rocuronium reversal without cholinergic side effects
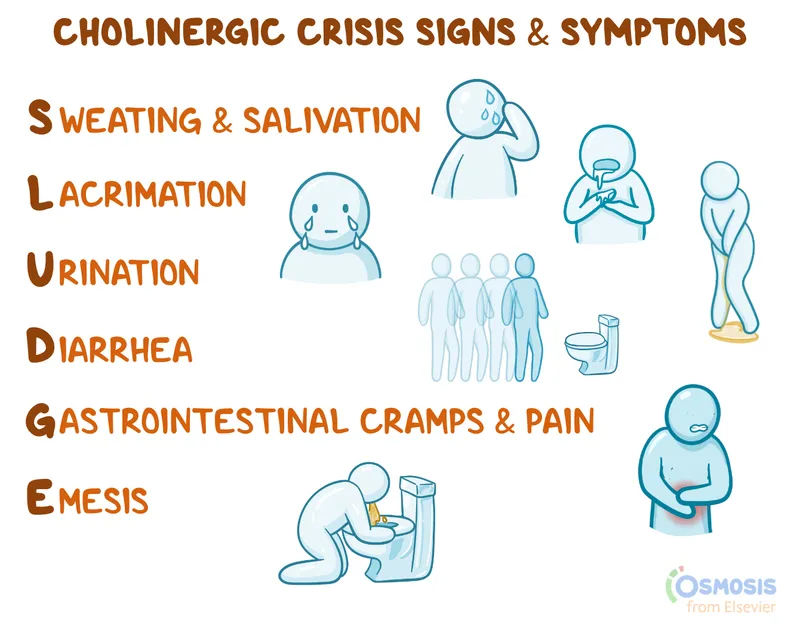
Cholinergic Crisis Management requires immediate recognition and intervention:
- DUMBBELSS syndrome: Diarrhea, Urination, Miosis, Bronchospasm, Bradycardia, Emesis, Lacrimation, Salivation, Sweating
- Atropine dosing: 2-4mg IV bolus, repeat q5-10 minutes until secretions dry
- Pralidoxime: 1-2g IV for organophosphate poisoning (within 24-48 hours)
- Supportive care: Airway management, mechanical ventilation if needed
⚠️ Warning: Edrophonium test for myasthenia gravis can precipitate cholinergic crisis in overdosed patients-always have atropine ready and monitor respiratory function
Connect cholinergic agonist mastery through muscarinic antagonist mechanisms to understand how anticholinergic drugs provide therapeutic balance.
⚡ Cholinergic Agonist Arsenal: Direct Strikes and Indirect Amplification
🛡️ Muscarinic Blockade Mastery: The Anticholinergic Arsenal
📌 Remember: DRY-FAST-HOT for anticholinergic toxicity - Dry mouth, Red skin, Yellow vision; Fever, Agitation, Seizures, Tachycardia; Hot skin, Ocular changes, Thermoregulation loss
- Atropine: The Gold Standard
- Mechanism: Competitive muscarinic antagonist (all subtypes)
- Onset: 1-2 minutes IV, 15-30 minutes IM/PO
- Duration: 4-6 hours (dose-dependent)
- Clinical applications:
- Bradycardia: 0.5-1mg IV (avoid <0.5mg paradoxical effect)
- Organophosphate poisoning: 2-4mg IV, repeat until secretions controlled
- Premedication: 0.01mg/kg to reduce secretions
- Ophthalmology: 1% drops for mydriasis/cycloplegia
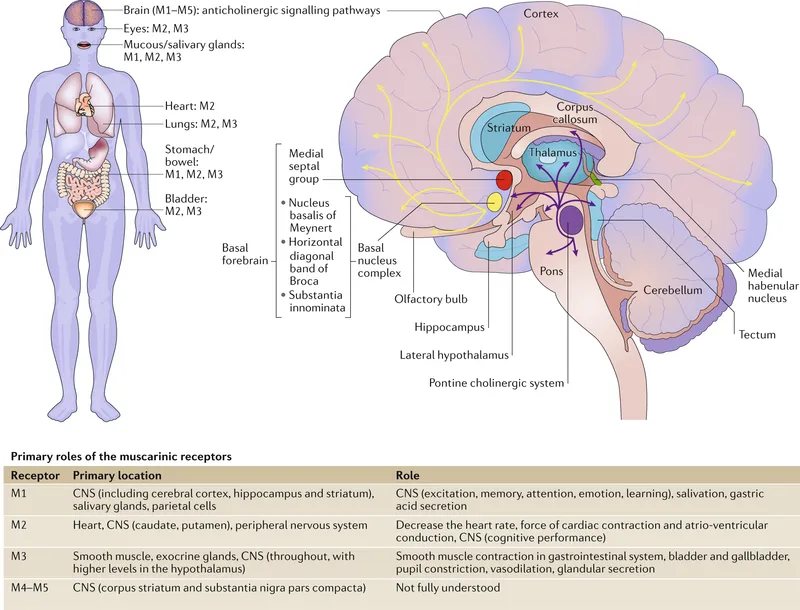
-
Scopolamine: CNS Specialist
- Advantage: Superior CNS penetration vs atropine
- Clinical use: Motion sickness (1.5mg transdermal patch)
- Duration: 72 hours (transdermal), 4-6 hours (IV)
- Sedation: More pronounced than atropine
- Amnesia: Anterograde memory impairment
-
Glycopyrrolate: Peripheral Precision
- Advantage: Quaternary ammonium = no CNS effects
- Clinical use: Perioperative antisialagogue
- Dosing: 0.1-0.2mg IV or 0.004mg/kg
- Duration: 2-4 hours
