V/Q mismatch

On this page
🌪️ V/Q Mismatch: The Lung's Perfect Storm
Every breath depends on an elegant match between air and blood, but when ventilation and perfusion fall out of sync, the body's oxygen delivery system falters in predictable yet diverse ways. You'll learn how V/Q mismatch creates a spectrum from wasted ventilation to dangerous shunting, master the clinical patterns that distinguish causes at the bedside, and command the therapeutic strategies that restore this critical balance across pulmonary, cardiac, and systemic disease.
📌 Remember: MATCH - Maintain airflow, Alveolar pressure, Tissue perfusion, Capillary flow, Hemoglobin saturation. Perfect V/Q requires all five components functioning optimally with 1:1 ratio at the alveolar level.
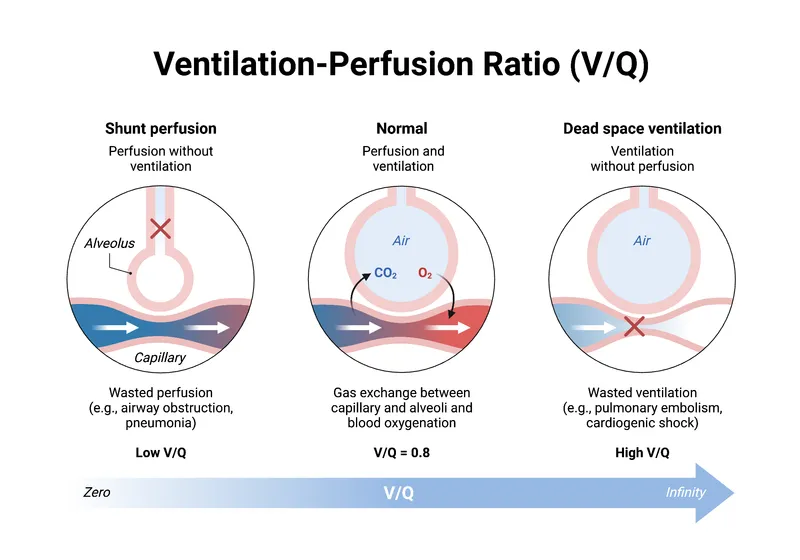
The normal V/Q ratio of 0.8 reflects the physiological reality that alveolar ventilation (4.2 L/min) slightly exceeds perfusion (5.0 L/min) when accounting for oxygen consumption and CO2 production. This seemingly simple ratio masks extraordinary complexity - each lung zone operates at different V/Q ratios, from 3.3 at the apex to 0.63 at the base.
-
Ventilation Components
- Alveolar ventilation: 4.2 L/min (total 6 L/min minus 1.8 L/min dead space)
- Respiratory rate: 12-16 breaths/min with 500 mL tidal volume
- Anatomical dead space: 150 mL (approximately 1 mL/lb body weight)
- Alveolar volume: 350 mL per breath reaching gas exchange units
- Regional distribution: 55% to bases, 45% to apices in upright position
-
Perfusion Components
- Cardiac output: 5.0 L/min through pulmonary circulation
- Pulmonary vascular resistance: 100-200 dynes·sec·cm⁻⁵ (10x lower than systemic)
- Capillary transit time: 0.75 seconds (equilibration complete in 0.25 seconds)
- Capillary surface area: 70 m² available for gas exchange
- Regional distribution: 75% to bases, 25% to apices due to gravity
⭐ Clinical Pearl: V/Q mismatch causes 95% of hypoxemia cases in clinical practice. Unlike diffusion defects or hypoventilation, V/Q mismatch creates widened A-a gradient while maintaining normal or low CO2 levels initially.
| Parameter | Normal Value | Apex (Zone 1) | Base (Zone 3) | Clinical Significance |
|---|---|---|---|---|
| V/Q Ratio | 0.8 | 3.3 | 0.63 | Determines gas exchange efficiency |
| PO2 (mmHg) | 100 | 132 | 89 | Reflects ventilation adequacy |
| PCO2 (mmHg) | 40 | 28 | 42 | Indicates perfusion matching |
| pH | 7.40 | 7.51 | 7.39 | Shows acid-base compensation |
| Saturation (%) | 97 | 99 | 95 | Practical oxygenation measure |
Understanding V/Q distribution patterns reveals why pulmonary embolism preferentially affects well-ventilated areas and why pneumonia typically develops in dependent lung zones. This knowledge transforms your approach to interpreting arterial blood gases and guides targeted therapeutic interventions.

