Anti-neoplastic drugs

On this page
🎯 The Anti-Neoplastic Arsenal: Cancer's Chemical Warfare
You'll master the complete anti-neoplastic arsenal-from broad-spectrum cytotoxic agents that dismantle rapidly dividing cells to precision-targeted therapies that exploit specific cancer vulnerabilities and immunotherapies that reprogram your patient's own defenses. Understanding how these drugs work, when to deploy them alone or in combination, and how to anticipate and manage their toxicities transforms you from passive observer to strategic commander in oncology care. This lesson builds your confidence to navigate treatment protocols, recognize mechanism-based side effects, and communicate clearly with patients facing cancer's most challenging battles.
📌 Remember: CAMP-VITA - Cell cycle, Alkylation, Metabolism, Protein synthesis, Vascular, Immune, Topoisomerase, Apoptosis - the 8 major anti-neoplastic targets
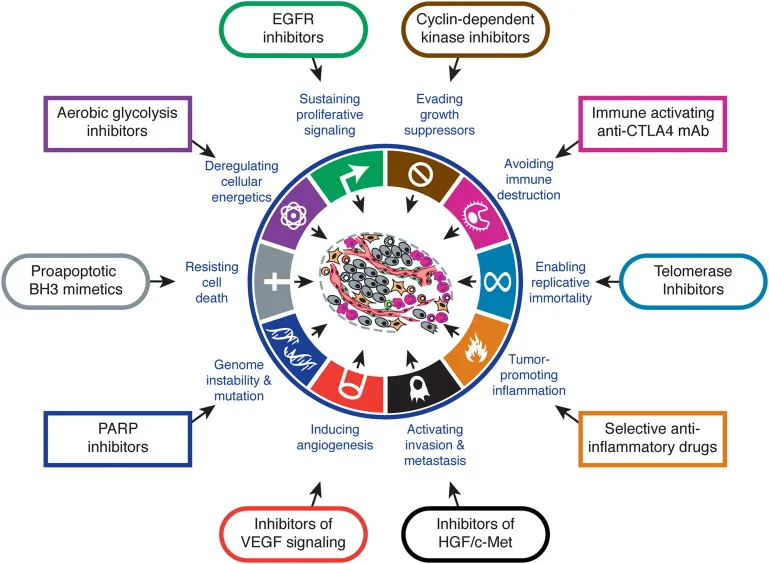
Mechanistic Classification Framework
-
Cell Cycle-Specific Agents (25% of all anti-neoplastics)
- S-phase: Antimetabolites (5-FU, methotrexate, cytarabine)
- M-phase: Microtubule inhibitors (paclitaxel, vincristine)
- Mitotic arrest occurs within 2-6 hours of administration
- Apoptosis triggered after 12-24 hours of arrest
- G1/S checkpoint: CDK inhibitors (palbociclib, ribociclib)
-
Cell Cycle-Nonspecific Agents (75% of all anti-neoplastics)
- DNA alkylators: Cyclophosphamide, cisplatin (>50 cross-links per cell)
- DNA intercalators: Doxorubicin, daunorubicin (1:10 drug:DNA ratio)
- Protein synthesis inhibitors: L-asparaginase (depletes asparagine to <1 μM)
⭐ Clinical Pearl: Cell cycle-specific drugs require prolonged exposure (>24 hours) for maximum efficacy, while cycle-nonspecific agents achieve therapeutic effect with brief, high-concentration exposure (1-4 hours).
