Antibiotics

On this page
🎯 Antibiotic Arsenal: Your Microbial Warfare Command Center
Antibiotics represent one of medicine's most powerful interventions, yet wielding them effectively demands far more than memorizing drug names. You'll master how antibiotics dismantle bacteria at the molecular level, recognize which pathogens and clinical syndromes call for specific agents, anticipate resistance patterns that threaten treatment success, and optimize therapy through pharmacokinetic principles and stewardship strategies. This lesson transforms antibiotic prescribing from guesswork into precision clinical reasoning, equipping you to match mechanism to microbe, drug to disease, and strategy to patient with confidence and accuracy.
The Antibiotic Classification Command Structure
-
Beta-Lactam Dynasty (40% of all antibiotic prescriptions)
- Penicillins: The original wall-breakers (1928 discovery revolutionized medicine)
- Cephalosporins: 4 generations of evolving spectrum
- Carbapenems: The "big guns" for resistant organisms
- Imipenem: 99% effective against Gram-positives
- Meropenem: Superior CNS penetration (CSF levels 20-30% of serum)
- Ertapenem: Once-daily dosing advantage
-
Protein Synthesis Inhibitors (30S and 50S ribosomal targets)
- Aminoglycosides: Concentration-dependent killing with post-antibiotic effect
- Macrolides: Tissue concentrations exceed serum by 10-100x
- Tetracyclines: Broad-spectrum with intracellular penetration
📌 Remember: CAMPFIRE for major antibiotic families - Cephalosporins, Aminoglycosides, Macrolides, Penicillins, Fluoroquinolones, Imidazoles, Rifamycins, Erythromycin derivatives. Each family targets different bacterial vulnerabilities with distinct resistance patterns.
| Antibiotic Class | Primary Target | Bactericidal/Static | Resistance Mechanism | Clinical Penetration | Cost Index |
|---|---|---|---|---|---|
| Penicillins | Cell Wall (PBPs) | Bactericidal | Beta-lactamases (60%) | Poor CNS | Low ($) |
| Fluoroquinolones | DNA Gyrase | Bactericidal | Target mutations (25%) | Excellent tissue | Medium ($) |
| Aminoglycosides | 30S Ribosome | Bactericidal | Enzymatic modification (40%) | Poor intracellular | Medium ($) |
| Macrolides | 50S Ribosome | Bacteriostatic | Efflux pumps (35%) | Excellent intracellular | High ($$) |
| Vancomycin | Cell Wall (D-Ala-D-Ala) | Bactericidal | Target modification (5%) | Poor tissue | High ($$) |
Understanding antibiotic pharmacodynamics unlocks the logic behind dosing strategies and combination therapy decisions.
🎯 Antibiotic Arsenal: Your Microbial Warfare Command Center
⚔️ Mechanism Mastery: The Bacterial Destruction Playbook
Cell Wall Synthesis Disruption Mechanisms
-
Beta-Lactam Action (Penicillins, Cephalosporins, Carbapenems)
- Target: Penicillin-binding proteins (PBPs) in peptidoglycan synthesis
- Mechanism: Irreversible acylation of serine residue in active site
- Result: Osmotic lysis due to weakened cell wall (2-4 hours post-exposure)
- Time-dependent killing: Efficacy correlates with %T>MIC
- Optimal effect: 40-50% of dosing interval above MIC
- Post-antibiotic effect: 1-2 hours for Gram-positives
-
Glycopeptide Mechanism (Vancomycin, Teicoplanin)
- Target: D-Alanyl-D-alanine terminus of peptidoglycan precursors
- Binding: Hydrogen bonding prevents transglycosylation
- Resistance: D-Ala-D-Lac substitution reduces binding 1000-fold
📌 Remember: WALL ATTACK for cell wall inhibitors - Weakens peptidoglycan, Acylates PBPs, Lyses bacteria, Leads to death, Affects growing cells, Time-dependent, Targets synthesis, Active against dividing organisms, Concentration above MIC critical, Kills through osmotic pressure.
