Growth/Development

On this page
🧬 The Pediatric Growth Engine: Decoding Development's Blueprint
Growth isn't just about getting taller-it's the most sensitive barometer of a child's overall health, reflecting everything from nutrition and hormones to chronic disease and psychosocial stress. You'll learn to decode growth patterns like a detective, master the hormonal machinery driving development, and build a systematic approach to evaluation and intervention. By integrating multi-system physiology with evidence-based diagnostics, you'll transform raw measurements into actionable clinical insights that can change a child's trajectory.

Growth represents the quantitative increase in body size, while development encompasses qualitative changes in function and capability. These processes interweave through complex feedback loops involving growth hormone, insulin-like growth factor-1 (IGF-1), thyroid hormones, and sex steroids, each contributing specific effects during critical developmental windows.
📌 Remember: GROWTH - Genetic potential, Recognize patterns, Optimal nutrition, Watch for red flags, Timing matters, Hormonal balance
The growth process follows predictable phases with distinct characteristics:
-
Infantile Phase (0-2 years)
- Rapid growth: 25 cm/year in first year, 12 cm/year in second year
- Nutrition-dependent growth predominates
- Brain growth: 75% of adult size by age 2
- Head circumference increases 12 cm in first year
- Fontanelles close: anterior by 18 months, posterior by 3 months
-
Childhood Phase (2 years-puberty)
- Steady growth: 5-7 cm/year and 2-3 kg/year
- Growth hormone-dependent linear growth
- Organ system maturation continues
- Kidney function reaches adult levels by 2 years
- Immune system develops through 6-8 years
-
Pubertal Phase (8-18 years)
- Growth spurt: 8-12 cm/year during peak velocity
- Sex hormone-driven development
- Final 15-20% of adult height achieved
- Girls: peak height velocity at Tanner stage 2-3
- Boys: peak height velocity at Tanner stage 4
| Growth Phase | Duration | Height Velocity | Primary Drivers | Key Milestones | Nutritional Needs |
|---|---|---|---|---|---|
| Infantile | 0-2 years | 25→12 cm/year | Nutrition, IGF-1 | Walking, language | High caloric density |
| Childhood | 2-puberty | 5-7 cm/year | Growth hormone | School readiness | Balanced macronutrients |
| Pubertal | 8-18 years | 8-12 cm/year | Sex hormones | Sexual maturation | Increased protein needs |
| Post-pubertal | 18+ years | <1 cm/year | Maintenance | Epiphyseal fusion | Adult requirements |
💡 Master This: Normal growth velocity varies by age, but any child growing <4 cm/year after age 3 or showing height velocity deceleration over 6-12 months needs comprehensive growth evaluation including bone age assessment.
The endocrine orchestration of growth involves multiple hormonal axes working in concert. Growth hormone secreted in pulsatile fashion (peak during stages 3-4 sleep) stimulates hepatic IGF-1 production, which mediates most growth-promoting effects. Thyroid hormones are essential for normal growth hormone action and skeletal maturation, while cortisol in excess inhibits growth through multiple mechanisms.
⚠️ Warning: Glucocorticoid therapy equivalent to >5 mg/day prednisolone for >3 months causes measurable growth suppression in >80% of children, requiring growth monitoring every 3 months during treatment.
Connect these foundational growth principles through hormonal regulation mechanisms to understand how disruptions create the clinical patterns you'll recognize in practice.
🧬 The Pediatric Growth Engine: Decoding Development's Blueprint
⚙️ The Hormonal Command Center: Growth's Molecular Machinery
Growth Hormone Secretion Patterns follow circadian rhythms with 60-70% of daily GH secreted during slow-wave sleep (stages 3-4). Peak concentrations occur 1-2 hours after sleep onset, reaching 10-40 ng/mL in healthy children compared to <5 ng/mL during waking hours.