| Drug Class | Mechanism | Cell Cycle Phase | Resistance Rate | Major Toxicity | Clinical Response |
|---|---|---|---|---|---|
| Alkylating Agents | DNA cross-linking | Non-specific | 15-30% | Myelosuppression | 60-80% |
| Antimetabolites | DNA synthesis block | S-phase | 20-40% | Mucositis | 40-70% |
| Topoisomerase Inhibitors | DNA strand breaks | S/G2-phase | 25-45% | Diarrhea | 30-60% |
| Microtubule Inhibitors | Mitotic arrest | M-phase | 10-25% | Neuropathy | 50-75% |
| Targeted Therapy | Specific pathways | Variable | 30-60% | Skin toxicity | 20-90% |
Resistance Mechanisms and Clinical Implications
-
Primary Resistance (present before treatment)
- P-glycoprotein overexpression: 40-60% of treatment failures
- DNA repair enhancement: BRCA1/2 mutations affect platinum sensitivity
- Apoptosis defects: p53 mutations in >50% of solid tumors
-
Acquired Resistance (develops during treatment)
- Multidrug resistance: Emerges in 70-90% of metastatic cancers
- Target mutations: T790M EGFR mutation in 60% of erlotinib resistance
- Bypass pathways: Alternative signaling in 80% of kinase inhibitor failures
⚠️ Warning: Tumor heterogeneity means <5% of cancer cells may harbor resistance mutations before treatment, expanding to >95% dominance within 6-12 months of selective pressure.
Understanding anti-neoplastic mechanisms provides the foundation for rational combination therapy, where synergistic drug interactions can overcome resistance and improve overall survival by 15-40% compared to monotherapy approaches.
🎯 The Anti-Neoplastic Arsenal: Cancer's Chemical Warfare
⚔️ Cytotoxic Chemotherapy: The Cellular Demolition Squad
📌 Remember: DNA-STOP - DNA alkylation, Nucleotide analogs, Antibiotics (intercalation), Spindle poisons, Topoisomerase inhibition, Oxidative damage, Protein synthesis block - the 7 cytotoxic mechanisms
DNA-Targeting Mechanisms
-
Alkylating Agents (Cross-linking Specialists)
- Nitrogen mustards: Cyclophosphamide requires hepatic activation to phosphoramide mustard
- Platinum compounds: Cisplatin forms 1,2-intrastrand cross-links in >80% of DNA adducts
- Carboplatin: 4-fold less nephrotoxic, equal efficacy in ovarian cancer
- Oxaliplatin: Unique neurotoxicity via calcium channel interference
- Nitrosoureas: BCNU, CCNU cross blood-brain barrier (>60% penetration)
-
Antimetabolites (Metabolic Mimics)
- Folate antagonists: Methotrexate inhibits dihydrofolate reductase (Ki = 0.1 nM)
- Pyrimidine analogs: 5-FU incorporates into RNA (60%) and DNA (40%)
- Capecitabine: Oral prodrug with tumor-selective activation
- Purine analogs: 6-Mercaptopurine requires HPRT for activation
⭐ Clinical Pearl: Leucovorin rescue must begin within 24-48 hours of high-dose methotrexate (>1 g/m²) to prevent life-threatening toxicity. Serum levels >10 μM at 24 hours indicate delayed clearance requiring extended rescue.
| Cytotoxic Class | DNA Lesion Type | Repair Pathway | Repair Time | Lethal Threshold | Selectivity Factor |
|---|---|---|---|---|---|
| Alkylating Agents | Cross-links | Homologous recombination | 6-12 hours | >1000 lesions/cell | 2-5x |
| Platinum Compounds | Intrastrand adducts | Nucleotide excision | 2-4 hours | >500 adducts/cell | 3-8x |
| Topoisomerase I Inhibitors | Single-strand breaks | Base excision | <1 hour | >10,000 breaks/cell | 5-15x |
| Topoisomerase II Inhibitors | Double-strand breaks | Non-homologous end joining | 4-8 hours | >50 breaks/cell | 10-25x |
| Antimetabolites | Replication fork stalling | Mismatch repair | 1-3 hours | >100 stalled forks | 8-20x |
Mitotic Targeting Strategies
-
Microtubule Inhibitors (Spindle Saboteurs)
- Vinca alkaloids: Vincristine, vinblastine prevent tubulin polymerization
- Dose-limiting neuropathy at >2 mg/m² cumulative dose
- Taxanes: Paclitaxel, docetaxel stabilize microtubules preventing depolymerization
- Hypersensitivity reactions in 10-15% without premedication
- Vinca alkaloids: Vincristine, vinblastine prevent tubulin polymerization
-
Topoisomerase Inhibitors (DNA Unraveling Specialists)
- Topoisomerase I: Irinotecan, topotecan trap cleavage complexes
- Topoisomerase II: Etoposide, doxorubicin prevent DNA religation
💡 Master This: Mitotic catastrophe occurs when cells attempt mitosis with >20 unrepaired DNA breaks, triggering p53-independent apoptosis within 1-2 cell cycles. This mechanism explains why combination chemotherapy achieves synergistic killing rather than additive effects.
