Genetic and Metabolic Disorders

On this page
🧬 The Genetic Blueprint: Decoding Life's Molecular Architecture
Genetic and metabolic disorders reveal how single molecular missteps cascade into multi-system disease, transforming abstract DNA sequences into tangible clinical presentations you'll recognize at the bedside. You'll decode inheritance patterns, trace biochemical disruptions from gene to symptom, and master the diagnostic reasoning that distinguishes phenylketonuria from maple syrup urine disease or differentiates storage disorders from mitochondrial syndromes. By integrating molecular mechanisms with pattern recognition and evidence-based treatment algorithms, you'll build the systematic framework needed to diagnose rare conditions confidently and intervene before irreversible damage occurs.

The genetic foundation encompasses 46 chromosomes containing 3.2 billion base pairs, where single nucleotide changes can trigger devastating metabolic cascades affecting millions of patients worldwide. Genetic disorders collectively affect 1 in 300 live births, making this knowledge essential for every practicing physician.
📌 Remember: GENETIC - Genotype determines phenotype, Enzyme defects cause metabolic blocks, Newborn screening saves lives, Ethical counseling guides families, Testing confirms diagnosis, Inheritance patterns predict risk, Carrier screening prevents disease
- Mendelian Disorders: Single gene defects following predictable inheritance patterns
- Autosomal dominant: 50% transmission risk per pregnancy
- Autosomal recessive: 25% affected risk with carrier parents
- X-linked recessive: 50% risk for male offspring of carrier mothers
- Chromosomal Disorders: Numerical or structural chromosome abnormalities
- Trisomy 21 (Down syndrome): 1 in 700 births overall
- Turner syndrome: 1 in 2,500 female births
- Klinefelter syndrome: 1 in 600 male births
- Multifactorial Disorders: Gene-environment interactions
- Neural tube defects: 40-80% reduction with folic acid supplementation
- Congenital heart disease: 3-5% recurrence risk in families
| Disorder Category | Prevalence | Key Features | Screening Method | Treatment Approach |
|---|---|---|---|---|
| Single Gene | 1 in 200 births | Mendelian inheritance | Family history, molecular testing | Gene therapy, enzyme replacement |
| Chromosomal | 1 in 150 births | Developmental delays, dysmorphism | Karyotype, microarray | Supportive care, early intervention |
| Multifactorial | 1 in 100 births | Complex inheritance | Risk assessment | Prevention, lifestyle modification |
| Mitochondrial | 1 in 5,000 births | Maternal inheritance | Muscle biopsy, genetic testing | Supportive care, cofactor therapy |
| Metabolic | 1 in 1,500 births | Enzyme deficiencies | Newborn screening | Dietary restriction, enzyme replacement |
💡 Master This: Genetic disorders follow molecular logic - understanding the disrupted pathway predicts clinical manifestations, inheritance patterns, and therapeutic targets. Every unexplained syndrome warrants genetic evaluation.
The molecular basis of genetic disorders reveals how single base pair changes can disrupt entire metabolic pathways, leading to toxic accumulations, deficient products, or altered cellular function that manifests as recognizable clinical syndromes requiring specialized management approaches.
🧬 The Genetic Blueprint: Decoding Life's Molecular Architecture
⚙️ Molecular Mechanisms: The Cellular Command Center Disruptions

📌 Remember: PATHWAYS - Protein defects block reactions, Accumulation causes toxicity, Transport defects trap substrates, Hormone resistance disrupts signaling, Waste products damage organs, Absent products cause deficiency, Yield determines severity, Storage overwhelms cells
- Enzyme Deficiencies: >400 known inborn errors of metabolism
- Complete deficiency: <1% normal activity, severe phenotype
- Partial deficiency: 1-10% activity, variable presentation
- Residual activity: 10-25% activity, mild or late-onset disease
- Transport Defects: Membrane protein dysfunction
- Cystic fibrosis: CFTR chloride channel defects in 1 in 3,500 births
- Glucose transporter defects: GLUT1 deficiency causing seizures
- Amino acid transporters: Cystinuria affecting 1 in 7,000 individuals
- Structural Protein Defects: Connective tissue and cellular architecture
- Marfan syndrome: Fibrillin-1 defects in 1 in 5,000 individuals
- Duchenne muscular dystrophy: Dystrophin defects in 1 in 3,500 males

| Mechanism Type | Molecular Defect | Clinical Consequence | Example Disorder | Therapeutic Target |
|---|---|---|---|---|
| Enzyme Loss | Catalytic site mutation | Substrate accumulation | Phenylketonuria | Substrate restriction |
| Transport Defect | Channel/carrier dysfunction | Cellular imbalance | Cystic fibrosis | Channel modulators |
| Structural Defect | Protein misfolding | Tissue weakness | Marfan syndrome | Structural support |
| Regulatory Defect | Transcription factor loss | Developmental failure | CHARGE syndrome | Growth factors |
| Storage Defect | Lysosomal enzyme loss | Cellular accumulation | Gaucher disease | Enzyme replacement |
💡 Master This: The molecular mechanism predicts inheritance pattern, age of onset, organ involvement, and therapeutic approach. Enzyme defects follow autosomal recessive inheritance, while structural proteins often show dominant patterns.