📌 Remember: GHRH AXIS - Growth hormone releasing hormone, Hypothalamic control, Rhythmic pulses, Hepatic IGF-1, Anterior pituitary, Xtra sleep needed, Insulin-like effects, Somatostatin inhibits
-
Hypothalamic Control
- GHRH neurons in arcuate nucleus
- Somatostatin from periventricular nucleus
- Ghrelin from stomach enhances GH release
- Peak ghrelin levels: 30 minutes before meals
- Fasting increases ghrelin 2-3 fold
-
Pituitary Response
- Somatotroph cells comprise 40-50% of anterior pituitary
- GH half-life: 20-30 minutes in circulation
- Binding proteins extend hormone availability
- GHBP carries 50% of circulating GH
- IGF-binding proteins modulate IGF-1 activity
-
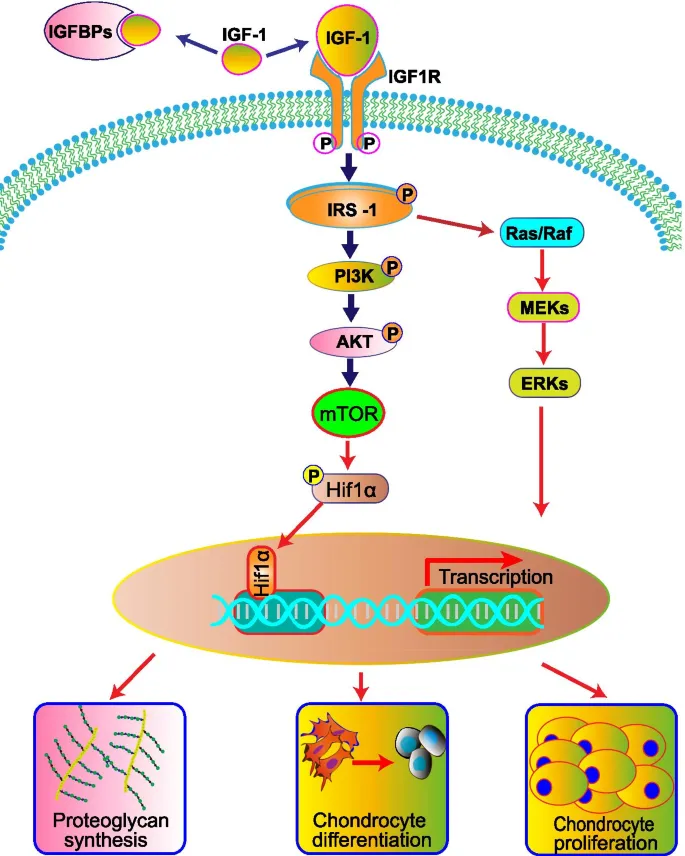
Peripheral Actions
- Direct effects: lipolysis, gluconeogenesis, protein synthesis
- Indirect effects: mediated through IGF-1 production
- Growth plate effects: chondrocyte proliferation and differentiation
- IGF-1 increases cell division 3-5 fold
- Collagen synthesis increases 200-300%
| Hormone | Source | Peak Levels | Half-life | Primary Actions | Growth Effects |
|---|---|---|---|---|---|
| GHRH | Hypothalamus | Sleep onset | 7-10 min | Stimulates GH | Indirect via GH |
| Somatostatin | Hypothalamus | Feeding | 2-3 min | Inhibits GH | Negative regulation |
| Growth Hormone | Pituitary | 1-2h post-sleep | 20-30 min | Metabolic, IGF-1 | Linear growth |
| IGF-1 | Liver/tissues | Continuous | 12-15 hours | Anabolic effects | Direct growth promotion |
| IGFBP-3 | Liver | Stable | 12-16 hours | IGF-1 transport | Modulates IGF-1 action |
⭐ Clinical Pearl: Hypothyroidism causes disproportionate growth failure with bone age delay >2 years compared to height age, while GH deficiency typically shows proportionate short stature with bone age delay 1-2 years.
Nutritional Modulation of the growth axis occurs through multiple mechanisms. Protein-energy malnutrition decreases IGF-1 levels by 40-60% despite normal or elevated GH concentrations, creating a state of GH resistance. Zinc deficiency impairs IGF-1 synthesis, while vitamin D deficiency affects growth plate mineralization.
💡 Master This: Nutritional growth failure presents with low IGF-1 (<50 ng/mL), elevated GH (>10 ng/mL), and rapid catch-up growth within 3-6 months of nutritional rehabilitation-distinguishing it from true GH deficiency.
Sex Hormone Interactions become critical during puberty when estrogen and testosterone amplify GH secretion 2-3 fold while simultaneously accelerating epiphyseal maturation. This creates the pubertal growth spurt but also limits final height potential through growth plate fusion.
⚠️ Warning: Precocious puberty before age 8 years (girls) or 9 years (boys) can result in adult short stature despite normal growth velocity during childhood, requiring GnRH agonist therapy to preserve height potential.
Connect these hormonal mechanisms through clinical pattern recognition to understand how different pathologies create characteristic growth disturbances you'll encounter in practice.