⚠️ Warning: Tumor lysis syndrome occurs in 10-30% of patients with high tumor burden (>10 cm masses, WBC >50,000) receiving rapid-acting cytotoxics. Hyperuricemia (>8 mg/dL), hyperkalemia (>6 mEq/L), and acute kidney injury can develop within 12-72 hours.
Cytotoxic chemotherapy remains the cornerstone of curative treatment for hematologic malignancies and adjuvant therapy for solid tumors, with combination regimens achieving complete response rates of 60-95% in chemosensitive cancers through complementary mechanisms of cellular destruction.
⚔️ Cytotoxic Chemotherapy: The Cellular Demolition Squad
🎯 Targeted Therapy: Precision-Guided Cancer Elimination
📌 Remember: TARGETS - Tyrosine kinases, Angiogenesis, Receptors, Growth factors, Epigenetics, Tumor suppressors, Signal transduction - the 7 major targeted therapy categories
Kinase Inhibitor Precision
-
EGFR Inhibitors (Lung Cancer Specialists)
- Erlotinib: Response rate 70% in EGFR-mutated NSCLC vs <10% in wild-type
- Osimertinib: Third-generation agent overcomes T790M resistance in 60% of cases
- CNS penetration >90% with CSF:plasma ratio 0.25
- Afatinib: Irreversible binding provides longer target engagement (>24 hours)
-
BCR-ABL Inhibitors (CML Game-Changers)
- Imatinib: Major molecular response in 85% of chronic phase CML
- Dasatinib: 325-fold more potent than imatinib, active against most mutations
- Pleural effusions in 20-30% due to platelet dysfunction
- Ponatinib: Only agent active against T315I gatekeeper mutation
⭐ Clinical Pearl: Companion diagnostics are mandatory for >30 targeted therapies. PD-L1 expression ≥50% predicts pembrolizumab response in 45% vs 15% in PD-L1 negative patients, representing a 3-fold difference in clinical benefit.
| Target | Drug Examples | Biomarker | Response Rate | Resistance Mechanism | Median PFS |
|---|---|---|---|---|---|
| EGFR mutation | Erlotinib, Osimertinib | Exon 19 del, L858R | 70-80% | T790M (60%) | 12-18 months |
| ALK fusion | Crizotinib, Alectinib | EML4-ALK | 60-90% | Secondary mutations | 10-25 months |
| BRAF V600E | Vemurafenib, Dabrafenib | V600E mutation | 50-60% | MEK activation | 6-8 months |
| HER2 amplification | Trastuzumab, T-DM1 | IHC 3+ or FISH+ | 35-80% | PI3K pathway | 6-15 months |
| BCR-ABL | Imatinib, Dasatinib | Philadelphia chromosome | 85-95% | Kinase mutations | >60 months |
Monoclonal Antibody Engineering
-
Checkpoint Inhibitors (Immune System Liberators)
- PD-1 inhibitors: Pembrolizumab, nivolumab block immune exhaustion
- Objective response in 20-40% across multiple tumor types
- PD-L1 inhibitors: Atezolizumab, durvalumab prevent T-cell inactivation
- CTLA-4 inhibitors: Ipilimumab enhances T-cell priming (earlier checkpoint)
- PD-1 inhibitors: Pembrolizumab, nivolumab block immune exhaustion
-
Growth Factor Receptor Targeting
- HER2 inhibitors: Trastuzumab + pertuzumab dual blockade
- Pathologic complete response increases from 30% to 60%
- VEGF inhibitors: Bevacizumab normalizes tumor vasculature
- Hypertension in >80% indicates on-target effect
- HER2 inhibitors: Trastuzumab + pertuzumab dual blockade
💡 Master This: Antibody-drug conjugates (ADCs) combine targeted delivery with cytotoxic payload, achieving therapeutic indices 10-100x higher than conventional chemotherapy. T-DM1 delivers DM1 cytotoxin specifically to HER2-positive cells, explaining superior efficacy with reduced systemic toxicity.