- Benefit: Cardiac effects without CNS toxicity
| Drug | CNS Penetration | Onset (IV) | Duration | Primary Uses | Dose Range | Key Advantage |
|---|---|---|---|---|---|---|
| Atropine | High | 1-2 min | 4-6 hrs | Bradycardia, Poisoning | 0.5-4mg | Universal antidote |
| Scopolamine | High | 15-30 min | 4-72 hrs | Motion sickness | 0.3-0.6mg | CNS sedation |
| Glycopyrrolate | None | 2-5 min | 2-4 hrs | Perioperative | 0.1-0.2mg | No CNS effects |
| Ipratropium | Minimal | 15-30 min | 4-6 hrs | Bronchodilation | 20-40 mcg | Respiratory selective |
| Tiotropium | None | 30-60 min | 24 hrs | COPD | 18 mcg daily | Long-acting |
💡 Master This: Physostigmine (1-2mg IV) specifically reverses CNS anticholinergic toxicity from atropine, scopolamine, tricyclics, or antihistamines-neostigmine cannot cross BBB and won't treat CNS symptoms

Anticholinergic Toxicity Recognition:
- Cardiovascular: Tachycardia >100 bpm, hypertension, arrhythmias
- CNS: Agitation, confusion, hallucinations, seizures, coma
- Thermoregulation: Hyperthermia (can reach >40°C)
- Ocular: Mydriasis, cycloplegia, photophobia
- Integumentary: Dry, flushed skin, absent sweating
- GI: Decreased motility, ileus, constipation
Clinical Mnemonic: "Red as a beet, dry as a bone, hot as a hare, blind as a bat, mad as a hatter"
⚠️ Warning: Tricyclic antidepressant overdose combines anticholinergic toxicity with sodium channel blockade-physostigmine treats CNS symptoms but sodium bicarbonate (1-2 mEq/kg) addresses cardiac conduction delays
Respiratory Applications:
- Ipratropium: Short-acting muscarinic antagonist (SAMA)
- Mechanism: M3 receptor blockade in bronchial smooth muscle
- Dosing: 20-40 mcg via MDI q6h or 0.5mg via nebulizer
- Onset: 15-30 minutes, Peak: 1-2 hours
- Tiotropium: Long-acting muscarinic antagonist (LAMA)
- Duration: 24 hours (once daily dosing)
- Selectivity: M1/M3 > M2 (preserves cardiac M2 function)
- COPD efficacy: 15-20% improvement in FEV1
Connect muscarinic antagonist mastery through adrenergic receptor architecture to understand sympathetic system pharmacology.