⚙️ The Hormonal Command Center: Growth's Molecular Machinery
🎯 The Growth Detective: Pattern Recognition Mastery
Constitutional Growth Patterns represent normal variants that account for 85-90% of children with height <3rd percentile. These patterns follow predictable trajectories that distinguish them from pathological causes:
-
Constitutional Delay of Growth and Puberty (CDGP)
- Family history of late bloomers in >80% of cases
- Bone age delay of 2-4 years compared to chronological age
- Normal growth velocity (5-7 cm/year) after age 3
- Height percentile remains stable over time
- Weight-for-height ratio stays normal
- Predicted adult height matches genetic potential
-
Familial Short Stature (FSS)
- Mid-parental height <10th percentile
- Bone age matches chronological age (±1 year)
- Growth velocity normal for age
- Height percentile tracks consistently
- Puberty timing appropriate for family
- Final height matches family pattern
📌 Remember: NORMAL VARIANTS - No red flags present, On track for family, Regular velocity maintained, Matching bone age pattern, Appropriate nutrition, Late bloomer history
Pathological Growth Patterns require systematic recognition and immediate evaluation. Key discriminating features include:
-
Growth Hormone Deficiency
- Height velocity <4 cm/year after age 3
- Bone age delay >2 years
- Truncal adiposity with immature facial features
- Growth deceleration crossing percentile lines
- Hypoglycemia episodes in 30-40% of cases
- Micropenis in males (<2.5 cm stretched length)
-
Hypothyroidism
- Severe bone age delay (>2-3 years)
- Disproportionate growth failure with weight gain
- Delayed dental eruption and intellectual impairment
- TSH >10 mIU/L with low T4
- Growth velocity <3 cm/year
- Myxedematous features in severe cases
-
Chronic Disease Growth Failure
- Weight loss preceding height deceleration
- Inflammatory markers elevated (ESR >30 mm/hr)
- Nutritional deficiencies with poor appetite
- Crohn's disease: 60% have growth failure at diagnosis
- Celiac disease: 40% present with isolated short stature
- Chronic kidney disease: growth failure when GFR <60 mL/min/1.73m²
| Pattern Type | Growth Velocity | Bone Age | Weight Pattern | Family History | Key Features |
|---|---|---|---|---|---|
| Constitutional Delay | 5-7 cm/year | 2-4 years delayed | Normal for height | Late bloomers | Stable percentiles |
| Familial Short | 5-7 cm/year | Age-appropriate | Normal for height | Short parents | Consistent tracking |
| GH Deficiency | <4 cm/year | >2 years delayed | Truncal obesity | Variable | Crossing percentiles |
| Hypothyroidism | <3 cm/year | >3 years delayed | Weight gain | Autoimmune disease | Developmental delay |
| Chronic Disease | Variable | Variable | Weight loss first | Disease-specific | Systemic symptoms |
Red Flag Recognition enables immediate identification of children requiring urgent evaluation:
-
Growth Velocity Red Flags
- <4 cm/year after age 3 years
- <5 cm/year during ages 4-8 years
- No pubertal growth spurt by age 14 (girls) or 15 (boys)
- Height velocity declining over 2 consecutive years
- Weight loss with height deceleration
- Severe short stature (<-3 SD or <1st percentile)
-
Dysmorphic Features
- Turner syndrome: webbed neck, shield chest, lymphedema
- Noonan syndrome: ptosis, pectus deformity, heart defects
- Russell-Silver syndrome: triangular facies, body asymmetry
- SHOX deficiency: Madelung deformity, mesomelic shortening
- Skeletal dysplasias: disproportionate limb shortening
💡 Master This: Growth failure with normal growth velocity suggests familial short stature, while growth failure with decreased velocity indicates pathological causes requiring hormonal, nutritional, or systemic disease evaluation.
Pubertal Pattern Recognition becomes critical for identifying precocious or delayed puberty:
-
Precocious Puberty Patterns
- Girls: breast development before age 8 years
- Boys: testicular enlargement before age 9 years
- Growth acceleration with advanced bone age
- Central precocious: LH-dominant gonadotropin response
- Peripheral precocious: suppressed gonadotropins
- McCune-Albright syndrome: café-au-lait spots, fibrous dysplasia
-
Delayed Puberty Patterns
- Girls: no breast development by age 13 years
- Boys: no testicular enlargement by age 14 years
- Growth deceleration without pubertal growth spurt
- Constitutional delay: family history, normal velocity
- Hypogonadotropic hypogonadism: anosmia (Kallmann syndrome)
- Hypergonadotropic hypogonadism: Turner syndrome, Klinefelter syndrome
⚠️ Warning: Rapid growth (>8 cm/year) in prepubertal children may indicate precocious puberty, hyperthyroidism, or growth hormone excess, requiring immediate endocrine evaluation to prevent compromised final height.
Connect these pattern recognition skills through systematic evaluation approaches to build comprehensive diagnostic frameworks for clinical practice.
