Neurobiology of addiction US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Neurobiology of addiction. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Neurobiology of addiction US Medical PG Question 1: A 32-year-old man is brought to the emergency department because he was found stumbling in the street heedless of oncoming traffic. On arrival, he is found to be sluggish and has slow and sometimes incoherent speech. He is also drowsy and falls asleep several times during questioning. Chart review shows that he has previously been admitted after getting a severe cut during a bar fight. Otherwise, he is known to be intermittently homeless and has poorly managed diabetes. Serum testing reveals the presence of a substance that increases the duration of opening for an important channel. Which of the following symptoms may be seen if the most likely substance in this patient is abruptly discontinued?
- A. Tremors
- B. Insomnia
- C. Delayed delirium
- D. Piloerection
- E. Seizures (Correct Answer)
Neurobiology of addiction Explanation: ***Seizures***
- This patient presents with symptoms of **central nervous system (CNS) depression** (sluggish, incoherent speech, drowsiness) and a history suggestive of **substance abuse** (homelessness, bar fight).
- The key clue is that the substance **increases the duration of opening** of the GABA-A receptor channel, which specifically describes **barbiturates** (benzodiazepines increase the **frequency** of opening, not duration).
- Abrupt discontinuation of barbiturates can lead to life-threatening **withdrawal seizures** due to CNS hyperexcitability when GABAergic inhibition is suddenly removed [1].
- This is the most critical and potentially fatal complication of barbiturate withdrawal.
*Tremors*
- While **tremors** can occur during withdrawal from CNS depressants, they are a less severe symptom compared to seizures.
- Tremors are common in withdrawal syndromes but do not represent the most life-threatening risk in acute barbiturate withdrawal.
*Insomnia*
- **Insomnia** is a common symptom of withdrawal from CNS depressants due to rebound CNS hyperactivity [1].
- However, compared to seizures, insomnia is not life-threatening and is a less critical feature of barbiturate withdrawal.
*Delayed delirium*
- **Delirium** can occur during severe withdrawal, particularly **delirium tremens** in alcohol withdrawal.
- While delirium may develop, the most immediate and severe risk for barbiturate withdrawal is seizures, which can occur within hours to days of cessation.
*Piloerection*
- **Piloerection** (goosebumps) is a classic symptom of **opioid withdrawal**, resulting from sympathetic nervous system activation.
- This symptom is **not** characteristic of withdrawal from barbiturates or other GABAergic substances, making it an incorrect choice.
Neurobiology of addiction US Medical PG Question 2: A genetic population study is being conducted to find the penetrance of a certain disease. This disease is associated with impaired iron metabolism and primarily affects the liver. Patients often present with diabetes and bronze skin pigmentation. After a genetic screening of 120 inhabitants with a family history of this disease, 40 were found to have the disease-producing genotype, but only 10 presented with symptoms. What are the chances of the screened patients with said genotype developing the disease phenotype?
- A. 0.4%
- B. 40%
- C. 3%
- D. 4%
- E. 25% (Correct Answer)
Neurobiology of addiction Explanation: ***25%***
- **Penetrance** is calculated as the proportion of individuals with a specific genotype who express the associated phenotype.
- In this case, 10 individuals out of 40 with the disease-producing genotype developed symptoms, so (10 / 40) * 100% = **25%**.
*0.4%*
- This value is significantly lower than the actual penetrance and likely results from an incorrect calculation or misinterpretation of the given data.
- It does not accurately reflect the proportion of genotypically affected individuals who express the phenotype.
*40%*
- This percentage represents the proportion of screened individuals with the disease-producing genotype (40 out of 120 are ~33%), not the penetrance itself.
- It incorrectly equates the presence of the genotype in the population with the expression of the phenotype.
*3%*
- This value is likely obtained by an erroneous calculation, possibly by dividing the symptomatic individuals by the total screened population (10/120 ≈ 8.3%), which does not represent penetrance.
- It does not account for the specific individuals who possess the genotype.
*4%*
- This percentage might arise from an incorrect division or a misunderstanding of what constitutes penetrance.