Resistance and Combination Strategies
-
Primary Resistance Mechanisms
- Pathway redundancy: >5 parallel pathways maintain cell survival
- Tumor heterogeneity: <50% of cells may express target antigen
- Pharmacokinetic barriers: Blood-brain barrier limits CNS penetration
-
Acquired Resistance Patterns
- Target mutations: Gatekeeper mutations in 40-60% of kinase inhibitor failures
- Bypass signaling: Alternative RTK activation in 30-50% of cases
- Downstream activation: PI3K/mTOR hyperactivation circumvents upstream blockade
⚠️ Warning: Immune-related adverse events (irAEs) occur in 60-90% of patients receiving checkpoint inhibitors, with grade 3-4 toxicity in 10-20%. Pneumonitis (3-5%), colitis (8-12%), and hepatitis (2-5%) can be life-threatening without prompt corticosteroid intervention.
Targeted therapy success depends on precise biomarker selection, optimal sequencing, and rational combinations that prevent resistance emergence while maximizing therapeutic benefit in molecularly-defined patient populations.
🎯 Targeted Therapy: Precision-Guided Cancer Elimination
🔬 Immunotherapy: Unleashing the Body's Cancer-Fighting Arsenal
📌 Remember: IMMUNE - Inhibitory checkpoints, Monoclonal antibodies, Modulatory cytokines, Universal CAR-T, Neoantigen vaccines, Effector cell transfer - the 6 immunotherapy modalities
Checkpoint Inhibitor Mechanisms
-
PD-1/PD-L1 Axis Blockade (T-Cell Reactivation)
- PD-1 expression: >50% of tumor-infiltrating lymphocytes in responsive tumors
- PD-L1 expression: Tumor proportion score ≥50% predicts response in 45% vs 15%
- Combined positive score (CPS) ≥10 expands eligible population by 40%
- Microsatellite instability: Response rate >80% in MSI-high tumors (any histology)
-
CTLA-4 Inhibition (T-Cell Priming Enhancement)
- Ipilimumab: Blocks CD80/CD86 binding to CTLA-4 on regulatory T-cells
- Combination benefit: Ipilimumab + nivolumab achieves 58% response rate vs 19% monotherapy
- Grade 3-4 toxicity increases from 15% to 55% with combination
⭐ Clinical Pearl: Pseudoprogression occurs in 5-10% of checkpoint inhibitor patients, where initial tumor enlargement (<20% increase) precedes delayed response. Continued treatment beyond progression can achieve response in 15-30% of carefully selected patients.
| Checkpoint Target | Drug Examples | Tumor Types | Response Rate | Median Duration | 5-Year Survival |
|---|---|---|---|---|---|
| PD-1 | Pembrolizumab, Nivolumab | Melanoma, NSCLC, RCC | 20-45% | 18-24 months | 25-50% |
| PD-L1 | Atezolizumab, Durvalumab | Urothelial, NSCLC | 15-25% | 12-18 months | 20-35% |
| CTLA-4 | Ipilimumab | Melanoma | 10-15% | >24 months | 18-20% |
| LAG-3 | Relatlimab | Melanoma (combination) | 43% | Not reached | Pending |
| TIGIT | Tiragolumab | NSCLC (combination) | 37% | 16 months | Pending |
Adoptive Cell Therapy Revolution
-
CAR-T Cell Engineering (Living Drug Manufacturing)
- CD19 CAR-T: Complete remission in 80-90% of relapsed/refractory B-ALL
- BCMA CAR-T: Response rate 85% in multiple myeloma with median PFS 12 months
- Manufacturing time: 14-28 days from leukapheresis to infusion
- Solid tumor CAR-T: Limited efficacy due to immunosuppressive microenvironment
-
Tumor-Infiltrating Lymphocytes (TIL)
- Melanoma TIL: Objective response 50% with durable remissions >2 years
- Cervical cancer TIL: 44% response rate in heavily pretreated patients
- Manufacturing challenges: 6-8 week ex vivo expansion required
💡 Master This: Cytokine release syndrome (CRS) occurs in 70-90% of CAR-T patients, with severe CRS (grade 3-4) in 10-30%. IL-6 levels >1000 pg/mL predict severe CRS, requiring tocilizumab (8 mg/kg) and corticosteroids for management.