🛡️ Muscarinic Blockade Mastery: The Anticholinergic Arsenal
🚀 Adrenergic Receptor Command: The Sympathetic Control Matrix
📌 Remember: α1-Gq-CONSTRICT (vasoconstriction, mydriasis, urinary retention) vs β1-Gs-CARDIAC (↑ HR, ↑ contractility) vs β2-Gs-RELAX (bronchodilation, vasodilation)
-
Alpha-1 Adrenergic Receptors
- G-protein coupling: Gq/11 → ↑ IP3/DAG → ↑ intracellular Ca2+
- Primary locations: Vascular smooth muscle (>80%), prostate, iris
- Physiological effects:
- Vasoconstriction: ↑ SVR, ↑ blood pressure
- Mydriasis: Pupil dilation (radial muscle contraction)
- Urinary retention: Bladder neck/prostate contraction
- Clinical significance: Hypertension target, BPH symptoms
-
Alpha-2 Adrenergic Receptors
- G-protein coupling: Gi/o → ↓ cAMP → ↓ Ca2+ influx
- Primary locations: Presynaptic terminals (negative feedback), CNS
- Physiological effects:
- Presynaptic inhibition: ↓ NE release (autoreceptor function)
- Central effects: ↓ sympathetic outflow, sedation
- Platelet aggregation: ↑ clotting (α2A subtype)
- Clinical significance: Hypertension, sedation, analgesia
-
Beta-1 Adrenergic Receptors
- G-protein coupling: Gs → ↑ cAMP → ↑ PKA → ↑ Ca2+ handling
- Primary locations: Heart (75% cardiac β-receptors), kidney (JGA)
- Physiological effects:
- ↑ Heart rate (SA node automaticity)
- ↑ Contractility (ventricular inotropy)
- ↑ Conduction velocity (AV node)
- ↑ Renin release (juxtaglomerular apparatus)
- Clinical significance: Heart failure, arrhythmias, hypertension
-
Beta-2 Adrenergic Receptors
- G-protein coupling: Gs → ↑ cAMP → smooth muscle relaxation
- Primary locations: Lungs (90% pulmonary β-receptors), vascular smooth muscle
- Physiological effects:
- Bronchodilation: Airway smooth muscle relaxation
- Vasodilation: Skeletal muscle blood vessels
- ↑ Glycogenolysis: Liver/muscle glucose mobilization
- ↑ Lipolysis: Adipose tissue fat breakdown
- Clinical significance: Asthma, COPD, preterm labor
| Receptor | G-Protein | Primary Location | Key Effects | Clinical Targets | Selectivity Drugs |
|---|---|---|---|---|---|
| α1 | Gq/11 | Vascular SM | Vasoconstriction | HTN, BPH | Prazosin, Doxazosin |
| α2 | Gi/o | Presynaptic, CNS | ↓ NE release | HTN, Sedation | Clonidine, Dexmedetomidine |
| β1 | Gs | Heart, Kidney | ↑ HR, ↑ Contractility | HF, Arrhythmias | Metoprolol, Atenolol |
| β2 | Gs | Lungs, Vessels | Bronchodilation | Asthma, COPD | Albuterol, Salmeterol |
| β3 | Gs | Adipose, Bladder | Lipolysis, Relaxation | Obesity, OAB | Mirabegron |
💡 Master This: Epinephrine reversal occurs with α-blockers (phentolamine)-β2 vasodilation predominates over α1 vasoconstriction, causing paradoxical hypotension instead of expected pressor response
Receptor Distribution Patterns:
- Cardiac: β1 > β2 (75:25 ratio), minimal α1
- Pulmonary: β2 >> β1 (90:10 ratio), α1 in vessels
- Vascular: α1 dominant (constriction), β2 in skeletal muscle
- Renal: β1 (renin), α1 (vasoconstriction), α2 (↓ renin)
- CNS: α2 (sedation), β1 (minimal), β2 (tremor)
⚠️ Warning: Non-selective β-blockers (propranolol) can precipitate severe bronchospasm in COPD/asthma patients through β2 blockade-use β1-selective agents (metoprolol) when β-blockade is essential
Clinical Selectivity Principles:
- α1-selective antagonists: Postural hypotension risk (first-dose effect)
- α2-agonists: Rebound hypertension with abrupt discontinuation
- β1-selective blockers: Preferred in pulmonary disease
- β2-selective agonists: Tremor/tachycardia from β1 spillover
Connect adrenergic receptor mastery through sympathomimetic drug mechanisms to understand how different agonists achieve therapeutic selectivity.