- It is an inaccurate representation of the ratio between phenotype expression and genotype presence.
Neurobiology of addiction US Medical PG Question 3: A 49-year-old woman presents to the clinic for a routine exam. She recently quit smoking after a 30 pack-year history and started exercising a little. Past medical history is noncontributory. She takes no medication. Her mother died at 65 from lung cancer. She rarely drinks alcohol and only uses nicotine gum as needed. She admits to having some cravings for a cigarette in the morning before work, and after work. Which of the following best describes this patient’s stage in overcoming her nicotine addiction?
- A. Relapse
- B. Contemplation
- C. Maintenance
- D. Precontemplation
- E. Action (Correct Answer)
Neurobiology of addiction Explanation: ***Action***
- The patient has **recently quit smoking** and is actively modifying her behavior to overcome the addiction, using **nicotine gum** and **starting to exercise**.
- The **action stage** lasts from the initial behavior change up to **6 months**, during which individuals actively work to change their behavior and environment.
- She is experiencing cravings but successfully resisting them, which is typical of the action stage as new behaviors are being established and reinforced.
*Maintenance*
- This stage begins **after 6 months** of sustained behavior change, focusing on preventing relapse and consolidating gains.
- The stem indicates she **recently quit**, suggesting she has not yet reached the 6-month threshold required for the maintenance stage.
- While she is working to sustain her change, the timeline places her in the earlier action phase.
*Contemplation*
- In this stage, individuals are **considering change** within the next 6 months but have not yet taken action.
- The patient has already **quit smoking** and started exercising, demonstrating she has moved beyond contemplation into active behavior modification.
*Precontemplation*
- This stage is characterized by **no intention to change** behavior in the foreseeable future, often due to denial or lack of awareness.
- The patient has clearly moved past this stage by successfully quitting smoking.
*Relapse*
- This stage involves a **return to the problematic behavior** after a period of abstinence.
- The patient has not relapsed; she is still abstinent from cigarettes and managing her cravings with nicotine replacement therapy.
Neurobiology of addiction US Medical PG Question 4: A 42-year-old man is brought in to the emergency department by his daughter. She reports that her father drank heavily for the last 16 years, but he stopped 4 days ago after he decided to quit drinking on his birthday. She also reports that he has been talking about seeing cats running in his room since this morning, although there were no cats. There is no history of any known medical problems or any other substance use. On physical examination, his temperature is 38.4ºC (101.2ºF), heart rate is 116/min, blood pressure is 160/94 mm Hg, and respiratory rate is 22/min. He is severely agitated and is not oriented to his name, time, or place. On physical examination, profuse perspiration and tremors are present. Which of the following best describes the pathophysiologic mechanism underlying his condition?
- A. Increased influx of chloride ions
- B. Increased inhibition of norepinephrine
- C. Functional increase in GABA
- D. Increased activity of NMDA receptors (Correct Answer)
- E. Increased inhibition of glutamate
Neurobiology of addiction Explanation: ***Increased activity of NMDA receptors***
- Chronic alcohol use leads to **downregulation of GABA receptors** and **upregulation of NMDA receptors** to compensate for alcohol's inhibitory effects.
- When alcohol is withdrawn, the unopposed upregulation of NMDA receptors (and decreased GABA activity) causes a state of **neuronal hyperexcitability**, leading to symptoms like agitation, hallucinations, and autonomic hyperactivity seen in **delirium tremens**.
*Increased influx of chloride ions*
- This describes the mechanism of action of **GABA-A agonists** (like benzodiazepines), which enhance GABA's inhibitory effects by increasing chloride influx and hyperpolarizing neurons.
- In alcohol withdrawal, there is a **functional decrease in GABAergic activity**, not an increase in chloride ion influx.
*Increased inhibition of norepinephrine*
- **Norepinephrine** is a neurotransmitter associated with wakefulness, alertness, and autonomic responses; increased activity is seen in alcohol withdrawal, contributing to sympathetic overdrive.
- Increased inhibition of norepinephrine would lead to sedation and reduced autonomic activity, which is the opposite of the patient's presentation.