Understanding molecular mechanisms enables precision medicine approaches, where gene therapy restores function, enzyme replacement bypasses defects, and substrate reduction prevents toxic accumulation in specific genetic disorders.
⚙️ Molecular Mechanisms: The Cellular Command Center Disruptions
🎯 Pattern Recognition: The Clinical Detective's Toolkit

📌 Remember: RECOGNIZE - Recurrent infections suggest immunodeficiency, Early seizures indicate metabolic disorders, Coarse features suggest storage diseases, Odors point to organic acidemias, Growth failure indicates chromosomal disorders, Neurologic regression suggests degenerative diseases, Intellectual disability requires genetic evaluation, Zone-specific malformations suggest sequence defects, Eye abnormalities indicate many syndromes
- Metabolic Crisis Patterns: Acute decompensation with specific triggers
- Protein intolerance: Urea cycle defects, organic acidemias
- Fasting intolerance: Fatty acid oxidation defects, glycogen storage diseases
- Infection-triggered: 80% of metabolic crises occur during illness
- Neonatal onset: >50% of severe metabolic disorders present in first month
- Dysmorphic Feature Patterns: Recognizable facial and physical characteristics
- Down syndrome: >99% accuracy with experienced clinicians
- Fetal alcohol syndrome: Smooth philtrum, thin upper lip, short palpebral fissures
- Williams syndrome: Elfin facies, supravalvular aortic stenosis in 75%
- Developmental Delay Patterns: Age-specific milestone failures
- Global delay: 25% have identifiable genetic cause
- Regression: >90% indicate degenerative disorders requiring urgent evaluation
- Selective delays: Language delay suggests specific genetic syndromes
| Clinical Pattern | Key Features | Diagnostic Yield | First-Line Testing | Urgent Interventions |
|---|---|---|---|---|
| Metabolic Crisis | Acidosis, hyperammonemia | 85% with targeted testing | Plasma amino acids, organic acids | Protein restriction, dialysis |
| Dysmorphic Features | >3 major anomalies | 70% with expert evaluation | Chromosomal microarray | Cardiac evaluation |
| Developmental Regression | Loss of milestones | 90% genetic cause | MRI, metabolic screen | Seizure control |
| Growth Failure | <3rd percentile | 40% genetic cause | Karyotype, IGF-1 | Nutritional support |
| Recurrent Infections | Unusual organisms | 60% immunodeficiency | Immunoglobulin levels | Prophylactic antibiotics |
💡 Master This: Pattern recognition requires systematic evaluation of dysmorphic features, developmental milestones, family history, and metabolic parameters. Three or more major anomalies indicate >70% likelihood of genetic syndrome.
The "genetic gestalt" develops through experience, where subtle feature combinations trigger immediate syndrome recognition, enabling rapid diagnosis and family counseling before confirmatory testing results.