🚀 Adrenergic Receptor Command: The Sympathetic Control Matrix
💥 Sympathomimetic Powerhouse: Direct, Indirect, and Mixed-Action Agents
📌 Remember: DIRECT-END for direct agonists - Epinephrine, Norepinephrine, Dopamine (high dose), Isoproterenol, Ritodrine, Ephedrine (partial), Clonidine, Terbutaline
-
Direct-Acting Sympathomimetics
- Epinephrine: Non-selective α/β agonist (equipotent α1/β1/β2)
- Cardiac arrest: 1mg IV q3-5min (1:10,000 concentration)
- Anaphylaxis: 0.3-0.5mg IM (1:1,000 concentration)
- Onset: 1-2 minutes IV, 5-10 minutes IM
- Duration: 5-10 minutes (rapid metabolism)
- Effects: ↑ HR, ↑ BP, bronchodilation, vasoconstriction
- Norepinephrine: α1 >> β1 > β2 (potent vasoconstrictor)
- Septic shock: 0.1-3 mcg/kg/min (first-line vasopressor)
- Mechanism: ↑ SVR with preserved cardiac output
- Advantage: Minimal β2 effects (less tachycardia)
- Dopamine: Dose-dependent receptor selectivity
- Low dose (2-5 mcg/kg/min): D1 receptors (renal vasodilation)
- Medium dose (5-10 mcg/kg/min): β1 receptors (inotropic)
- High dose (>10 mcg/kg/min): α1 receptors (vasopressor)
- Epinephrine: Non-selective α/β agonist (equipotent α1/β1/β2)
-
Indirect-Acting Sympathomimetics
- Amphetamine: NE reuptake inhibition + vesicular release
- Mechanism: Blocks NET and reverses transporter
- Duration: 4-8 hours (longer than direct agonists)
- Clinical use: ADHD, narcolepsy (controlled substance)
- Cocaine: Triple reuptake inhibition (NET/DAT/SERT)
- Toxicity: Coronary spasm, arrhythmias, seizures
- Treatment: Avoid β-blockers (unopposed α-stimulation)
- Tyramine: Vesicular NE release (cheese effect)
- MAOI interaction: Hypertensive crisis risk
- Mechanism: Accumulated tyramine → massive NE release
- Amphetamine: NE reuptake inhibition + vesicular release
-
Mixed-Acting Sympathomimetics
- Ephedrine: Direct α/β agonist + NE release
- Hypotension: 5-25mg IV bolus (especially spinal anesthesia)
- Onset: 2-5 minutes, Duration: 30-60 minutes
- Advantage: Oral bioavailability, CNS penetration
- Tachyphylaxis: Decreased response with repeated dosing
- Pseudoephedrine: Nasal decongestant (OTC restrictions)