*Functional increase in GABA*
- **GABA** (gamma-aminobutyric acid) is the primary inhibitory neurotransmitter in the brain; alcohol enhances GABAergic activity.
- In alcohol withdrawal, there is a **functional decrease in GABAergic activity**, contributing to neuronal hyperexcitability and withdrawal symptoms.
*Increased inhibition of glutamate*
- **Glutamate** is the primary excitatory neurotransmitter, and its receptors (like NMDA) are implicated in alcohol withdrawal.
- Alcohol withdrawal is characterized by **increased excitatory activity**, including increased glutamate release and NMDA receptor activation, not increased inhibition of glutamate.
Neurobiology of addiction US Medical PG Question 5: A 40-year-old man is brought to the emergency department after sustaining multiple lacerations during a bar fight. The patient’s wife says that he has been showing worsening aggression and has been involved in a lot of arguments and fights for the past 2 years. The patient has no significant past medical or psychiatric history and currently takes no medications. The patient cannot provide any relevant family history since he was adopted as an infant. His vitals are within normal limits. On physical examination, the patient looks apathetic and grimaces repeatedly. Suddenly, his arms start to swing by his side in an uncontrolled manner. Which area of the brain is most likely affected in this patient?
- A. Cerebral cortex
- B. Caudate nucleus (Correct Answer)
- C. Cerebellum
- D. Medulla oblongata
- E. Substantia nigra
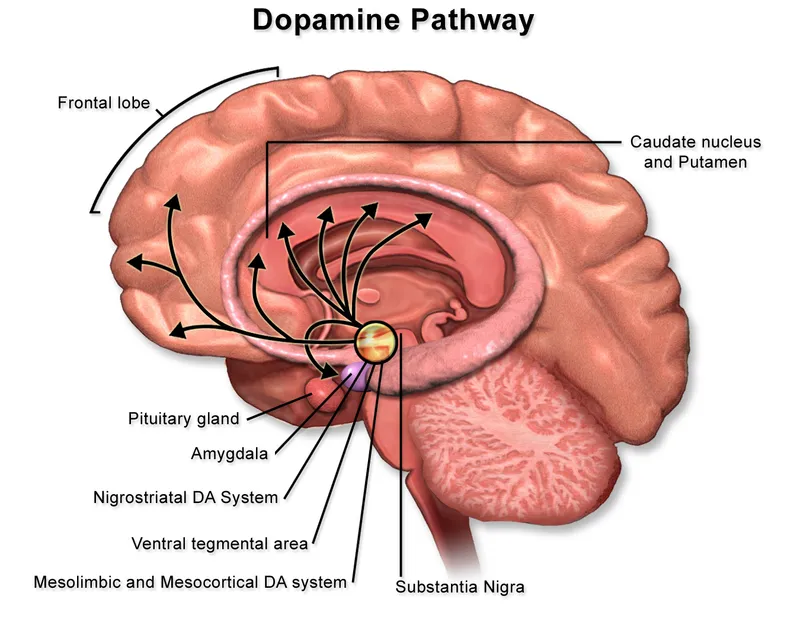
Neurobiology of addiction Explanation: **Caudate nucleus**
- The patient exhibits features like **worsening aggression**, **apathy**, and **uncontrolled, sudden movements** of the limbs, which are characteristic of Huntington's disease, a condition primarily affecting the **caudate nucleus**.
- **Huntington's disease** is an autosomal dominant neurodegenerative disorder linked to a trinucleotide repeat expansion (CAG) on chromosome 4, leading to atrophy of the **caudate and putamen**.
*Cerebral cortex*
- While damage to the cerebral cortex can cause personality changes and motor deficits, the specific combination of **choreiform movements** and progressive cognitive/behavioral decline seen here is more indicative of a basal ganglia disorder like Huntington's.
- Cortical lesions more commonly present with **focal neurological deficits** such as hemiparesis, aphasia, or sensory loss, which are not the primary features described.
*Cerebellum*
- Damage to the cerebellum typically results in **ataxia**, **dysmetria**, **intention tremor**, and problems with balance and coordination.