🌪️ V/Q Mismatch: The Lung's Perfect Storm
⚖️ The Spectrum: From Dead Space to Shunt Territory
📌 Remember: SHUNT - Silent zones (no ventilation), Hypoxemia refractory, Unresponsive to O2, No improvement 100% FiO2, True anatomical or physiological bypass. Shunt fraction >30% causes severe hypoxemia resistant to supplemental oxygen.
-
High V/Q Spectrum (Dead Space Effect)
- V/Q ratio: >1.5 progressing toward infinity
- Pathophysiology: Ventilated alveoli with reduced/absent perfusion
- Wasted ventilation: >30% of minute ventilation in severe cases
- CO2 elimination impaired: Requires increased respiratory drive
- Compensatory hyperventilation: PaCO2 <35 mmHg initially
- Clinical manifestations: Increased work of breathing, normal oxygenation initially
-
Low V/Q Spectrum (Shunt Effect)
- V/Q ratio: <0.5 progressing toward zero
- Pathophysiology: Perfused alveoli with reduced/absent ventilation
- Venous admixture: >15% creates clinically significant hypoxemia
- Oxygen content mixing: Reduces arterial saturation proportionally
- Refractory hypoxemia: Minimal response to supplemental oxygen
- Clinical manifestations: Cyanosis, dyspnea, A-a gradient >30 mmHg

⭐ Clinical Pearl: Shunt equation quantifies severity: Qs/Qt = (CcO2 - CaO2)/(CcO2 - CvO2). Normal shunt <5%, physiological shunt 5-10%, pathological shunt >10%. Values >30% indicate life-threatening respiratory failure requiring immediate intervention.
| V/Q Category | Ratio Range | Primary Effect | A-a Gradient | O2 Response | Clinical Example |
|---|---|---|---|---|---|
| Dead Space | >2.0 | CO2 retention | Normal-mild ↑ | Good | Pulmonary embolism |
| High Normal | 1.0-2.0 | Mild inefficiency | <20 mmHg | Excellent | Upper lung zones |
| Normal | 0.5-1.0 | Optimal exchange | <15 mmHg | Excellent | Healthy lung |
| Low V/Q | 0.1-0.5 | Hypoxemia | 20-50 mmHg | Moderate | Pneumonia |
| True Shunt | 0 | Severe hypoxemia | >50 mmHg | Poor | Consolidation |
Regional V/Q heterogeneity explains why COPD patients develop CO2 retention (high V/Q areas) while pneumonia patients present with isolated hypoxemia (low V/Q areas). This pathophysiological understanding directs appropriate oxygen therapy and ventilator management strategies.

⚖️ The Spectrum: From Dead Space to Shunt Territory
🎯 Pattern Recognition: The Clinical Detective Framework
-
Dead Space Pattern Recognition
- Clinical presentation: Tachypnea without cyanosis, increased work of breathing
- ABG signature: Normal PaO2, low-normal PaCO2, respiratory alkalosis
- A-a gradient: <25 mmHg (normal to mildly elevated)
- End-tidal CO2: Decreased due to increased dead space ventilation
- Minute ventilation: Increased >10 L/min to maintain CO2 elimination
- Imaging correlates: Oligemia on chest CT, perfusion defects on V/Q scan
-
Shunt Pattern Recognition
- Clinical presentation: Cyanosis, dyspnea, refractory hypoxemia
- ABG signature: Low PaO2, normal/low PaCO2, widened A-a gradient
- A-a gradient: >30 mmHg (often >50 mmHg in severe cases)
- Oxygen response: Poor improvement with supplemental O2
- Shunt fraction: >15% calculated using shunt equation
- Imaging correlates: Consolidation, atelectasis, ground-glass opacities
📌 Remember: DETECTIVE - Dyspnea assessment, End-tidal CO2, Tachypnea evaluation, Examination findings, Cyanosis presence, Timing of symptoms, Imaging correlation, Ventilation response, Exercise tolerance. Systematic evaluation prevents missed diagnoses.