🎯 Pattern Recognition: The Clinical Detective's Toolkit
🔬 Systematic Discrimination: The Differential Diagnosis Matrix
📌 Remember: DISTINGUISH - DNA testing confirms diagnosis, Inheritance patterns guide counseling, Symptom onset indicates severity, Testing algorithms save time, Imaging reveals organ involvement, Newborn screening catches early cases, Gender affects X-linked disorders, Unique features distinguish syndromes, Incidence varies by ethnicity, Severity correlates with residual function, History reveals family patterns
- Inheritance Pattern Discrimination: Critical for family counseling and risk assessment
- Autosomal dominant: 50% transmission, vertical pattern, variable expressivity
- Autosomal recessive: 25% risk, horizontal pattern, consanguinity increases risk 8-fold
- X-linked recessive: Male predominance, maternal transmission, no male-to-male transmission
- Mitochondrial: Maternal inheritance, variable penetrance, multi-system involvement
- Biochemical Pattern Discrimination: Specific metabolite profiles guide diagnosis
- Organic acidemias: Elevated organic acids, metabolic acidosis, ketosis
- Amino acidopathies: Elevated plasma amino acids, normal organic acids
- Fatty acid oxidation defects: Hypoketotic hypoglycemia, elevated acylcarnitines
- Urea cycle defects: Hyperammonemia with normal or low glucose
| Disorder Category | Inheritance | Key Biochemical Marker | Age of Onset | Distinguishing Feature | Treatment Response |
|---|---|---|---|---|---|
| PKU | AR | Elevated phenylalanine >20mg/dL | Neonatal | Musty odor, fair skin | Excellent with diet |
| Galactosemia | AR | Elevated galactose >20mg/dL | Neonatal | Cataracts, hepatomegaly | Good with restriction |
| MSUD | AR | Elevated branched-chain amino acids | Neonatal | Maple syrup odor | Moderate with diet |
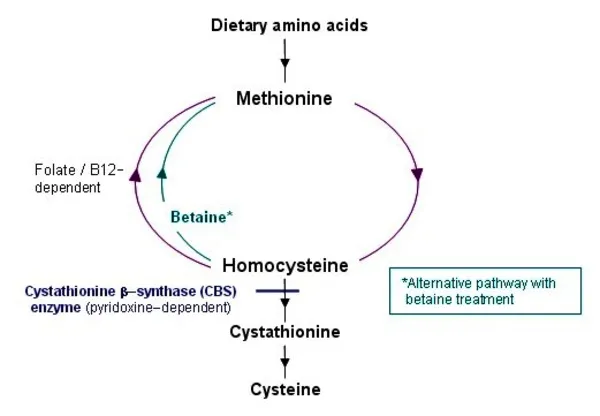
| Homocystinuria | AR | Elevated homocystine | Childhood | Lens dislocation, thrombosis | Variable with B6 |
| Marfan Syndrome | AD | Normal biochemistry | Variable | Aortic dilatation, lens dislocation | Supportive only |
| Duchenne MD | XLR | Elevated CK >10,000 U/L | Early childhood | Progressive weakness | Steroids slow progression |
- Intellectual disability: >1,000 genetic causes, requires tiered testing approach
- Short stature: >300 genetic causes, growth hormone response varies
- Seizures: >500 genetic causes, treatment resistance indicates genetic etiology
- Cardiomyopathy: >100 genetic causes, family screening essential
⭐ Clinical Pearl: Metabolic disorders typically follow autosomal recessive inheritance and present with acute crises, while structural disorders follow dominant inheritance with progressive manifestations.
💡 Master This: Systematic discrimination requires understanding that similar phenotypes can result from different molecular mechanisms, while different phenotypes can result from mutations in the same gene (allelic heterogeneity).
The key to accurate genetic diagnosis lies in recognizing that >80% of genetic disorders have overlapping features, requiring systematic evaluation of inheritance patterns, biochemical markers, and molecular testing to achieve precise diagnosis.