- The patient's **uncontrolled, sudden limb movements** are characteristic of chorea, not cerebellar dysfunction.
*Medulla oblongata*
- The medulla oblongata is crucial for vital autonomic functions such as **breathing, heart rate, and blood pressure regulation**.
- Lesions in this area would likely cause life-threatening symptoms, including **respiratory failure** or severe cardiovascular instability, which are not present in this patient.
*Substantia nigra*
- Damage or degeneration of the substantia nigra is primarily associated with **Parkinson's disease**, leading to symptoms like **bradykinesia**, **rigidity**, **resting tremor**, and **postural instability**.
- The patient's **hyperkinetic movements** (choreiform movements) are opposite to the hypokinetic presentation of Parkinson's disease.
Neurobiology of addiction US Medical PG Question 6: A 22-year-old man is brought to the physician by his mother because of concerns about his recent behavior. Three months ago, the patient first reported hearing loud voices coming from the ceiling of his room. During this time, he has also become increasingly worried that visitors to the house were placing secret surveillance cameras. Mental status examination shows tangential speech with paranoid thoughts. Treatment for this patient's condition predominantly targets which of the following dopaminergic pathways?
- A. Mesocortical pathway
- B. Thalamocortical pathway
- C. Nigrostriatal pathway
- D. Corticostriatal pathway
- E. Mesolimbic pathway (Correct Answer)
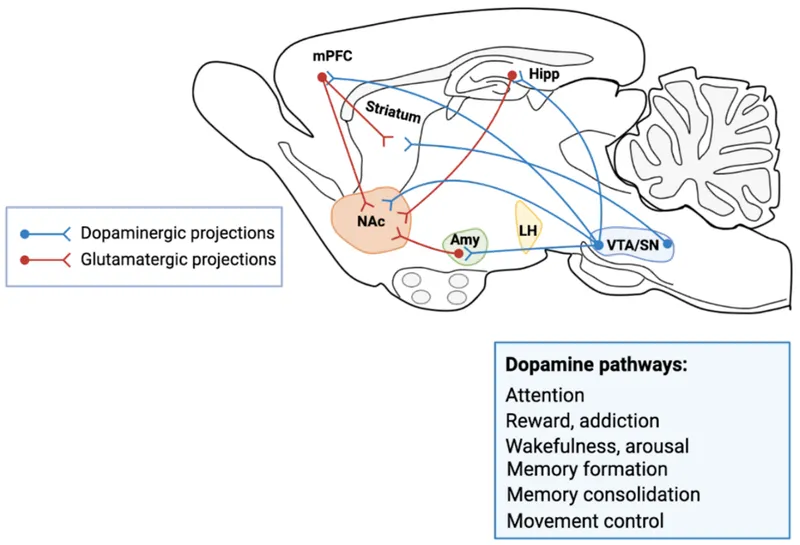
Neurobiology of addiction Explanation: ***Mesolimbic pathway***
- The patient's symptoms of **auditory hallucinations** and **paranoid delusions** are **positive symptoms** of psychosis consistent with **schizophrenia**.
- **Hyperactivity** of the **mesolimbic dopaminergic pathway** is strongly associated with the positive symptoms of schizophrenia, making it the primary target for antipsychotic treatment.
*Mesocortical pathway*
- The **mesocortical pathway** is primarily involved in **cognition, motivation, and executive functions**, originating from the ventral tegmental area and projecting to the prefrontal cortex.
- **Hypoactivity** in this pathway is thought to contribute to the **negative and cognitive symptoms** of schizophrenia, not the positive symptoms described.
*Thalamocortical pathway*
- The **thalamocortical pathway** connects the **thalamus to the cerebral cortex** and is crucial for sensory processing, arousal, and consciousness.
- While involved in neural circuits, it is not considered a primary dopaminergic pathway targeted for the treatment of positive psychotic symptoms.
*Nigrostriatal pathway*
- The **nigrostriatal pathway** projects from the **substantia nigra to the striatum** and is primarily involved in **motor control**.