🎯 The Growth Detective: Pattern Recognition Mastery
🔬 The Diagnostic Arsenal: Systematic Growth Evaluation
Initial Assessment Protocol establishes the foundation for all subsequent investigations:
-
Anthropometric Precision
- Height measurement: stadiometer accuracy to ±0.1 cm
- Weight assessment: calibrated scale to ±0.1 kg
- Head circumference: non-stretchable tape to ±0.1 cm
- Arm span measurement: fingertip-to-fingertip with arms extended
- Upper-to-lower segment ratio: sitting height vs leg length
- Growth velocity calculation: minimum 6-month intervals
-
Family History Quantification
- Mid-parental height: (maternal height + paternal height ± 13 cm) ÷ 2
- Target height range: mid-parental height ± 8.5 cm
- Pubertal timing: age at menarche (mother), growth spurt (father)
- Genetic conditions: consanguinity, growth disorders
- Chronic diseases: autoimmune, malabsorption, endocrine
📌 Remember: MEASURE TWICE - Multiple measurements, Equipment calibrated, Accurate technique, Same time of day, Uniform conditions, Record precisely, Evaluate trends, Track velocity, Watch for errors, Interpret carefully, Compare standards, Examine patterns
Laboratory Investigation Hierarchy follows evidence-based protocols that maximize diagnostic yield:
-
First-Line Screening (all children with growth failure)
- Complete blood count: anemia, chronic disease markers
- Comprehensive metabolic panel: renal function, liver function
- Inflammatory markers: ESR, CRP for chronic disease
- Thyroid function: TSH, free T4 (hypothyroidism in 5-10%)
- Celiac screening: tissue transglutaminase IgA (positive in 3-5%)
- Urinalysis: proteinuria, hematuria for renal disease
-
Second-Line Endocrine Testing (abnormal velocity or clinical suspicion)
- IGF-1 level: age-adjusted reference ranges
- IGFBP-3: less variable than IGF-1, better screening
- Bone age X-ray: left hand and wrist using Greulich-Pyle atlas
- Karyotype (girls): Turner syndrome screening
- 25-hydroxyvitamin D: deficiency in 30-40% of children
- Prolactin: elevated in craniopharyngioma, pituitary adenoma
| Investigation | Indication | Normal Values | Abnormal Findings | Next Steps |
|---|---|---|---|---|
| IGF-1 | Growth velocity <4 cm/year | Age-specific percentiles | <10th percentile | GH stimulation test |
| Bone Age | All growth failure | ±1 year chronological | >2 years delayed | Endocrine evaluation |
| TSH/Free T4 | Universal screening | TSH 0.5-4.5 mIU/L | TSH >10 mIU/L | Thyroid replacement |
| Celiac Antibodies | Short stature workup | tTG-IgA <20 units | >20 units | Intestinal biopsy |
| Karyotype | Girls with short stature | 46,XX | Turner variants | Cardiology evaluation |
-
Indications for GH Stimulation Testing
- Height <-2 SD with growth velocity <25th percentile
- IGF-1 <-2 SD for age and Tanner stage
- Clinical features suggestive of GH deficiency
- Bone age delay >2 years
- Neonatal hypoglycemia with micropenis
- Cranial irradiation or pituitary surgery history
-
Stimulation Test Methodology
- Two different stimuli required for diagnosis
- Peak GH response <10 ng/mL indicates deficiency
- Common stimuli: clonidine, arginine, glucagon, insulin
- Priming with sex steroids if bone age >10 years
- Overnight fasting required for accurate results
- Normal cortisol response confirms pituitary function
⭐ Clinical Pearl: IGF-1 levels <-2 SD for age have 95% sensitivity for severe GH deficiency, but normal IGF-1 doesn't exclude partial GH deficiency, requiring stimulation testing in symptomatic children.
Advanced Imaging Studies provide crucial diagnostic information in specific clinical contexts:
-
Brain MRI Indications
- Confirmed GH deficiency (evaluate pituitary anatomy)
- Multiple pituitary hormone deficiencies
- Visual field defects or neurological symptoms
- Craniopharyngioma: calcified suprasellar mass
- Pituitary adenoma: sellar enlargement, stalk deviation
- Empty sella syndrome: flattened pituitary gland
-
Skeletal Imaging
- Bone age assessment: standard for growth evaluation
- Skeletal survey: suspected skeletal dysplasia
- DEXA scan: bone density in chronic disease
- Metaphyseal changes: rickets, leukemia
- Spinal abnormalities: vertebral compression, scoliosis
💡 Master This: Bone age delay >2 years suggests endocrine causes (GH deficiency, hypothyroidism), while bone age matching chronological age with short stature indicates skeletal dysplasia or familial short stature.
Genetic Testing Considerations become important when syndromic features or family history suggest hereditary causes:
- Chromosomal Analysis
- Turner syndrome: 45,X or mosaic patterns
- Noonan syndrome: PTPN11, SOS1, RAF1 mutations
- SHOX deficiency: Xp22.33 deletions
- Russell-Silver syndrome: chromosome 7 or 11p15 abnormalities
- Prader-Willi syndrome: 15q11-q13 deletions
⚠️ Warning: Multiple pituitary hormone deficiencies in neonates may indicate congenital hypopituitarism requiring immediate cortisol replacement to prevent life-threatening adrenal crisis.
Connect these diagnostic approaches through evidence-based treatment algorithms to understand how accurate diagnosis guides optimal therapeutic interventions.