- Mechanism: α1 vasoconstriction in nasal mucosa
- Systemic effects: ↑ BP, ↑ HR (less than ephedrine)
- Ephedrine: Direct α/β agonist + NE release
| Drug | Mechanism | α1 | β1 | β2 | Clinical Use | Dose Range | Duration |
|---|---|---|---|---|---|---|---|
| Epinephrine | Direct | +++ | +++ | +++ | Cardiac arrest, Anaphylaxis | 0.3-1mg | 5-10 min |
| Norepinephrine | Direct | ++++ | ++ | + | Septic shock | 0.1-3 mcg/kg/min | 2-5 min |
| Dopamine | Direct | +/++++ | ++/+++ | + | Cardiogenic shock | 2-20 mcg/kg/min | 5-10 min |
| Isoproterenol | Direct | 0 | ++++ | ++++ | Heart block, Asthma | 1-10 mcg/min | 5-10 min |
| Ephedrine | Mixed | ++ | ++ | ++ | Hypotension | 5-25mg | 30-60 min |
💡 Master This: Norepinephrine is first-line vasopressor for septic shock because α1 selectivity provides vasoconstriction without excessive β2 vasodilation that can worsen distributive shock patterns
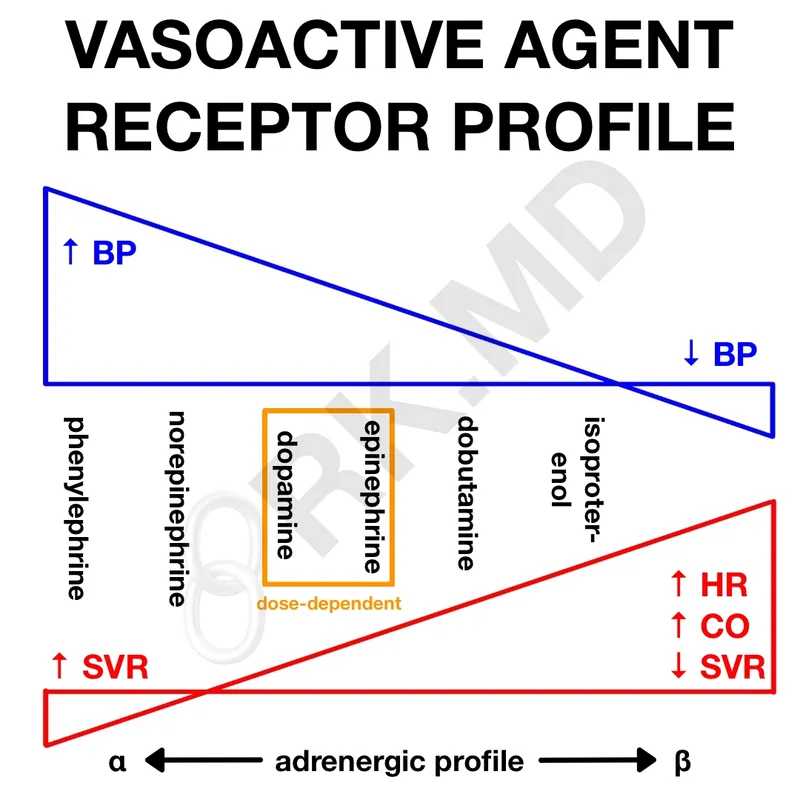
Clinical Application Principles:
- Cardiac arrest: Epinephrine 1mg IV → α1 effects improve coronary/cerebral perfusion pressure
- Anaphylaxis: Epinephrine IM → β2 bronchodilation + α1 vasoconstriction + β1 inotropy
- Septic shock: Norepinephrine → α1 vasoconstriction restores SVR without excessive chronotropy
- Cardiogenic shock: Dopamine/dobutamine → β1 inotropy improves cardiac output
Receptor-Specific Therapeutic Targets:
- α1 agonists: Hypotension, nasal congestion, urinary incontinence
- α2 agonists: Hypertension, sedation, opioid withdrawal
- β1 agonists: Heart failure, cardiogenic shock, heart block
- β2 agonists: Asthma, COPD, preterm labor, hyperkalemia
⚠️ Warning: Cocaine toxicity requires avoiding β-blockers due to unopposed α-stimulation risk-use phentolamine for hypertension and benzodiazepines for agitation/seizures
Tachyphylaxis Considerations:
- Ephedrine: Depletes NE stores with repeated dosing
- Indirect agents: Lose efficacy as vesicular NE becomes depleted
- Direct agents: Maintain efficacy through direct receptor activation
Connect sympathomimetic mastery through adrenergic antagonist mechanisms to understand how α and β-blockers provide therapeutic balance in cardiovascular disease.