⭐ Clinical Pearl: Exercise testing unmasks subclinical V/Q mismatch. Normal individuals maintain A-a gradient <25 mmHg during maximal exercise, while patients with underlying V/Q mismatch show gradient widening >40 mmHg with exertion.
| Clinical Scenario | V/Q Pattern | Key Discriminator | Immediate Action | Expected Response |
|---|---|---|---|---|
| Sudden dyspnea, clear lungs | High V/Q | Normal PaO2, low ETCO2 | CT-PA, anticoagulation | Improved ventilation |
| Gradual dyspnea, crackles | Low V/Q | Hypoxemia, infiltrates | Supplemental O2 | Moderate improvement |
| Acute dyspnea, wheezing | Mixed V/Q | Variable response | Bronchodilators | Rapid improvement |
| Chronic dyspnea, clubbing | Fixed shunt | Poor O2 response | Echocardiogram | Minimal improvement |
| Post-op dyspnea | Atelectasis | Position-dependent | Incentive spirometry | Good improvement |
The temporal pattern of V/Q mismatch provides crucial diagnostic information. Acute onset suggests embolism or pneumothorax, subacute progression indicates pneumonia or heart failure, while chronic patterns suggest COPD or interstitial lung disease.
🎯 Pattern Recognition: The Clinical Detective Framework
🔬 Pathophysiological Mechanisms: The Cellular Battlefield
-
Ventilation Impairment Mechanisms
- Airway obstruction: Mucus plugging, bronchospasm, foreign body aspiration
- Critical diameter: <2 mm airway caliber causes significant V/Q mismatch
- Resistance increase: Exponential relationship with diameter reduction (Poiseuille's law)
- Collateral ventilation: Pores of Kohn provide 10-15% backup ventilation
- Alveolar filling: Pneumonia, pulmonary edema, hemorrhage
- Surfactant dysfunction: Increases surface tension from 25 to 70 dynes/cm
- Compliance reduction: <50 mL/cmH2O indicates significant alveolar disease
- Gas diffusion barrier: Thickened membrane >0.5 μm impairs gas exchange
- Airway obstruction: Mucus plugging, bronchospasm, foreign body aspiration
-
Perfusion Impairment Mechanisms
- Vascular obstruction: Thromboembolism, air embolism, tumor emboli
- Critical occlusion: >50% cross-sectional area reduction causes symptoms
- Pressure gradient: Mean PAP >25 mmHg indicates pulmonary hypertension
- Recruitment failure: Capillary bed reduction >30% limits perfusion reserve
- Hypoxic vasoconstriction: Adaptive mechanism becomes maladaptive in disease
- Threshold PO2: <60 mmHg triggers vasoconstriction response
- Magnitude: 300-400% increase in pulmonary vascular resistance
- Reversibility: Oxygen therapy can normalize pressures within minutes
- Vascular obstruction: Thromboembolism, air embolism, tumor emboli

📌 Remember: BARRIER - Blood flow obstruction, Airway narrowing, Resistance increase, Recruitement failure, Inflammation cascade, Edema formation, Respiratory mechanics altered. Each mechanism requires specific therapeutic targeting.
⭐ Clinical Pearl: Inflammatory mediators create bidirectional V/Q mismatch. TNF-α and IL-1β levels >100 pg/mL correlate with shunt fraction >20%, while endothelin-1 elevation >5 pmol/L predicts dead space fraction >40%.
| Mechanism | Mediator | Time Course | Reversibility | Therapeutic Target |
|---|---|---|---|---|
| Inflammation | TNF-α, IL-1β | Hours-days | Moderate | Anti-inflammatory |
| Vasoconstriction | Endothelin-1 | Minutes-hours | High | Vasodilators |
| Thrombosis | Thromboxane A2 | Minutes-hours | Variable | Anticoagulation |
| Fibrosis | TGF-β | Days-weeks | Low | Antifibrotic |
| Edema | VEGF | Hours-days | High | Diuretics |
The time course of V/Q mismatch development guides therapeutic timing. Acute changes (minutes to hours) suggest reversible mechanisms amenable to immediate intervention, while chronic changes (days to weeks) indicate structural alterations requiring long-term management strategies.