Protein Synthesis Interference Patterns
-
30S Ribosomal Subunit Inhibitors (Aminoglycosides, Tetracyclines)
-
Aminoglycosides: Bind 16S rRNA causing misreading of mRNA
- Concentration-dependent: Cmax/MIC >8-10 for optimal killing
- Post-antibiotic effect: 2-8 hours duration
- Synergy: With cell wall inhibitors (enhanced uptake)
-
Tetracyclines: Block A-site of ribosome, prevent tRNA binding
- Bacteriostatic: Reversible binding allows recovery
- Broad spectrum: Effective against intracellular pathogens
-
-
50S Ribosomal Subunit Inhibitors (Macrolides, Chloramphenicol, Lincosamides)
- Macrolides: Bind 23S rRNA, block peptide exit tunnel
- Chloramphenicol: Inhibits peptidyl transferase activity
- Lincosamides: Prevent peptide bond formation
⭐ Clinical Pearl: Ribosomal binding sites determine cross-resistance patterns. MLSB resistance (Macrolides, Lincosamides, Streptogramins B) occurs through 23S rRNA methylation, affecting all 50S inhibitors simultaneously with >90% resistance rates.
💡 Master This: Bacteriostatic vs bactericidal distinction matters in immunocompromised patients. Bacteriostatic drugs require functional host immunity for bacterial clearance, while bactericidal agents achieve >99.9% killing independent of immune status.
Mechanism knowledge predicts drug interactions, resistance development, and optimal dosing strategies for clinical success.
⚔️ Mechanism Mastery: The Bacterial Destruction Playbook
🎯 Clinical Pattern Recognition: The Diagnostic Decision Matrix
Infection Syndrome Recognition Patterns
-
Respiratory Tract Infections (RTI Pattern Recognition)
-
Community-Acquired Pneumonia (CAP)
- Typical pathogens: S. pneumoniae (35%), H. influenzae (15%)
- First-line: Amoxicillin 1g TID or macrolide
- Severe CAP: Beta-lactam + macrolide (mortality reduction 15%)
-
Healthcare-Associated Pneumonia (HCAP)
- Risk factors: Hospitalization >2 days in past 90 days
- Pathogens: MRSA (25%), Pseudomonas (20%), Klebsiella (15%)
- Empirical: Vancomycin + anti-pseudomonal beta-lactam
-
-
Urinary Tract Infections (UTI Spectrum Analysis)
-
Uncomplicated Cystitis (Young, healthy females)
- E. coli dominance: 75-85% of cases
- Resistance rates: TMP-SMX (20%), fluoroquinolones (15%)
- First-line: Nitrofurantoin 100mg BID x 5 days (95% efficacy)
-
Complicated UTI/Pyelonephritis
- Broader spectrum: Enterococcus (15%), Pseudomonas (10%)
- Empirical: Fluoroquinolone or ceftriaxone
- Duration: 7-14 days vs 3 days for simple cystitis
-
📌 Remember: SITE BUGS for infection-specific pathogens - Skin (Staph/Strep), Intra-abdominal (E. coli/Bacteroides), Thoracic (Pneumococcus), Endocarditis (Staph/Enterococcus), Blood (Staph/E. coli), Urinary (E. coli), Genitourinary (Chlamydia/Gonorrhea), Surgical site (Staph).
| Infection Site | Most Common Pathogen | Resistance Rate | First-Line Therapy | Alternative | Duration |
|---|---|---|---|---|---|
| CAP (Outpatient) | S. pneumoniae (35%) | Penicillin (15%) | Amoxicillin 1g TID | Macrolide | 5-7 days |
| UTI (Simple) | E. coli (80%) | TMP-SMX (20%) | Nitrofurantoin | Fosfomycin | 3-5 days |
| Cellulitis | S. pyogenes (40%) | Clindamycin (10%) | Cephalexin 500mg QID | Clindamycin | 7-10 days |
| IAI (Mild) | E. coli + Bacteroides | Amp/Sulb (25%) | Amox/Clav 875mg BID | Ciprofloxacin + Metro | 5-7 days |
| Endocarditis | S. aureus (30%) | MRSA (45%) | Vancomycin + Gentamicin | Daptomycin | 4-6 weeks |
-
MRSA Risk Stratification (Methicillin-Resistant S. aureus)
- High-risk factors: ICU admission, prior antibiotics (30 days), dialysis
- Prevalence: 25-50% in healthcare settings vs 1-2% community
- Empirical coverage: Required when >10-20% local prevalence
- Agents: Vancomycin, linezolid, daptomycin, ceftaroline
-
ESBL Pattern Recognition (Extended-Spectrum Beta-Lactamases)
- Risk factors: Recent hospitalization, nursing home, prior fluoroquinolones
- Organisms: E. coli (15-25%), Klebsiella (20-40%)
- Resistance: All penicillins, cephalosporins (except cephamycins)
- Treatment: Carbapenems (meropenem preferred)
⭐ Clinical Pearl: Local antibiograms guide empirical therapy more accurately than national guidelines. Hospital-specific resistance rates vary 2-5 fold from published averages, making institutional data critical for >80% empirical success rates.