🔬 Systematic Discrimination: The Differential Diagnosis Matrix
⚕️ Treatment Algorithms: Evidence-Based Therapeutic Pathways
📌 Remember: TREATMENT - Timing determines outcome, Restriction prevents toxicity, Enzyme replacement restores function, Antioxidants protect organs, Transplantation cures severe cases, Monitoring prevents complications, Education empowers families, Nutrition supports growth, Therapy improves function
- Emergency Management Protocols: Time-sensitive interventions for metabolic crises
- Hyperammonemia: Immediate protein restriction, sodium benzoate/phenylacetate
- Hypoglycemia: IV glucose 10-15 mg/kg/min, avoid fasting >4-6 hours
- Metabolic acidosis: Sodium bicarbonate if pH <7.2, address underlying cause
- Seizures: Standard anticonvulsants, pyridoxine trial if refractory
- Chronic Management Strategies: Long-term therapeutic approaches
- Dietary restriction: PKU requires phenylalanine <6 mg/kg/day for optimal outcomes
- Enzyme replacement: Gaucher disease shows >90% improvement in organomegaly
- Gene therapy: >15 approved therapies with $2-3 million per treatment cost
- Substrate reduction: Miglustat reduces storage in Niemann-Pick type C
| Treatment Category | Mechanism | Success Rate | Cost (Annual) | Monitoring Requirements |
|---|---|---|---|---|
| Dietary Restriction | Substrate limitation | 85-95% | $5,000-15,000 | Monthly amino acids |
| Enzyme Replacement | Functional restoration | 70-90% | $200,000-500,000 | Quarterly assessments |
| Gene Therapy | Genetic correction | 60-80% | $2-3 million | Lifelong monitoring |
| Organ Transplant | Organ replacement | 80-95% | $500,000-1 million | Immunosuppression |
| Supportive Care | Symptom management | Variable | $10,000-50,000 | Condition-specific |
- Pharmacogenomics: >200 FDA-approved drug labels include genetic information
- Antisense oligonucleotides: Nusinersen for spinal muscular atrophy shows >80% motor improvement
- Small molecule therapies: Ivacaftor for specific CFTR mutations improves lung function >10%
- CRISPR gene editing: >40 clinical trials ongoing for genetic disorders
⭐ Clinical Pearl: Early intervention is critical - PKU patients starting diet within 3 weeks of birth have normal IQ, while delayed treatment results in irreversible intellectual disability.
💡 Master This: Treatment success depends on understanding the molecular mechanism - enzyme deficiencies respond to substrate restriction or enzyme replacement, while structural defects require supportive care and organ-specific interventions.
The future of genetic medicine lies in personalized therapeutic algorithms where individual genetic profiles guide customized treatment protocols, maximizing efficacy while minimizing adverse effects through precision medicine approaches.
⚕️ Treatment Algorithms: Evidence-Based Therapeutic Pathways
🌐 Multi-System Integration: The Genetic Network Architecture
📌 Remember: SYSTEMS - Syndromic features cluster together, Young age suggests genetic cause, Severe phenotypes indicate complete deficiency, Timing of symptoms guides diagnosis, Early intervention prevents complications, Multiple specialists coordinate care, Screening identifies at-risk relatives
- Cardiovascular-Genetic Integration: Cardiac manifestations in genetic syndromes
- Marfan syndrome: Aortic root dilatation in >80%, requires annual echocardiography
- 22q11.2 deletion: Conotruncal heart defects in 75%, requires cardiothoracic surgery
- Noonan syndrome: Pulmonary stenosis in 50%, hypertrophic cardiomyopathy in 25%
- Glycogen storage disease: Cardiomyopathy in Pompe disease requires enzyme replacement
- Neurological-Metabolic Integration: Brain involvement in metabolic disorders
- Phenylketonuria: Irreversible intellectual disability without dietary restriction
- Leukodystrophies: Progressive white matter degeneration, >40 genetic subtypes
- Mitochondrial disorders: Multi-system involvement, maternal inheritance pattern
- Peroxisomal disorders: Severe developmental delays, characteristic brain MRI findings
| Genetic Syndrome | Primary System | Secondary Systems | Specialist Requirements | Monitoring Frequency |
|---|---|---|---|---|
| Marfan Syndrome | Connective Tissue | Cardiac, Ocular, Skeletal | Cardiology, Ophthalmology | Annual imaging |
| Down Syndrome | Chromosomal | Cardiac, GI, Endocrine | Multiple subspecialists | Condition-specific |
| Cystic Fibrosis | Pulmonary | GI, Endocrine, Reproductive | Pulmonology, GI, Endocrine | Quarterly visits |
| Duchenne MD | Neuromuscular | Cardiac, Pulmonary, Orthopedic | Neurology, Cardiology | Every 6 months |
| Prader-Willi | Hypothalamic | Endocrine, Behavioral, GI | Endocrine, Psychiatry | Every 3-6 months |
- Turner syndrome: Short stature in >95%, growth hormone therapy improves final height
- Prader-Willi syndrome: Hyperphagia and obesity require strict dietary management
- McCune-Albright syndrome: Precocious puberty in >50%, requires endocrine intervention
- Beckwith-Wiedemann syndrome: Macrosomia and hypoglycemia require glucose monitoring
⭐ Clinical Pearl: >70% of genetic syndromes involve multiple organ systems, requiring coordinated subspecialty care and comprehensive management protocols to optimize outcomes.