- Blocking dopamine receptors in this pathway by antipsychotic medications can cause **extrapyramidal symptoms (EPS)**, but it is not the main pathway responsible for positive psychotic symptoms or their treatment.
*Corticostriatal pathway*
- The **corticostriatal pathway** is **predominantly a glutamatergic pathway** connecting the **cerebral cortex to the striatum**, playing a role in motor control and habit formation.
- This is not a primary dopaminergic pathway and is not directly implicated in the positive symptoms of schizophrenia or their pharmacological treatment.
Neurobiology of addiction US Medical PG Question 7: A neuroscientist is delivering a lecture on the electrophysiology of the brain. He talks about neuroreceptors which act as ion channels in the neurons. He mentions a specific receptor, which is both voltage-gated and ligand-gated ion channel. Which of the following receptors is most likely to be the one mentioned by the neuroscientist?
- A. NMDA receptor (Correct Answer)
- B. GABAA receptor
- C. AMPA receptor
- D. Nicotinic acetylcholine receptor
- E. Glycine receptor
Neurobiology of addiction Explanation: ***NMDA receptor***
- The **NMDA receptor** is unique among ionotropic glutamate receptors as it functions as both a **ligand-gated** and **voltage-gated** ion channel.
- It requires both the binding of an excitatory neurotransmitter (like **glutamate**) and a sufficient **depolarization** of the postsynaptic membrane to remove a **magnesium ion (Mg2+) block** from its pore.
*GABAA receptor*
- The **GABAA receptor** is a **ligand-gated ion channel** that opens upon binding of the neurotransmitter **GABA**, leading to an influx of chloride ions and neuronal hyperpolarization.
- It is primarily responsible for **inhibitory synaptic transmission** in the central nervous system.
*AMPA receptor*
- The **AMPA receptor** is an ionotropic glutamate receptor that is primarily **ligand-gated**, opening swiftly upon binding of **glutamate** to allow sodium and potassium ion flow.
- While it contributes to depolarization, it is generally not considered to have a significant **voltage-gating** mechanism like the NMDA receptor.
*Nicotinic acetylcholine receptor*
- The **nicotinic acetylcholine receptor** is a **ligand-gated ion channel** that opens in response to the binding of **acetylcholine**, initiating fast excitatory synaptic transmission.
- It is **not voltage-gated** in the same manner as the NMDA receptor; its opening is primarily dependent on neurotransmitter binding.
*Glycine receptor*
- The **glycine receptor** is a **ligand-gated chloride channel** that mediates fast inhibitory synaptic transmission in the spinal cord and brainstem.
- Its activation by **glycine** leads to an influx of chloride ions, causing hyperpolarization, and it does not exhibit significant voltage-gating properties.
Neurobiology of addiction US Medical PG Question 8: A 19-year-old man presents to a psychiatrist for the management of substance abuse. He reports that he started using the substance 2 years ago and that he smokes it after sprinkling it on his cigarette. He describes that after smoking the substance, he feels excited and as if he does not belong to himself. He also reports that when he is in his room, he sees vivid colors on the walls after using the substance; if he listens to his favorite music, he clearly sees colors and shapes in front of his eyes. There is no history of alcohol or nicotine abuse. The psychiatrist goes through his medical records and notes that he had presented with acute substance intoxication 1 month prior. At that point, his clinical features included delusions, amnesia, generalized erythema of his skin, tachycardia, hypertension, dilated pupils, dysarthria, and ataxia. Which of the following signs is also most likely to have been present on physical examination while the man was intoxicated with the substance?
- A. Increased sensitivity to pain
- B. Excessive perspiration
- C. Hyporeflexia
- D. Generalized hypotonia
- E. Nystagmus (Correct Answer)
Neurobiology of addiction Explanation: ***Nystagmus***
- The patient's symptoms of **dissociation** ("feels as if he does not belong to himself"), **visual hallucinations** (seeing vivid colors and shapes), delusions, amnesia, tachycardia, hypertension, dilated pupils, dysarthria, and ataxia are highly characteristic of **phencyclidine (PCP) intoxication**.