💡 Master This: De-escalation strategy improves outcomes and reduces resistance. Start broad-spectrum empirical therapy, then narrow based on cultures within 48-72 hours. This approach reduces C. difficile risk by 30-50% while maintaining clinical efficacy.
Pattern recognition enables rapid, evidence-based antibiotic selection before definitive pathogen identification.
🎯 Clinical Pattern Recognition: The Diagnostic Decision Matrix
🔬 Resistance Analysis: The Bacterial Counterstrike Intelligence
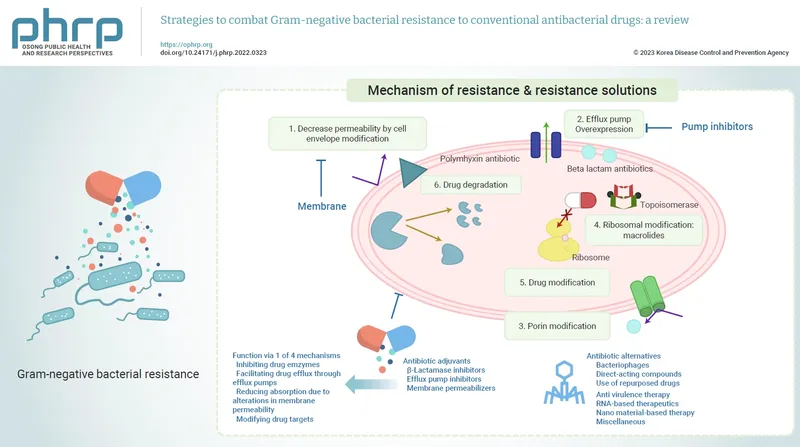
Enzymatic Destruction Mechanisms
-
Beta-Lactamase Classification (Ambler System)
-
Class A (Serine beta-lactamases): TEM, SHV, CTX-M families
- TEM-1: >80% E. coli resistance to ampicillin
- ESBL variants: CTX-M-15 most common (>50% of ESBLs globally)
- Inhibition: Clavulanate, sulbactam, tazobactam effective
-
Class B (Metallo-beta-lactamases): NDM, VIM, IMP types
- Carbapenem resistance: >95% hydrolysis of all beta-lactams
- No inhibitors: Current beta-lactamase inhibitors ineffective
- Treatment: Polymyxins, tigecycline, newer agents (ceftazidime-avibactam)
-
-
Aminoglycoside-Modifying Enzymes (AMEs)
- AAC (acetyltransferases): 6'-AAC confers gentamicin resistance
- ANT (nucleotidyltransferases): 2''-ANT affects tobramycin
- APH (phosphotransferases): 3'-APH causes kanamycin resistance
- Clinical impact: >70% Enterobacteriaceae carry multiple AMEs
📌 Remember: BETA BLAST for beta-lactamase types - Broad spectrum (TEM/SHV), Extended spectrum (CTX-M), Target carbapenems (KPC), All beta-lactams (MBL), Blocks inhibitors (OXA), Large plasmids (spread), Ambler classes (A-D), Serine vs metallo, Treatment challenges.
| Resistance Mechanism | Enzyme Family | Substrate Spectrum | Inhibitor Susceptible | Prevalence | Clinical Impact |
|---|---|---|---|---|---|
| TEM-1 | Class A ESBL | Penicillins | Clavulanate (Yes) | >80% E. coli | Ampicillin resistance |
| CTX-M-15 | Class A ESBL | Cephalosporins | Clavulanate (Yes) | 50% ESBLs | 3rd gen ceph resistance |
| KPC-2 | Class A Carbapenemase | All beta-lactams | Clavulanate (Partial) | 25% Klebsiella | Carbapenem resistance |
| NDM-1 | Class B MBL | All beta-lactams | None (No) | 15% Enterobacteriaceae | Pan-beta-lactam resistance |
| OXA-48 | Class D Carbapenemase | Carbapenems | None (No) | 30% Mediterranean | Carbapenem resistance |
-
PBP Alterations (Penicillin-Binding Protein Changes)
- MRSA mechanism: mecA gene encodes PBP2a with low beta-lactam affinity
- Binding reduction: >1000-fold decreased penicillin binding
- Cross-resistance: All beta-lactams except ceftaroline (maintains PBP2a activity)
-
Ribosomal Modifications (rRNA Methylation)
- MLSB resistance: erm genes methylate 23S rRNA A2058 position
- Cross-resistance: Macrolides, lincosamides, streptogramins B
- Prevalence: >60% S. pneumoniae in some regions
-
DNA Gyrase Mutations (Fluoroquinolone Resistance)
- Primary targets: gyrA and parC genes (QRDR regions)
- Stepwise accumulation: 2-4 mutations for high-level resistance
- Clinical threshold: >4-fold MIC increase predicts treatment failure
⭐ Clinical Pearl: Heteroresistance complicates susceptibility testing. Vancomycin-intermediate S. aureus (VISA) shows subpopulations with reduced susceptibility that standard testing may miss, requiring population analysis profiling for detection.