🔬 Pathophysiological Mechanisms: The Cellular Battlefield
🎛️ Therapeutic Algorithms: The Treatment Command Center
-
Dead Space Management Algorithm
- Immediate interventions: Anticoagulation within 4 hours if PE suspected
- Heparin dosing: 80 units/kg bolus, then 18 units/kg/hr infusion
- Target aPTT: 60-80 seconds (1.5-2.5x normal)
- Monitoring: Platelet count every 48 hours for HIT surveillance
- Perfusion optimization: Fluid management and cardiac output support
- Fluid challenge: 500 mL crystalloid over 15 minutes
- Vasopressor support: Norepinephrine 0.1-2.0 μg/kg/min if needed
- Target MAP: >65 mmHg to maintain pulmonary perfusion pressure
- Immediate interventions: Anticoagulation within 4 hours if PE suspected
-
Shunt Management Algorithm
- PEEP optimization: Incremental PEEP trial from 5-20 cmH2O
- PEEP titration: 2 cmH2O increments every 15 minutes
- Optimal PEEP: Best compliance or lowest driving pressure
- Maximum PEEP: Plateau pressure <30 cmH2O safety limit
- Recruitment strategies: Sustained inflation or decremental PEEP
- Recruitment maneuver: 40 cmH2O for 40 seconds if tolerated
- Response criteria: PaO2 increase >20 mmHg within 30 minutes
- Contraindications: Hemodynamic instability or pneumothorax risk
- PEEP optimization: Incremental PEEP trial from 5-20 cmH2O
📌 Remember: OPTIMIZE - Oxygen delivery, PEEP titration, Tidal volume limitation, Inspiratory pressure, Monitoring response, Individualized strategy, Zero complications, Evidence-based protocols. Systematic approach prevents ventilator-induced lung injury.
⭐ Clinical Pearl: Driving pressure (Plateau pressure - PEEP) <15 cmH2O correlates with improved survival in ARDS patients. This parameter integrates lung compliance and PEEP effects, providing superior guidance compared to plateau pressure alone.
| Intervention | Indication | Target Parameter | Success Criteria | Monitoring Frequency |
|---|---|---|---|---|
| PEEP increase | Shunt >15% | Driving pressure <15 | PaO2 ↑ >20 mmHg | Every 4 hours |
| Recruitment | Atelectasis | Compliance ↑ >20% | Shunt ↓ >5% | Every 12 hours |
| Prone positioning | P/F ratio <150 | P/F ratio ↑ >20% | Mortality benefit | Daily assessment |
| iNO therapy | RV dysfunction | PAP ↓ >10 mmHg | Improved RV function | Every 6 hours |
| ECMO consideration | P/F ratio <80 | Lung rest | Bridge to recovery | Continuous |
Personalized medicine approaches use electrical impedance tomography and volumetric capnography to guide individualized PEEP and tidal volume settings, optimizing regional V/Q matching while minimizing ventilator-induced lung injury.
🎛️ Therapeutic Algorithms: The Treatment Command Center
🌐 Multi-System Integration: The Physiological Network
-
Cardiovascular Compensation Mechanisms
- Right heart adaptation: Acute cor pulmonale develops with >40% dead space
- RV dilation: RVEDD >42 mm indicates significant strain
- Tricuspid regurgitation: Velocity >3.4 m/s suggests PASP >50 mmHg
- Interventricular dependence: Septal shift impairs LV filling by 15-25%
- Cardiac output optimization: Increased stroke volume compensates for V/Q inefficiency
- Frank-Starling mechanism: Preload optimization maintains CO >4 L/min/m²
- Chronotropic response: Heart rate 100-120 bpm maximizes oxygen delivery
- Contractility enhancement: Sympathetic stimulation increases ejection fraction
- Right heart adaptation: Acute cor pulmonale develops with >40% dead space
-
Renal-Metabolic Integration
- Acid-base compensation: Renal bicarbonate handling adapts to respiratory changes
- Metabolic alkalosis: HCO3- retention compensates for chronic hypocapnia
- Expected compensation: HCO3- increases 3.5 mEq/L per 10 mmHg CO2 decrease
- Renal response time: 24-72 hours for complete compensation
- Fluid balance optimization: RAAS activation maintains intravascular volume
- Aldosterone elevation: 2-3x normal in chronic respiratory disease
- ADH response: Maintains osmolality despite altered ventilation patterns
- Electrolyte management: K+ and Mg2+ optimization improves respiratory muscle function
- Acid-base compensation: Renal bicarbonate handling adapts to respiratory changes

📌 Remember: NETWORK - Neurological drive, Endocrine response, Tissue perfusion, Work of breathing, Oxygen delivery, Renal compensation, Kinetic energy balance. Multi-system approach optimizes patient outcomes.