💥 Sympathomimetic Powerhouse: Direct, Indirect, and Mixed-Action Agents
🛑 Adrenergic Antagonist Arsenal: Precision Blockade for Cardiovascular Control
📌 Remember: α1-BLOCK-DROPS (blood pressure, peripheral resistance, urinary obstruction) vs β1-BLOCK-SLOWS (heart rate, contractility, conduction, renin)
- Alpha-1 Selective Antagonists
- Prazosin: Selective α1 antagonist (>1000:1 α1:α2 selectivity)
- Mechanism: Competitive α1 blockade → vasodilation
- Clinical use: Hypertension, BPH symptoms
- Dosing: 1mg BID initially → up to 20mg daily
- First-dose effect: Orthostatic hypotension (30-90 minutes)
- Doxazosin: Long-acting α1 antagonist
- Half-life: 12-22 hours (once daily dosing)
- BPH efficacy: 30-40% improvement in symptom scores
- Cardiovascular: ↓ BP without reflex tachycardia
- Tamsulosin: α1A-selective (prostate-specific)
- Selectivity: α1A > α1D >> α1B (minimal cardiovascular effects)
- BPH dosing: 0.4mg daily (no titration needed)
- Advantage: Reduced orthostatic hypotension risk
- Prazosin: Selective α1 antagonist (>1000:1 α1:α2 selectivity)
-
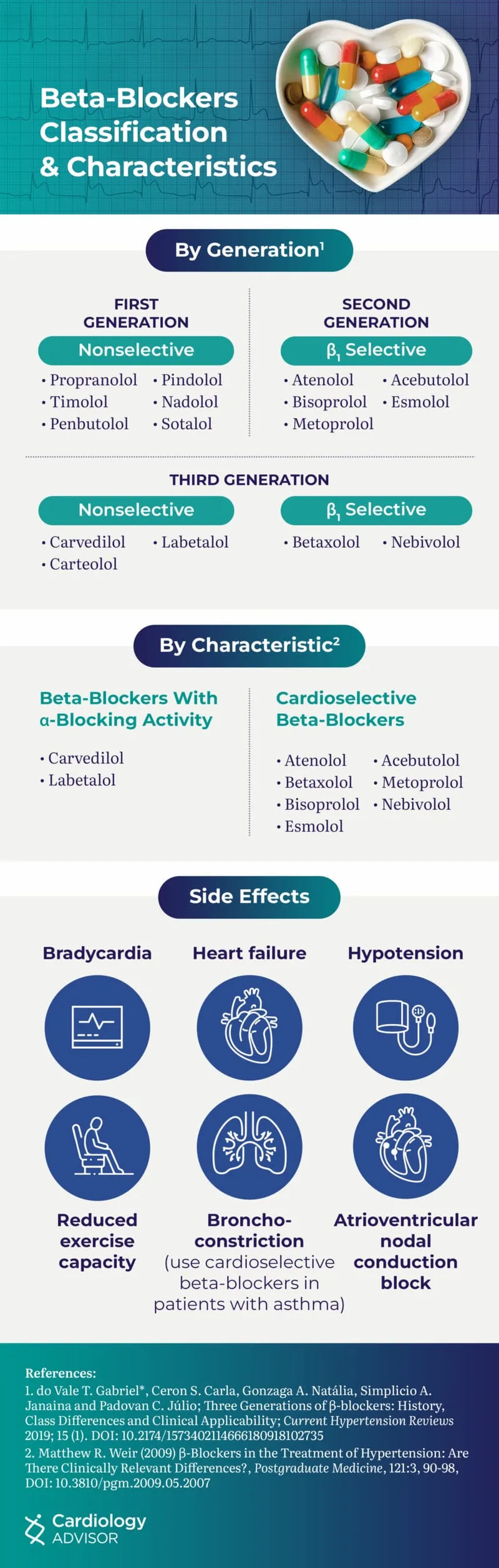
Beta-1 Selective Antagonists (Cardioselective)
- Metoprolol: β1-selective (β1:β2 = 75:1 at therapeutic doses)
- Heart failure: 12.5-25mg BID → target 200mg BID
- Mortality benefit: 34% reduction in HF death (MERIT-HF)
- Formulations: Immediate-release vs extended-release
- Atenolol: β1-selective with renal elimination
- Half-life: 6-7 hours (adjust in renal impairment)
- Hypertension: 25-100mg daily
- Limitation: Poor CNS penetration (hydrophilic)
- Bisoprolol: Highly β1-selective (β1:β2 = 120:1)
- Heart failure: 1.25mg daily → target 10mg daily
- CIBIS-II: 34% mortality reduction in HF patients
- Metoprolol: β1-selective (β1:β2 = 75:1 at therapeutic doses)
-
Non-Selective Beta Antagonists
- Propranolol: Equal β1/β2 blockade + membrane stabilizing
- Clinical uses: Migraine, essential tremor, portal hypertension
- CNS effects: Lipophilic → crosses BBB → anxiety reduction
- Contraindications: Asthma, COPD (β2 blockade)
- Nadolol: Long-acting non-selective (24-hour dosing)
- Half-life: 20-24 hours
- Renal elimination: Dose adjustment in kidney disease
- Propranolol: Equal β1/β2 blockade + membrane stabilizing
| Drug Class | Selectivity | Primary Uses | Mortality Benefit | Key Advantage | Major Limitation |
|---|---|---|---|---|---|
| α1 Blockers | α1 selective | HTN, BPH | No CV benefit | ↓ Urinary symptoms | First-dose hypotension |
| β1 Selective | β1 > β2 | HF, HTN, MI | 34% HF mortality ↓ | Safe in lung disease | Lose selectivity at high doses |