🔬 The Diagnostic Arsenal: Systematic Growth Evaluation
⚖️ The Treatment Command Center: Evidence-Based Growth Interventions
Growth Hormone Replacement Therapy represents the gold standard for confirmed GH deficiency with established protocols for dosing, monitoring, and outcome optimization:
-
Dosing Protocols
- Starting dose: 0.18-0.3 mg/kg/week divided into daily injections
- Injection timing: bedtime to mimic physiological secretion
- Dose adjustments: every 3-6 months based on growth response and IGF-1 levels
- Target IGF-1: 0 to +2 SD for age and Tanner stage
- Maximum dose: 0.47 mg/kg/week in prepubertal children
- Pubertal dosing: increase by 25-50% during growth spurt
-
Expected Growth Response
- First year: 8-12 cm height gain (catch-up growth)
- Subsequent years: 6-8 cm/year until pubertal growth spurt
- Total height gain: 1.2-1.8 SD improvement in final height
- Response predictors: younger age, more severe deficiency, better compliance
- Poor response: <4 cm in first year requires re-evaluation
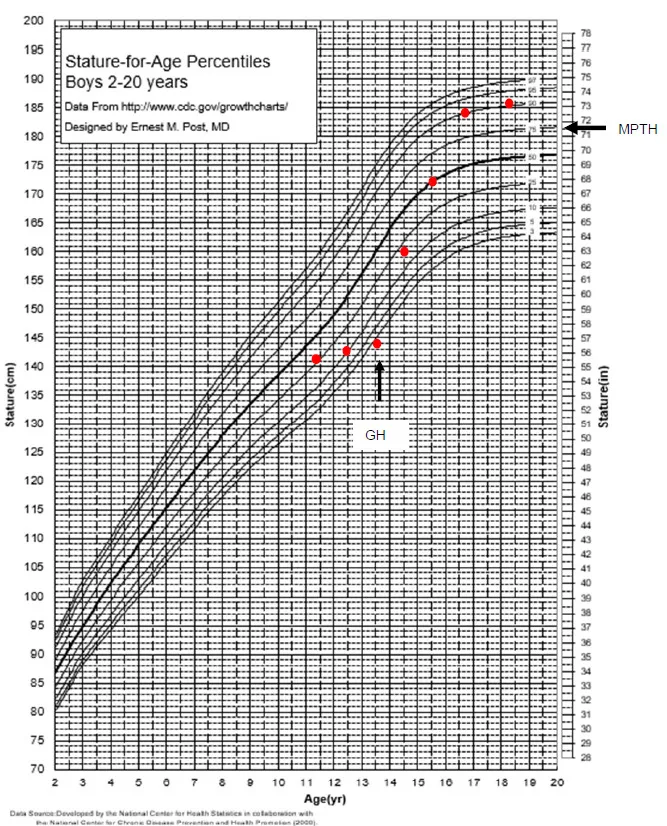
📌 Remember: GH SUCCESS - Good compliance essential, Height velocity monitoring, Side effects surveillance, Understanding expectations, Catch-up growth first year, Continue until fusion, Endocrine follow-up, Stop at final height, Safety monitoring always
Monitoring and Safety Protocols ensure optimal outcomes while detecting potential complications:
-
Growth Monitoring
- Height measurement: every 3 months during treatment
- Growth velocity calculation: 6-month intervals minimum
- Bone age assessment: annually to predict remaining growth
- IGF-1 levels: every 6 months to guide dose adjustments
- Pubertal staging: every 6 months during adolescence
- Final height prediction: Bayley-Pinneau method using bone age
-
Safety Surveillance
- Glucose tolerance: annual fasting glucose and HbA1c
- Thyroid function: TSH and free T4 every 6-12 months
- Scoliosis screening: clinical examination every 6 months
- Intracranial pressure: fundoscopy, headache assessment
- Hip problems: slipped capital femoral epiphysis risk
- Injection site rotation: prevent lipodystrophy
| Monitoring Parameter | Frequency | Normal Response | Concerning Findings | Action Required |
|---|---|---|---|---|
| Height Velocity | Every 3 months | >6 cm/year | <4 cm/year | Dose adjustment |
| IGF-1 Level | Every 6 months | 0 to +2 SD | >+2 SD | Reduce dose |
| Bone Age | Annually | Appropriate advancement | Rapid advancement | Consider stopping |
| Glucose Tolerance | Annually | Normal fasting glucose | Diabetes mellitus | Endocrine consultation |
| Thyroid Function | Every 6-12 months | Normal TSH/T4 | Hypothyroidism | Thyroid replacement |
-
Levothyroxine Dosing
- Infants: 10-15 mcg/kg/day for congenital hypothyroidism
- Children: 4-6 mcg/kg/day for acquired hypothyroidism
- Adolescents: 2-3 mcg/kg/day approaching adult dosing
- Dose adjustments: 12.5-25 mcg increments every 6-8 weeks
- Target TSH: 0.5-2.0 mIU/L for optimal growth
- Free T4: upper half of normal range
-
Growth Response to Thyroid Treatment
- Catch-up growth: begins within 3-6 months of adequate replacement
- Growth velocity: normalizes to 6-8 cm/year within 12 months
- Bone age advancement: accelerated initially, then normalizes
- Final height: usually normal if treated early
- Cognitive recovery: depends on duration of hypothyroidism
⭐ Clinical Pearl: Overtreatment with levothyroxine (TSH <0.1 mIU/L) can cause accelerated bone maturation and compromised final height, requiring careful dose titration to maintain TSH 0.5-2.0 mIU/L.