Immune-Related Adverse Events
-
Autoimmune Toxicity Spectrum
- Dermatologic: Rash (40-50%), vitiligo (5-10%) - favorable prognostic sign
- Gastrointestinal: Colitis (8-15%), hepatitis (5-10%) - dose-limiting
- Endocrine: Thyroiditis (15-20%), hypophysitis (5-8%) - often permanent
- Pulmonary: Pneumonitis (3-5%) - potentially fatal (1-2% mortality)
-
Management Algorithms
- Grade 1-2: Continue treatment + topical/systemic steroids
- Grade 3: Hold treatment + prednisone 1-2 mg/kg
- Grade 4: Permanently discontinue + methylprednisolone 1-4 mg/kg
⚠️ Warning: Immune-related adverse events can occur months to years after treatment discontinuation. Thyroid dysfunction develops in 15-50% of patients, requiring lifelong monitoring and hormone replacement in >80% of hypothyroid cases.
Immunotherapy success requires careful patient selection, biomarker-guided treatment, and expert management of immune-related toxicities to maximize therapeutic benefit while minimizing life-threatening complications in this paradigm-shifting treatment modality.
🔬 Immunotherapy: Unleashing the Body's Cancer-Fighting Arsenal
⚖️ Combination Strategies: Orchestrating Multi-Modal Cancer Destruction
📌 Remember: COMBINE - Cell cycle complementarity, Overlapping toxicity avoidance, Mechanistic synergy, Biomarker selection, Immune priming, Non-cross resistance, Effect scheduling - the 7 combination principles
Synergistic Mechanism Design
-
Chemotherapy Combinations (Cytotoxic Synergy)
- FOLFOX: 5-FU + leucovorin + oxaliplatin - thymidylate synthase inhibition + DNA cross-linking
- Response rate 50% vs 20% 5-FU monotherapy in colorectal cancer
- ABVD: Doxorubicin + bleomycin + vinblastine + dacarbazine - 4 distinct mechanisms
- Cure rate >90% in early-stage Hodgkin lymphoma
- R-CHOP: Rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone
- 5-year OS 80% vs 60% CHOP alone in DLBCL
- FOLFOX: 5-FU + leucovorin + oxaliplatin - thymidylate synthase inhibition + DNA cross-linking
-
Targeted Combination Strategies
- Vertical pathway inhibition: MEK + BRAF inhibitors in BRAF-mutant melanoma
- Median PFS 14.9 months vs 7.3 months BRAF monotherapy
- Horizontal pathway blocking: CDK4/6 + endocrine therapy in HR+ breast cancer
- Median PFS 28 months vs 14 months endocrine monotherapy
- Vertical pathway inhibition: MEK + BRAF inhibitors in BRAF-mutant melanoma
⭐ Clinical Pearl: Combination index (CI) quantifies drug interactions: CI <0.8 = synergism, CI 0.8-1.2 = additive, CI >1.2 = antagonism. Optimal scheduling can convert antagonistic combinations (CI >1.5) to synergistic (CI <0.7) through temporal separation.