| Non-selective β | β1 = β2 | Migraine, Tremor | Variable | CNS penetration | Bronchospasm risk |
| α + β Block | α1 + β1/β2 | HTN, HF | Mixed evidence | Dual mechanism | Complex titration |
💡 Master This: Carvedilol combines α1 blockade (vasodilation) with β1/β2 blockade (cardiac depression), providing unique hemodynamic profile with ↓ afterload and ↓ preload for advanced heart failure
Evidence-Based Clinical Applications:
-
Heart Failure with Reduced EF:
- Metoprolol succinate: Target 200mg daily
- Carvedilol: Target 25mg BID (50mg BID if >85kg)
- Bisoprolol: Target 10mg daily
- Mortality reduction: 30-35% across major trials
-
Post-MI Secondary Prevention:
- β-blockers reduce mortality by 23% (long-term therapy)
- Start within 24 hours if hemodynamically stable
- Continue indefinitely unless contraindicated
-
Hypertension Management:
- β-blockers no longer first-line (2017 ACC/AHA guidelines)
- Consider in specific indications: HF, post-MI, arrhythmias
- α1-blockers reserved for resistant HTN or BPH comorbidity
Contraindications and Cautions:
- Absolute contraindications: Cardiogenic shock, severe bradycardia (<50 bpm), high-grade AV block
- Relative contraindications: Asthma (β2 blockade), severe COPD, cocaine use
- Withdrawal syndrome: Rebound hypertension and MI risk with abrupt discontinuation
⚠️ Warning: Abrupt β-blocker withdrawal can precipitate rebound hypertension, angina, and MI within 24-48 hours-taper over 1-2 weeks when discontinuing chronic therapy
Special Populations:
- Diabetes: β1-selective agents preferred (mask hypoglycemia less)
- Peripheral vascular disease: Avoid non-selective β-blockers
- Pregnancy: Labetalol and methyldopa are preferred agents
- Elderly: Start low doses (increased sensitivity to hypotension)
This comprehensive mastery of cholinergic and adrenergic pharmacology provides the foundation for precision autonomic medicine, enabling optimal therapeutic outcomes while minimizing adverse effects through mechanistic understanding and evidence-based application.
🛑 Adrenergic Antagonist Arsenal: Precision Blockade for Cardiovascular Control
Practice Questions: Cholinergic/Adrenergic drugs
Test your understanding with these related questions
In patients with chronic obstructive pulmonary disease, stimulation of muscarinic acetylcholine receptors results in an increase in mucus secretion, smooth muscle contraction and bronchoconstriction. The end result is an increase in airway resistance. Which of the following pharmacologic agents interferes directly with this pathway?