- **Nystagmus**, particularly **horizontal and vertical nystagmus**, is a classic and frequently observed sign in PCP intoxication due to its effects on the **cerebellum** and vestibular system.
*Increased sensitivity to pain*
- PCP is known for its **analgesic** and **anesthetic** properties, leading to **decreased sensitivity to pain**, not increased.
- This effect contributes to the potential for self-injurious behavior during intoxication.
*Excessive perspiration*
- While other stimulants can cause diaphoresis, PCP intoxication more typically presents with **dry skin** or normal perspiration despite **hyperthermia** as it interferes with cholinergic thermoregulation.
- The described **generalized erythema** suggests **vasodilation**, but **dry skin** is more often associated with the anticholinergic effects that can accompany PCP.
*Hyporeflexia*
- PCP intoxication commonly causes **hyperreflexia** and **spasticity**, not hyporeflexia, due to its excitatory effects on the **central nervous system**.
- **Muscle rigidity** and **seizures** are also possible, further indicating CNS excitation.
*Generalized hypotonia*
- PCP typically leads to **increased muscle tone** and **rigidity**, not generalized hypotonia.
- The patient's presentation with **ataxia** and **dysarthria** suggests cerebellar involvement, but this usually manifests with motor incoordination rather than widespread flaccidity.
Neurobiology of addiction US Medical PG Question 9: A 30-year-old man presents to his family physician admitting to using heroin. He says he started using about 6-months ago when his back pain medication ran out. At first, he says he would borrow his wife’s Percocet but, eventually, that ran out and he had to find a different source. Since then, he has been having more and more issues related to his heroin use, and it has started to affect his work and home life. He is concerned that, if he continues like this, he might end up in real trouble. He denies sharing needles and is sincerely interested in quitting. He recalls trying to quit last month but recounts how horrible the withdrawal symptoms were. Because of this and the strong cravings, he relapsed shortly after his initial attempt. Methadone maintenance therapy is prescribed. Which of the following would most likely be the most important benefit of this new treatment plan in this patient?
- A. Decreases methadone dependence
- B. Euphoria without the side effects
- C. Prevention of withdrawal symptoms and reduced cravings (Correct Answer)
- D. Reduced risk of hepatitis B and C transmission
- E. Improved interpersonal relationships
Neurobiology of addiction Explanation: ***Prevention of withdrawal symptoms and reduced cravings***
- **Methadone maintenance therapy** is a long-acting μ-opioid receptor agonist that prevents withdrawal symptoms and reduces cravings—this is the **primary therapeutic benefit** and mechanism of action.
- By providing a stable, long-acting opioid, methadone eliminates the cycle of withdrawal and drug-seeking behavior that characterizes heroin addiction.
- This patient's previous quit attempt failed specifically due to **"horrible withdrawal symptoms"** and **strong cravings**, making this the most directly relevant benefit for his situation.
- All other benefits of methadone maintenance (improved functioning, better relationships, reduced risk behaviors) are **secondary consequences** that stem from this primary pharmacological effect.
- Evidence-based guidelines consistently identify withdrawal prevention and craving reduction as the core therapeutic goals of opioid agonist therapy.
*Improved interpersonal relationships*
- While this is an important **downstream benefit** of successful methadone maintenance, it is an indirect consequence rather than the primary therapeutic effect.
- Improved relationships result FROM the stabilization achieved through withdrawal prevention and craving reduction, not as a direct pharmacological action.
- Though clinically meaningful, this represents a **psychosocial outcome** rather than the most important direct benefit of the medication itself.
*Decreases methadone dependence*
- This is **incorrect**—methadone itself is an opioid agonist and patients on maintenance therapy develop **physical dependence** on methadone.
- The goal is to substitute unstable illicit opioid use (heroin) with stable, medically supervised opioid therapy (methadone), not to eliminate opioid dependence immediately.
- Methadone maintenance is harm reduction, not abstinence-based treatment initially.
*Euphoria without the side effects*
- Methadone is **not intended to produce euphoria**—it is administered at stable doses to maintain normal functioning without intoxication.