| Combination Type | Example Regimen | Mechanism | Response Rate | Toxicity Grade 3-4 | Survival Benefit |
|---|---|---|---|---|---|
| Chemo + Chemo | FOLFIRI | Topoisomerase I + antimetabolite | 45-55% | 60-70% | +4-6 months |
| Targeted + Targeted | Dabrafenib + Trametinib | BRAF + MEK inhibition | 70-80% | 25-35% | +8-12 months |
| Chemo + Targeted | Carboplatin + Trastuzumab | DNA damage + HER2 blockade | 80-90% | 45-55% | +12-18 months |
| Immuno + Targeted | Pembrolizumab + Axitinib | PD-1 + VEGFR inhibition | 60-70% | 40-50% | +6-10 months |
| Immuno + Immuno | Nivolumab + Ipilimumab | PD-1 + CTLA-4 blockade | 55-65% | 55-65% | +15-25 months |
Resistance Prevention Strategies
-
Non-Cross Resistant Combinations
- Alternating regimens: ABVD × 2 cycles → BEACOPP × 2 cycles prevents single-mechanism resistance
- Concurrent administration: Simultaneous pathway blockade prevents compensatory activation
- Sequential therapy: Induction → consolidation → maintenance phases
-
Immune System Integration
- Chemotherapy immune priming: Anthracyclines induce immunogenic cell death
- Calreticulin exposure increases dendritic cell uptake by 300%
- Targeted therapy + immunotherapy: Anti-angiogenic agents normalize tumor vasculature
- T-cell infiltration increases 5-10 fold with bevacizumab + atezolizumab
- Chemotherapy immune priming: Anthracyclines induce immunogenic cell death
💡 Master This: Fractional kill hypothesis explains log-linear cell kill where each treatment cycle kills a constant fraction (90-99%) rather than absolute number of cancer cells. Combination therapy increases fractional kill from 1-2 logs to 3-5 logs per cycle, explaining superior cure rates.
Toxicity Management and Optimization
-
Overlapping Toxicity Avoidance
- Myelosuppression: Stagger nadir timing (days 7-14 vs days 14-21)
- Organ-specific toxicity: Avoid multiple nephrotoxic or cardiotoxic agents
- Growth factor support: G-CSF maintains dose intensity in >80% of patients
-
Pharmacokinetic Interactions
- CYP3A4 inhibition: Ketoconazole increases docetaxel AUC by 300%
- P-glycoprotein competition: Verapamil reverses doxorubicin resistance
- Renal clearance: Cisplatin reduces methotrexate elimination by 40%
⚠️ Warning: Treatment-related mortality increases exponentially with combination complexity: 2-drug combinations (1-3% mortality), 3-drug combinations (3-8% mortality), 4+ drug combinations (8-15% mortality). Risk-benefit assessment requires careful patient selection and expert supportive care.
Successful combination therapy requires mechanistic rationale, optimal sequencing, toxicity management expertise, and biomarker-guided patient selection to maximize therapeutic benefit while minimizing treatment-related morbidity in complex multi-modal treatment regimens.
⚖️ Combination Strategies: Orchestrating Multi-Modal Cancer Destruction
🛡️ Toxicity Management: Mastering the Therapeutic Battlefield
📌 Remember: TOXICITY - Thrombocytopenia, Organ damage, Xerostomia/mucositis, Infection risk, Cardiac dysfunction, Immune suppression, Tumor lysis, Yield preservation - the 8 major toxicity categories
Hematologic Toxicity Management
-
Myelosuppression Patterns (Predictable Nadir Timing)
- Neutropenia: Nadir days 7-14, recovery days 14-21 for most agents
- ANC <500: Infection risk 40% per day, mortality 5-15%
- Febrile neutropenia: Empiric antibiotics within 1 hour of fever >38.3°C
- Thrombocytopenia: Nadir days 10-14, bleeding risk at platelets <20,000
- Platelet transfusion threshold: <10,000 (prophylactic) or <50,000 (bleeding)
- Anemia: Gradual onset, transfusion at Hgb <7-8 g/dL or symptomatic
- Neutropenia: Nadir days 7-14, recovery days 14-21 for most agents
-
Growth Factor Support
- G-CSF: Filgrastim 5 μg/kg daily starting day +1 post-chemotherapy
- Pegfilgrastim 6 mg single dose 24-72 hours post-chemotherapy
- Thrombopoietin agonists: Romiplostim, eltrombopag for persistent thrombocytopenia
- ESAs: Epoetin alfa 40,000 units weekly if Hgb <10 g/dL
- G-CSF: Filgrastim 5 μg/kg daily starting day +1 post-chemotherapy
⭐ Clinical Pearl: Tumor lysis syndrome occurs in 10-30% of high-burden hematologic malignancies. Prophylactic allopurinol (300-600 mg daily) or rasburicase (0.2 mg/kg) prevents hyperuricemia, while aggressive hydration (3-4 L/day) prevents acute kidney injury.