Nutritional Intervention Protocols address growth failure secondary to malnutrition or chronic disease:
-
Caloric Requirements
- Catch-up growth: 120-150% of recommended daily allowance
- Protein needs: 1.5-2.0 g/kg/day during active growth
- Micronutrient supplementation: zinc, iron, vitamin D
- Enteral nutrition: nasogastric or gastrostomy if oral intake inadequate
- Growth monitoring: weekly weights, monthly heights during rehabilitation
-
Disease-Specific Interventions
- Celiac disease: strict gluten-free diet with dietitian support
- Crohn's disease: anti-inflammatory therapy plus nutritional support
- Chronic kidney disease: phosphate binders, growth hormone if indicated
- Growth response: catch-up growth within 6-12 months if underlying disease controlled
- Monitoring: albumin, prealbumin, growth velocity
💡 Master This: Treatment success requires >6 cm/year growth velocity in prepubertal children and appropriate pubertal progression-failure to achieve these targets within 12 months indicates inadequate treatment or incorrect diagnosis.
Pubertal Intervention Strategies optimize timing and progression of sexual maturation:
-
Delayed Puberty Treatment
- Constitutional delay: low-dose sex steroids for 4-6 months
- Hypogonadism: full hormone replacement therapy
- Monitoring: pubertal staging, growth velocity, bone age
- Boys: testosterone enanthate 50-100 mg monthly
- Girls: estradiol 5-10 mcg daily for 6 months
- Growth spurt: expected within 6-12 months of treatment initiation
-
Precocious Puberty Management
- GnRH agonist therapy: leuprolide or histrelin implants
- Treatment duration: until appropriate pubertal age
- Height preservation: prevents early epiphyseal fusion
- Monitoring: LH suppression, growth velocity, bone age
- Final height: improved by 4-7 cm with appropriate treatment
⚠️ Warning: Growth hormone therapy in children with closed epiphyses provides no height benefit and increases risk of diabetes and joint problems-bone age assessment is mandatory before treatment initiation.
Connect these treatment protocols through long-term outcome monitoring to understand how therapeutic success translates into optimal adult health and function.
⚖️ The Treatment Command Center: Evidence-Based Growth Interventions
🔗 The Integration Matrix: Multi-System Growth Orchestration
Neuroendocrine-Metabolic Integration demonstrates how brain-hormone-metabolism networks coordinate growth responses to environmental challenges:
-
Hypothalamic-Pituitary-Growth Axis
- Circadian rhythms: GH secretion peaks during stages 3-4 sleep
- Nutritional sensing: leptin and ghrelin modulate GHRH release
- Stress responses: cortisol inhibits GH action and IGF-1 production
- Sleep deprivation: reduces GH secretion by 30-50%
- Chronic stress: elevates cortisol, suppressing linear growth
- Malnutrition: creates GH resistance despite elevated GH levels
-
Thyroid-Growth Hormone Synergy
- T3 regulates GH gene transcription and IGF-1 receptor expression
- Hypothyroidism impairs GH secretion and growth plate responsiveness
- Combined deficiency: more severe growth failure than either alone
- Treatment synergy: thyroid replacement enhances GH therapy response
- Monitoring complexity: both hormones affect bone age advancement
📌 Remember: INTEGRATION - Interconnected systems, Neural control central, Thyroid synergy essential, Environmental factors, Genetic programming, Rhythmic patterns, Adaptive responses, Timing critical, Influence multifactorial, Orchestrated precisely, Nutrition fundamental
Genetic-Epigenetic Programming reveals how inherited factors and environmental influences shape growth trajectories:
-
Genetic Height Determinants
- >700 genetic variants influence adult height
- SHOX gene: X-linked, affects 10-15% of idiopathic short stature
- IGF-1 pathway genes: GHR, IGF1, IGFALS mutations
- Polygenic scores: predict 60-70% of height variation
- Rare variants: large effect sizes in syndromic short stature
- Population differences: genetic architecture varies between ethnicities
-
Epigenetic Modifications
- DNA methylation: IGF-1 promoter regulation by nutritional status
- Histone modifications: GH gene expression influenced by sleep patterns
- MicroRNA regulation: post-transcriptional control of growth factors
- Maternal nutrition: affects offspring growth through epigenetic programming
- Early life stress: methylation changes persist into adulthood
- Reversibility: some modifications respond to therapeutic interventions
| Integration Level | Key Components | Clinical Manifestations | Therapeutic Targets | Monitoring Parameters |
|---|---|---|---|---|
| Neuroendocrine | GH, thyroid, cortisol | Growth velocity changes | Hormone replacement | IGF-1, TSH, cortisol |
| Metabolic | Nutrition, insulin, leptin | Weight-height dissociation | Nutritional support | Albumin, glucose, lipids |
| Genetic | SHOX, IGF pathway | Syndromic features | Gene therapy (future) | Genetic testing |
| Environmental | Sleep, stress, toxins | Variable growth patterns | Lifestyle modification | Growth velocity trends |
| Immune | Cytokines, inflammation | Chronic disease effects | Anti-inflammatory | ESR, CRP, cytokines |
-
Cytokine-Mediated Growth Suppression
- TNF-α and IL-1β inhibit IGF-1 synthesis and growth plate function
- IL-6 induces IGF-1 resistance and accelerated protein catabolism
- Chronic inflammation increases cortisol production, further suppressing growth
- Inflammatory bowel disease: 60% have growth failure at diagnosis
- Juvenile arthritis: growth suppression correlates with disease activity
- Anti-TNF therapy: improves growth velocity in responsive patients
-
Nutritional-Immune Interactions
- Malnutrition impairs immune function, increasing infection risk
- Chronic infections increase metabolic demands and nutrient losses
- Micronutrient deficiencies affect both growth and immune responses
- Zinc deficiency: impairs T-cell function and IGF-1 synthesis
- Vitamin D deficiency: affects immune regulation and bone mineralization
- Iron deficiency: reduces growth velocity and cognitive development
⭐ Clinical Pearl: Chronic inflammatory diseases cause growth failure through IGF-1 resistance rather than GH deficiency-anti-inflammatory therapy often restores growth more effectively than growth hormone treatment.