💡 Master This: Collateral sensitivity offers therapeutic opportunities. Bacteria developing fluoroquinolone resistance often become hypersusceptible to beta-lactams through fitness costs, enabling targeted therapy selection based on resistance history.
Resistance intelligence guides combination therapy decisions and predicts treatment success patterns.
🔬 Resistance Analysis: The Bacterial Counterstrike Intelligence
⚖️ Treatment Optimization: The Therapeutic Strategy Engine
Pharmacokinetic/Pharmacodynamic Optimization
-
Time-Dependent Antibiotics (Beta-lactams, Vancomycin)
- Target: %T>MIC of 40-70% for bacteriostatic/bactericidal effect
- Optimization strategies:
- Extended infusions: 3-4 hour beta-lactam infusions vs 30 minutes
- Continuous infusions: 24-hour vancomycin for 100% T>MIC
- Frequent dosing: Q6H vs Q8H for severe infections
- Clinical benefit: 15-25% mortality reduction in severe infections
-
Concentration-Dependent Antibiotics (Fluoroquinolones, Aminoglycosides)
- Targets: Cmax/MIC >8-10 and AUC/MIC >125 for fluoroquinolones
- Aminoglycoside optimization:
- Once-daily dosing: 7mg/kg gentamicin vs divided doses
- Extended interval: Q24-48H based on renal function
- Therapeutic monitoring: Peak 5-10 mg/L, trough <2 mg/L
-
AUC-Dependent Antibiotics (Vancomycin, Linezolid)
- Vancomycin: AUC/MIC >400 for efficacy, <600 to avoid nephrotoxicity
- Monitoring: Bayesian dosing software improves target attainment >85%
- Loading doses: 25-30 mg/kg for rapid therapeutic levels
📌 Remember: PK/PD TARGETS for optimization - Peak levels (concentration-dependent), Keep above MIC (time-dependent), Pharmacokinetic monitoring, Dose adjustments, Timing optimization, AUC calculations, Renal adjustments, Goal-directed therapy, Extended infusions, Through monitoring, Steady state achievement.
| Antibiotic Class | PK/PD Parameter | Target Value | Optimization Strategy | Monitoring | Clinical Benefit |
|---|---|---|---|---|---|
| Beta-lactams | %T>MIC | 40-70% | Extended infusions | Clinical response | 20% ↓ mortality |
| Vancomycin | AUC/MIC | 400-600 | Bayesian dosing | AUC monitoring | 15% ↓ nephrotoxicity |
| Aminoglycosides | Cmax/MIC | >8-10 | Once-daily dosing | Peak/trough levels | 25% ↓ toxicity |
| Fluoroquinolones | AUC/MIC | >125 | High-dose therapy | Clinical response | 30% ↓ resistance |
| Linezolid | AUC | >200 mg·h/L | Standard dosing | Therapeutic monitoring | 10% ↓ toxicity |
-
Synergistic Combinations (Enhanced Efficacy)
-
Beta-lactam + Aminoglycoside: Cell wall + protein synthesis
- Mechanism: Beta-lactam enhances aminoglycoside uptake
- Indications: Enterococcal endocarditis, severe Pseudomonas infections
- Synergy testing: >2-log reduction in CFU with combination
-
Trimethoprim-Sulfamethoxazole: Sequential folate blockade
- Synergy ratio: 1:20 TMP:SMX for optimal effect
- Resistance prevention: >100-fold reduction vs monotherapy
-
-
Resistance Prevention Combinations
- Anti-tuberculosis therapy: 4-drug regimen prevents resistance
- H. pylori eradication: Triple/quadruple therapy for >90% success
- Antifungal combinations: Amphotericin B + flucytosine for cryptococcal meningitis
⭐ Clinical Pearl: Antibiotic cycling vs mixing strategies show different resistance impacts. Cycling (temporal rotation) may select for resistance, while mixing (simultaneous use) often prevents resistance development through collateral sensitivity mechanisms.