| Toxicity Grade | ANC (cells/μL) | Platelets (×10³/μL) | Management Strategy | Infection Risk | Bleeding Risk |
|---|---|---|---|---|---|
| Grade 1 | 1500-1999 | 75-99 | Monitor closely | <5% | <2% |
| Grade 2 | 1000-1499 | 50-74 | Consider G-CSF | 10-15% | 5-10% |
| Grade 3 | 500-999 | 25-49 | G-CSF + monitoring | 20-30% | 15-25% |
| Grade 4 | <500 | <25 | Hospitalization + support | 40-60% | 30-50% |
| Life-threatening | <100 | <10 | ICU + transfusions | >80% | >70% |
-
Cardiotoxicity Management (Anthracycline-Induced Cardiomyopathy)
- Baseline ECHO/MUGA: LVEF >50% required for anthracycline therapy
- Cumulative dose limits: Doxorubicin >450 mg/m² = >20% cardiomyopathy risk
- Dexrazoxane: Cardioprotectant reducing cardiomyopathy by 65%
- Monitoring schedule: ECHO at 250 mg/m², 350 mg/m², then every 100 mg/m²
-
Nephrotoxicity Prevention (Cisplatin-Induced AKI)
- Pre-hydration: 1-2 L normal saline + magnesium/potassium supplementation
- Mannitol diuresis: 12.5-25 g during cisplatin infusion
- Amifostine: Cytoprotectant reducing nephrotoxicity by 40%
-
Pulmonary Toxicity (Bleomycin-Induced Pneumonitis)
- Cumulative dose limit: >400 units = >10% pneumonitis risk
- Pulmonary function monitoring: DLCO baseline and every 2 cycles
- Oxygen restriction: FiO₂ <25% during anesthesia (prevents oxygen toxicity)
💡 Master This: Mucositis severity correlates with infection risk and nutritional status. Grade 3-4 mucositis (unable to swallow) occurs in 40-60% of high-dose chemotherapy patients, requiring parenteral nutrition and **aggressive oral care to prevent secondary infections.
Emergency Toxicity Interventions
-
Febrile Neutropenia Protocol
- Blood cultures × 2 sets + urine culture + chest X-ray
- Empiric antibiotics: Cefepime 2g q8h or piperacillin-tazobactam 4.5g q6h
- Antifungal therapy: Add after 96 hours if persistent fever
-
Tumor Lysis Syndrome Management
- Rasburicase 0.2 mg/kg daily for uric acid >8 mg/dL
- Calcium gluconate for symptomatic hypocalcemia (<7 mg/dL)
- Hemodialysis for refractory hyperkalemia (>6.5 mEq/L)
⚠️ Warning: Extravasation of vesicant chemotherapy (doxorubicin, vincristine) can cause tissue necrosis requiring surgical debridement. Immediate intervention with cold compresses (vinca alkaloids) or warm compresses (anthracyclines) plus antidotes (hyaluronidase, dimethyl sulfoxide) within 30 minutes minimizes tissue damage.
Expert toxicity management requires proactive monitoring, rapid intervention protocols, and multidisciplinary coordination to maintain treatment efficacy while minimizing patient morbidity and treatment-related mortality in complex anti-neoplastic regimens.