Cardiovascular-Renal-Growth Networks illustrate how organ system dysfunction creates complex growth disturbances:
-
Chronic Kidney Disease Effects
- Metabolic acidosis impairs protein synthesis and bone mineralization
- Phosphate retention causes secondary hyperparathyroidism
- Anemia reduces oxygen delivery to growing tissues
- Growth hormone resistance: elevated GH with low IGF-1
- Bone disease: renal osteodystrophy affects growth plate function
- Treatment complexity: requires multiple interventions for optimal growth
-
Congenital Heart Disease Impact
- Chronic hypoxemia impairs cellular metabolism and growth
- Increased caloric demands: cardiac work increases energy requirements
- Feeding difficulties: poor oral intake compounds growth problems
- Catch-up growth: possible after surgical correction in young children
- Nutritional support: high-calorie formulas and feeding assistance
- Growth monitoring: weight-for-length more reliable than absolute percentiles
💡 Master This: Multi-system growth failure requires comprehensive evaluation addressing underlying disease, nutritional status, hormonal function, and psychosocial factors-isolated interventions rarely achieve optimal outcomes.
Psychosocial-Growth Interactions reveal how emotional stress and social factors influence physical development:
-
Psychosocial Short Stature
- Emotional deprivation suppresses GH secretion through stress pathways
- Reversible growth failure: catch-up growth occurs with improved environment
- Behavioral manifestations: sleep disturbances, appetite changes, developmental delays
- Prevalence: 5-10% of children with short stature
- Diagnosis: normal GH testing with environmental risk factors
- Treatment: psychosocial intervention more effective than hormone therapy
-
Socioeconomic Determinants
- Food insecurity: affects 25% of children in low-income families
- Healthcare access: delayed diagnosis and treatment of growth disorders
- Educational outcomes: short stature correlates with academic performance
- Intervention programs: comprehensive support improves multiple outcomes
- Community resources: nutrition programs, healthcare coordination
⚠️ Warning: Psychosocial short stature can mimic GH deficiency with abnormal stimulation tests-careful history and trial of environmental intervention may avoid unnecessary hormone therapy.
Connect these integration principles through clinical mastery frameworks to develop comprehensive approaches that optimize growth outcomes across all domains of child development.
🔗 The Integration Matrix: Multi-System Growth Orchestration
🎯 The Growth Mastery Toolkit: Clinical Excellence Arsenal
Rapid Assessment Protocol enables comprehensive growth evaluation in <10 minutes:
-
The 5-Minute Growth Screen
- Height percentile: plot on age-appropriate chart
- Growth velocity: calculate cm/year over 6-12 months
- Family history: mid-parental height and pubertal timing
- Red flag symptoms: headaches, visual changes, polyuria
- Medication review: steroids, stimulants, chemotherapy
- Nutritional assessment: appetite, weight changes, dietary restrictions
-
Physical Examination Essentials
- Proportionality: arm span-to-height ratio (normal 0.97-1.03)
- Dysmorphic features: Turner stigmata, skeletal dysplasia signs
- Pubertal staging: Tanner stages for age-appropriate development
- Fundoscopy: papilledema suggests increased intracranial pressure
- Thyroid palpation: goiter or nodules
- Abdominal examination: hepatosplenomegaly, masses
📌 Remember: RAPID GROWTH - Record measurements accurately, Assess velocity trends, Plot on appropriate charts, Identify red flags, Document family history, Genetic factors, Review medications, Organ system examination, Watch for syndromes, Tanner staging, Hormonal clues
Critical Threshold Reference provides immediate decision-making support:
-
Growth Velocity Thresholds
- Ages 2-4 years: <5 cm/year requires evaluation
- Ages 4-8 years: <4 cm/year indicates pathology
- Pubertal ages: <6 cm/year suggests delayed puberty
- Crossing percentiles: >2 major lines downward abnormal
- Height-for-age: <-2 SD warrants investigation
- Weight-for-height: discordance suggests nutritional issues
-
Laboratory Decision Points
- IGF-1: <10th percentile for age/Tanner stage → GH testing
- TSH: >10 mIU/L → immediate thyroid replacement
- Bone age delay: >2 years → endocrine evaluation
- Celiac antibodies: >20 units → gastroenterology referral
- Inflammatory markers: ESR >30 mm/hr → chronic disease workup
| Clinical Scenario | Threshold Value | Immediate Action | Follow-up Timeline | Expected Outcome |
|---|---|---|---|---|
| Growth velocity decline | <4 cm/year | Laboratory workup | 3 months | Diagnosis established |