💡 Master This: Biofilm infections require combination therapy with extended durations. Standard MICs underestimate resistance by 10-1000 fold in biofilms, necessitating high-dose combinations and >6-week treatment courses for device-related infections.
Treatment optimization algorithms enable precision antibiotic therapy tailored to individual patient and pathogen characteristics.
⚖️ Treatment Optimization: The Therapeutic Strategy Engine
🌐 Systems Integration: The Multi-Pathogen Battlefield Network
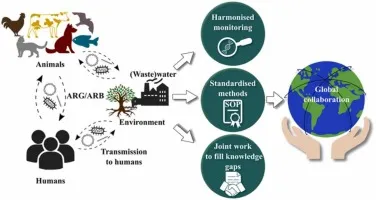
Hospital Microbiome Ecosystem Dynamics
-
Selective Pressure Networks (Antibiotic-Driven Ecology)
- C. difficile emergence: Fluoroquinolone use increases risk 3-5 fold
- MRSA selection: Cephalosporin pressure selects for mecA-positive strains
- Carbapenem resistance: Carbapenem use drives KPC/NDM emergence within 6-12 months
- Microbiome recovery: 6-24 months for normal flora restoration post-antibiotics
-
Cross-Transmission Amplification (Horizontal Gene Transfer)
- Plasmid conjugation: 10^-2 to 10^-6 transfer frequency per donor cell
- Transposon mobility: Tn916 (tetracycline resistance) transfers across >40 species
- Integron cassettes: Class 1 integrons carry multiple resistance genes
- Biofilm facilitation: 100-1000 fold increased transfer rates in biofilms
Global Surveillance Integration Networks
-
WHO Global Antimicrobial Resistance Surveillance System (GLASS)
- Participating countries: >100 nations reporting standardized data
- Priority pathogens: 12 bacterial families with critical/high priority
- Resistance trends: Carbapenem resistance increasing 5-10% annually
- Data standardization: WHONET software for global comparability
-
National Surveillance Programs
- NARMS (USA): >15,000 isolates annually from humans, animals, food
- EARS-Net (Europe): >500,000 isolates from 30 countries
- ANSORP (Asia-Pacific): Regional resistance monitoring across 15 countries
📌 Remember: GLOBAL WATCH for surveillance systems - Global coordination (WHO), Local implementation (national programs), One Health approach (human-animal-environment), Benchmarking standards, Antibiotic consumption data, Laboratory networks, WHONET software, Animal surveillance, Trend analysis, Cross-border collaboration, Horizontal gene transfer monitoring.
| Surveillance System | Geographic Scope | Annual Isolates | Key Metrics | Resistance Trends | Data Integration |
|---|---|---|---|---|---|
| GLASS | Global (>100 countries) | >1 million | AMR prevalence | 5-10% annual ↑ | WHONET |
| NARMS | USA | >15,000 | Food-human link | Salmonella resistance | CDC integration |
| EARS-Net | Europe (30 countries) | >500,000 | Hospital surveillance | MRSA declining | ECDC platform |
| ANSORP | Asia-Pacific (15 countries) | >50,000 | Regional patterns | ESBL increasing | Regional database |
| CAESAR | Central Asia (5 countries) | >10,000 | Emerging resistance | MDR-TB rising | WHO coordination |
-
Human-Animal-Environment Interface (Resistance Ecology)
- Agricultural antibiotic use: >70% of global antibiotic consumption
- Resistance transfer: Livestock to human via food chain and environmental contamination
- Colistin resistance: mcr-1 gene emerged from agricultural polymyxin use
- Environmental reservoirs: Wastewater treatment plants as resistance hotspots
-
Stewardship Integration Strategies
- Hospital stewardship: 20-30% reduction in C. difficile infections
- Outpatient optimization: >50% of prescriptions potentially inappropriate
- Agricultural restrictions: EU ban on growth promotion antibiotics
- Global action plans: >70 countries with national action plans
⭐ Clinical Pearl: Antibiotic heterogeneity within hospitals reduces resistance pressure more effectively than cycling programs. Unit-specific protocols with different first-line agents create spatial diversity that limits clonal expansion of resistant organisms.
💡 Master This: Resistance fitness costs create opportunities for targeted interventions. Carbapenem-resistant organisms often show reduced virulence and competitive disadvantage when carbapenem pressure is removed, enabling ecological restoration through targeted de-escalation.