💡 Master This: Multi-system involvement follows predictable patterns based on gene function and protein distribution - understanding these patterns enables anticipatory guidance and preventive interventions.
The emerging field of systems genetics reveals how genetic networks interact with environmental factors to create complex phenotypes, enabling personalized medicine approaches that address individual patient needs through precision interventions.
🌐 Multi-System Integration: The Genetic Network Architecture
🎯 Clinical Mastery Arsenal: The Genetic Medicine Toolkit
📌 Remember: MASTERY - Molecular mechanisms predict phenotypes, Anticipatory guidance prevents complications, Screening identifies at-risk individuals, Testing confirms diagnoses, Ethical counseling guides families, Risk assessment enables planning, Yield optimization maximizes diagnostic success
- Essential Diagnostic Thresholds: Critical values for immediate recognition
- Phenylalanine >20 mg/dL: PKU requiring immediate dietary restriction
- Ammonia >150 μmol/L: Urea cycle defect requiring emergency intervention
- CK >10,000 U/L: Muscular dystrophy requiring cardiac evaluation
- Homocysteine >100 μmol/L: Homocystinuria requiring B6 trial and anticoagulation
- Rapid Pattern Recognition Framework: "See this, think that" clinical correlations
- Coarse facial features + hepatosplenomegaly = Lysosomal storage disease
- Intellectual disability + seizures + fair skin = Phenylketonuria
- Tall stature + lens dislocation + aortic dilatation = Marfan syndrome
- Short stature + webbed neck + cardiac defects = Turner syndrome
| Clinical Scenario | Immediate Action | Diagnostic Test | Treatment Priority | Specialist Referral |
|---|---|---|---|---|
| Neonatal seizures + metabolic acidosis | IV glucose, stop feeds | Plasma amino acids | Protein restriction | Genetics urgent |
| Developmental delay + dysmorphic features | Photograph, measure | Chromosomal microarray | Early intervention | Genetics routine |
| Recurrent infections + failure to thrive | Immunoglobulin levels | Flow cytometry | Prophylactic antibiotics | Immunology urgent |
| Progressive weakness + elevated CK | Cardiac evaluation | Genetic testing | Steroid therapy | Neurology urgent |
| Lens dislocation + tall stature | Echocardiogram | Marfan testing | Activity restriction | Cardiology urgent |
- Recurrence risks: 25% for AR, 50% for AD, variable for multifactorial
- Penetrance concepts: Complete vs incomplete expression of genetic defects
- Anticipatory guidance: Age-specific screening and preventive interventions
- Reproductive options: Preimplantation diagnosis, prenatal testing, donor gametes
⭐ Clinical Pearl: Genetic testing yield reaches >50% when three or more major anomalies are present, making systematic phenotyping essential for diagnostic success.
💡 Master This: Pattern recognition combined with molecular understanding enables predictive medicine - knowing the genotype predicts phenotype evolution, treatment response, and family recurrence risks.
The future of genetic medicine lies in artificial intelligence-assisted diagnosis, gene therapy expansion, and precision medicine protocols that transform genetic disorders from devastating diagnoses to manageable conditions through early intervention and targeted therapies.
🎯 Clinical Mastery Arsenal: The Genetic Medicine Toolkit
Practice Questions: Genetic and Metabolic Disorders
Test your understanding with these related questions
A 28-year-old woman comes to the physician for genetic counseling prior to conception. For the past year, she has had intermittent episodes of headache, nausea, abdominal pain, and tingling of her fingers. She also complains of dark urine during the episodes. Her mother and maternal uncle have similar symptoms and her father is healthy. Her husband is healthy and there is no history of serious illness in his family. Serum studies show elevated concentrations of porphobilinogen and δ-aminolevulinic acid. What is the probability of this patient having a child with the same disease as her?