| Severe short stature | <-3 SD height | Urgent endocrine referral | 2 weeks | Treatment initiated |
| Abnormal IGF-1 | <10th percentile | GH stimulation testing | 4 weeks | GH status determined |
| Delayed puberty | No signs by 13F/14M | Hormone evaluation | 6 weeks | Pubertal assessment |
| Rapid growth | >8 cm/year prepubertal | Precocity evaluation | 2 weeks | Intervention if needed |
-
Growth Hormone Therapy Benchmarks
- First 6 months: >4 cm height gain expected
- First year: 8-12 cm total height gain
- Subsequent years: 6-8 cm/year until pubertal growth spurt
- IGF-1 monitoring: target 0 to +2 SD for age
- Side effect surveillance: glucose tolerance, scoliosis, headaches
- Dose optimization: adjust based on growth response and IGF-1 levels
-
Thyroid Replacement Monitoring
- Initial response: TSH normalization within 6-8 weeks
- Growth improvement: velocity increase within 3-6 months
- Long-term goals: TSH 0.5-2.0 mIU/L for optimal growth
- Dose adjustments: every 6-8 weeks until stable
- Growth monitoring: every 3 months during catch-up phase
- Bone age: annual assessment to monitor advancement
⭐ Clinical Pearl: Poor growth response to appropriate therapy within 12 months indicates non-compliance (40% of cases), incorrect diagnosis (30%), or additional pathology (30%)-systematic re-evaluation required.
Differential Diagnosis Framework enables rapid pattern recognition:
-
Short Stature with Normal Velocity
- Familial short stature: family history, normal bone age
- Constitutional delay: delayed bone age, late bloomer family
- Skeletal dysplasia: disproportionate measurements, dysmorphic features
- SHOX deficiency: mesomelic shortening, Madelung deformity
- Turner syndrome: female, webbed neck, cardiac defects
-
Short Stature with Decreased Velocity
- Growth hormone deficiency: proportionate short stature, delayed bone age
- Hypothyroidism: severe bone age delay, developmental delay
- Chronic disease: weight loss, systemic symptoms, inflammatory markers
- Malnutrition: low IGF-1, elevated GH, rapid catch-up with nutrition
- Psychosocial deprivation: environmental stressors, behavioral changes
Emergency Recognition Patterns identify life-threatening conditions:
- Immediate Intervention Required
- Neonatal hypoglycemia with micropenis → hypopituitarism
- Severe dehydration with salt craving → adrenal insufficiency
- Rapid growth with headaches → brain tumor
- Visual field defects → craniopharyngioma, pituitary adenoma
- Diabetes insipidus → posterior pituitary dysfunction
- Multiple hormone deficiencies → panhypopituitarism
💡 Master This: Growth disorders rarely present as isolated findings-systematic evaluation of associated symptoms, family history, and physical findings enables accurate diagnosis and optimal treatment in >95% of cases.
Long-term Outcome Optimization ensures sustained therapeutic success:
-
Final Height Prediction
- Bayley-Pinneau method: uses bone age and current height
- Genetic target: mid-parental height ± 8.5 cm
- Treatment goals: achieve genetic potential while minimizing risks
- Growth hormone: 1.2-1.8 SD improvement possible
- Early intervention: better outcomes when started young
- Compliance monitoring: essential for success
-
Transition to Adult Care
- Growth hormone: continue until growth velocity <2 cm/year
- Thyroid replacement: lifelong therapy for permanent hypothyroidism
- Monitoring protocols: establish adult endocrine follow-up
- Bone health: DEXA scanning for osteoporosis risk
- Cardiovascular health: lipid monitoring in GH-treated patients
- Reproductive health: fertility assessment if indicated
⚠️ Warning: Premature discontinuation of growth hormone therapy before growth velocity drops below 2 cm/year can result in loss of 3-5 cm of potential final height-bone age assessment guides optimal timing.
This comprehensive growth mastery toolkit provides the clinical excellence framework needed to recognize, evaluate, and treat pediatric growth disorders with expert-level precision and optimal patient outcomes.
🎯 The Growth Mastery Toolkit: Clinical Excellence Arsenal
Practice Questions: Growth/Development
Test your understanding with these related questions
A 45 year-old gentleman presents to his primary care physician complaining of wrist pain and is diagnosed with carpal tunnel syndrome. Upon further questioning, the patient admits that he has recently been outgrowing his gloves and shoes and has had to purchase a new hat as well due to increased head size. Upon exam, he is found to have new mild hypertension and on basic labs he is found to be hyperglycemic. Which of the following is the best blood test to diagnose his suspected disorder?