- Its slow onset and long duration of action when taken orally minimize the "rush" or euphoric effects associated with rapid-acting opioids like heroin.
- The goal is stabilization and normal functioning, not achieving a "high."
*Reduced risk of hepatitis B and C transmission*
- This is a valuable **harm reduction benefit**, particularly for those who inject drugs and share needles.
- However, this patient specifically **denies sharing needles**, making this less relevant to his individual case.
- More importantly, this is a secondary benefit that occurs as a result of reduced injection drug use, which itself results from the primary effect of withdrawal prevention and craving reduction.
Neurobiology of addiction US Medical PG Question 10: A 68-year-old man, accompanied by his wife, presents to his physician with cognitive decline and hallucinations. The patient’s wife tells that his cognitive impairment progressed gradually over the past 6 years, and first began with problems counting and attention. The hallucinations began approximately a year ago. The patient describes them as realistic and non-frightening; most often, he sees his cat accompanying him everywhere he goes. The patient’s wife also notes frequent episodes of staring spells in her husband and prolonged daytime napping. The blood pressure is 130/80 mm Hg with the orthostatic change to 110/60 mm Hg, heart rate is 75/min, respiratory rate is 13/min, and the temperature is 36.6°C (97.8°F). The patient is alert and responsive, but he is disoriented to time and place. He is pale and hypomimic. The cardiac, lung, and abdominal examinations are within normal limits for the patient’s age. The neurological examination is significant for a bilateral symmetrical cogwheel rigidity in the upper extremities. What would you most likely see on additional radiological investigations?
- A. Multiple lacunar infarcts on MRI
- B. Marked hippocampal atrophy on MRI
- C. Hypoperfusion and hypometabolism in frontal lobes on SPECT
- D. Decreased perfusion and dopaminergic activity in occipital lobes on PET (Correct Answer)
- E. Pontine 'hot-cross bun' sign on MRI
Neurobiology of addiction Explanation: ***Decreased perfusion and dopaminergic activity in occipital lobes on PET***
- This finding is characteristic of **dementia with Lewy bodies (DLB)**, which is strongly suggested by the patient's presentation with **cognitive fluctuations**, **visual hallucinations** (non-frightening, realistic), **parkinsonism** (cogwheel rigidity), and **REM sleep behavior disorder** (daytime napping/staring spells could be a manifestation). PET scans in DLB often show reduced occipital lobe uptake.
- The combination of **parkinsonism** (cogwheel rigidity) and **visual hallucinations** preceding or appearing early in the course of cognitive decline is a hallmark of DLB, which differentiates it from other dementias.
*Multiple lacunar infarcts on MRI*
- While lacunar infarcts can cause cognitive decline (**vascular dementia**), the clinical picture of prominent, well-formed visual hallucinations, parkinsonism, and cognitive fluctuations is less typical for purely vascular dementia.
- Vascular dementia usually presents with a step-wise decline in cognition and focal neurological deficits, which are not the primary features here.
*Marked hippocampal atrophy on MRI*
- **Hippocampal atrophy** is a hallmark of **Alzheimer's disease**, which typically presents with insidious memory loss as the primary symptom.
- The prominent early visual hallucinations and parkinsonism are not typical initial features of Alzheimer's disease.
*Hypoperfusion and hypometabolism in frontal lobes on SPECT*
- **Frontal lobe hypoperfusion/hypometabolism** on SPECT/PET is characteristic of **frontotemporal dementia (FTD)**.
- FTD typically presents with early behavioral changes or language deficits, not prominent visual hallucinations, parkinsonism, or significant cognitive fluctuations in the way seen in this patient.
*Pontine 'hot-cross bun' sign on MRI*
- The **'hot-cross bun' sign** on MRI is pathognomonic for **multiple system atrophy (MSA)**, specifically the **MSA-C subtype (cerebellar)**.
- While MSA can cause parkinsonism and autonomic dysfunction, it typically does not feature prominent visual hallucinations or significant cognitive decline as early and striking features as seen in this patient.
More Neurobiology of addiction US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.