🛡️ Toxicity Management: Mastering the Therapeutic Battlefield
🎯 Clinical Mastery Arsenal: Your Anti-Neoplastic Command Center
📌 Essential Numbers Arsenal: ANC <500 = infection emergency, platelets <20K = bleeding risk, LVEF drop >10% = cardiotoxicity, creatinine ↑50% = nephrotoxicity, grade 3-4 toxicity = dose modification required
Rapid Assessment Framework
-
Treatment Selection Hierarchy
- Curative intent: >90% cure rate in testicular cancer, Hodgkin lymphoma, pediatric ALL
- Adjuvant therapy: 20-30% survival benefit in high-risk solid tumors
- Palliative treatment: Symptom control + quality of life preservation
- Performance status ≥2 = limited treatment options
-
Biomarker-Guided Decisions
- HER2 amplification: Trastuzumab mandatory (response rate 80% vs 30%)
- EGFR mutation: Osimertinib first-line (PFS 18.9 vs 10.2 months)
- PD-L1 ≥50%: Pembrolizumab monotherapy (response rate 45% vs 28%)
⭐ Clinical Command: Combination index <0.8 = synergistic benefit, dose intensity >85% = optimal outcomes, treatment-related mortality <5% = acceptable risk-benefit ratio
| Clinical Scenario | First-Line Choice | Response Rate | Key Monitoring | Resistance Pattern |
|---|---|---|---|---|
| EGFR+ NSCLC | Osimertinib | 80% | T790M mutation | C797S (20%) |
| HER2+ Breast | TCH regimen | 85% | LVEF q3 months | PI3K activation |
| BRAF+ Melanoma | Dabrafenib + Trametinib | 70% | Skin toxicity | MEK bypass |
| BCR-ABL+ CML | Imatinib | 95% | BCR-ABL levels | Kinase mutations |
| CD20+ Lymphoma | R-CHOP | 80% | Tumor lysis | CD20 loss |
-
Emergency Interventions (<1 Hour Response)
- Febrile neutropenia: Blood cultures + empiric antibiotics
- Tumor lysis syndrome: Rasburicase + aggressive hydration
- Anaphylaxis: Epinephrine + corticosteroids + H1/H2 blockers
-
Dose Modification Rules
- Grade 2 toxicity: Continue with close monitoring
- Grade 3 toxicity: 25% dose reduction or treatment delay
- Grade 4 toxicity: 50% dose reduction or regimen change
💡 Master This: Fractional kill = log₁₀(initial cells/surviving cells). 3-log kill (99.9% reduction) requires combination therapy achieving CI <0.7 with optimal scheduling to prevent resistance emergence and maximize therapeutic benefit.
Resistance Management Strategies
-
Predictive Resistance Markers
- KRAS mutation: Anti-EGFR resistance in colorectal cancer
- T790M mutation: First-generation EGFR inhibitor resistance (60% of cases)
- MDR1 overexpression: Multi-drug resistance in >70% of refractory tumors
-
Sequential Therapy Planning
- First-line failure: Mechanism-based second-line selection
- Cross-resistance avoidance: Non-overlapping pathways preferred
- Salvage options: Clinical trials for >2 prior regimens
⚠️ Critical Alert: Treatment-related secondary malignancies occur in 2-10% of long-term survivors, with latency periods of 5-15 years. Alkylating agents increase AML risk 10-fold, while topoisomerase II inhibitors increase risk 50-fold within 2-5 years.
Anti-neoplastic mastery integrates mechanistic understanding, clinical experience, and evidence-based protocols to optimize therapeutic outcomes while minimizing toxicity through personalized treatment strategies and expert supportive care management.
🎯 Clinical Mastery Arsenal: Your Anti-Neoplastic Command Center
Practice Questions: Anti-neoplastic drugs
Test your understanding with these related questions
A research team develops a new monoclonal antibody checkpoint inhibitor for advanced melanoma that has shown promise in animal studies as well as high efficacy and low toxicity in early phase human clinical trials. The research team would now like to compare this drug to existing standard of care immunotherapy for advanced melanoma. The research team decides to conduct a non-randomized study where the novel drug will be offered to patients who are deemed to be at risk for toxicity with the current standard of care immunotherapy, while patients without such risk factors will receive the standard treatment. Which of the following best describes the level of evidence that this study can offer?