Systems integration enables population-level antibiotic stewardship that addresses resistance as a global health security threat.
🌐 Systems Integration: The Multi-Pathogen Battlefield Network
🎯 Clinical Mastery Arsenal: The Rapid-Fire Reference Engine
Essential Clinical Decision Matrix
-
Rapid Pathogen-Antibiotic Matching (First-Line Selections)
- MRSA coverage: Vancomycin 15-20 mg/kg Q8-12H (target trough 15-20 mg/L)
- Pseudomonas coverage: Piperacillin-tazobactam 4.5g Q6H or cefepime 2g Q8H
- Anaerobe coverage: Metronidazole 500mg Q8H or clindamycin 600mg Q8H
- Atypical coverage: Azithromycin 500mg daily or levofloxacin 750mg daily
-
Critical Dosing Adjustments (Renal/Hepatic Impairment)
- CrCl 30-50 mL/min: Reduce dose 50% for renally eliminated drugs
- CrCl 10-30 mL/min: Reduce dose 75% or extend intervals
- Hemodialysis: Post-dialysis dosing for dialyzable antibiotics
- Hepatic impairment: Avoid chloramphenicol, reduce macrolide doses
📌 Remember: STAT BUGS for emergency antibiotic selection - Sepsis (broad spectrum), Tissue penetration (CNS/bone), Allergy history (penicillin), Toxicity profile (renal/hepatic), Bacterial resistance (local patterns), Urgency level (empirical vs targeted), Gram stain results (if available), Site of infection (specific coverage needs).
| Clinical Scenario | First-Line Agent | Dose | Duration | Alternative | Key Monitoring |
|---|---|---|---|---|---|
| Septic Shock | Pip-Tazo + Vancomycin | 4.5g Q6H + 15mg/kg Q12H | 7-10 days | Meropenem + Linezolid | Lactate clearance |
| Meningitis | Ceftriaxone + Vancomycin | 2g Q12H + 15mg/kg Q8H | 10-14 days | Meropenem + Vancomycin | CSF sterilization |
| Endocarditis | Vancomycin + Gentamicin | 15mg/kg Q12H + 3mg/kg Q24H | 4-6 weeks | Daptomycin + Gentamicin | Blood culture clearance |
| Neutropenic Fever | Cefepime | 2g Q8H | Until ANC >500 | Meropenem | ANC recovery |
| C. diff Colitis | Vancomycin (PO) | 125mg QID | 10-14 days | Fidaxomicin | Symptom resolution |
-
High-Risk Resistance Indicators (Red Flag Patterns)
- Prior carbapenem use: >50% risk of carbapenem-resistant Enterobacteriaceae
- ICU stay >7 days: >30% risk of multidrug-resistant organisms
- Recent hospitalization: <90 days increases MRSA/ESBL risk 3-5 fold
- Nursing home residence: >40% MRSA colonization rates
-
Empirical Coverage Decision Tree
- Low resistance risk: Standard spectrum (ceftriaxone, ampicillin-sulbactam)
- Moderate risk: Extended spectrum (piperacillin-tazobactam, cefepime)
- High resistance risk: Broad spectrum (carbapenem + vancomycin)
- Extreme risk: Last-resort agents (colistin, tigecycline, ceftazidime-avibactam)
⭐ Clinical Pearl: 48-72 hour reassessment is mandatory for all empirical therapy. De-escalation based on culture results reduces C. difficile risk by 30-50% while maintaining clinical efficacy in >95% of appropriately selected cases.
💡 Master This: Antibiotic allergies are over-reported in >90% of cases. Penicillin allergy labels lead to inferior outcomes and increased resistance. Allergy testing or graded challenges can safely de-label >80% of patients, enabling optimal beta-lactam therapy.
This clinical arsenal enables rapid, evidence-based antibiotic decisions across the full spectrum of infectious disease emergencies and routine care scenarios.
🎯 Clinical Mastery Arsenal: The Rapid-Fire Reference Engine
Practice Questions: Antibiotics
Test your understanding with these related questions
An 8-year-old girl is brought to the emergency room for a 6-hour history of fever, sore throat, and difficulty swallowing. Physical examination shows pooling of oral secretions and inspiratory stridor. Lateral x-ray of the neck shows thickening of the epiglottis and aryepiglottic folds. Throat culture with chocolate agar shows small, gram-negative coccobacilli. The patient's brother is started on the recommended antibiotic for chemoprophylaxis. Which of the following is the primary mechanism of action of this drug?