⭐ Clinical Pearl: Brain natriuretic peptide (BNP) levels >400 pg/mL in acute V/Q mismatch indicate significant right heart strain requiring aggressive afterload reduction and careful fluid management. Serial BNP monitoring guides therapeutic intensity.
| System | Compensation | Time Course | Monitoring Parameter | Therapeutic Target |
|---|---|---|---|---|
| Cardiac | CO increase | Minutes-hours | Cardiac index >2.5 | Optimize preload |
| Renal | HCO3- retention | Hours-days | Base excess ±3 | Maintain pH 7.35-7.45 |
| Neurologic | Respiratory drive | Seconds-minutes | Respiratory rate <30 | Reduce work of breathing |
| Hematologic | Hgb increase | Days-weeks | Hgb 10-12 g/dL | Optimize O2 carrying |
| Metabolic | Energy substrate | Hours-days | Lactate <2 mmol/L | Maintain aerobic metabolism |
Cutting-edge research demonstrates that mesenchymal stem cell therapy and extracellular vesicle treatment can restore V/Q matching by promoting alveolar regeneration and reducing inflammatory responses, offering future therapeutic options for refractory cases.
🌐 Multi-System Integration: The Physiological Network
🎯 Clinical Mastery Arsenal: The Expert's Toolkit
📌 Remember: ARSENAL - Assessment tools, Rapid diagnosis, Systematic approach, Evidence-based therapy, Nonitoring parameters, Advanced techniques, Long-term outcomes. Complete toolkit ensures clinical excellence.
- Rapid Assessment Protocol
- 5-Minute V/Q Assessment: Clinical examination + ABG + chest imaging
- Respiratory rate >24/min suggests significant mismatch
- A-a gradient >30 mmHg indicates pathological V/Q distribution
- Chest X-ray identifies >80% of structural causes
- Advanced Diagnostics: CT-PA, V/Q scan, echocardiography within 2 hours
- CT-PA sensitivity 95% for central PE, 83% for subsegmental
- V/Q scan specificity 97% when clinical probability considered
- Echo RV/LV ratio >0.9 indicates acute right heart strain
- 5-Minute V/Q Assessment: Clinical examination + ABG + chest imaging
| Clinical Scenario | First-Line Test | Diagnostic Accuracy | Time to Result | Alternative Test |
|---|---|---|---|---|
| Acute dyspnea | CT-PA | 95% sensitivity | 30 minutes | V/Q scan |
| Chronic dyspnea | PFTs + imaging | 90% specificity | 2 hours | Cardiopulmonary exercise |
| Post-operative | Chest X-ray | 75% sensitivity | 15 minutes | CT chest |
| ICU patient | ABG + compliance | 85% accuracy | 10 minutes | EIT monitoring |
| Suspected PE | D-dimer + Wells | 98% NPV if low risk | 1 hour | CT-PA |
💡 Master This: Volumetric capnography measures dead space fraction continuously, providing real-time V/Q monitoring. VD/VT >0.6 indicates significant dead space, while sudden increases >0.1 suggest acute PE or cardiovascular collapse.
Essential Clinical Thresholds for immediate decision-making:
- A-a gradient >50 mmHg: Severe V/Q mismatch requiring intensive monitoring
- Shunt fraction >30%: Consider ECMO evaluation for refractory hypoxemia
- Dead space >60%: Investigate massive PE or cardiovascular collapse
- P/F ratio <200: Initiate lung-protective ventilation strategies
- Driving pressure >15 cmH2O: Optimize PEEP and tidal volume settings
This comprehensive understanding of V/Q mismatch pathophysiology, recognition patterns, and therapeutic strategies provides the foundation for expert-level respiratory care and optimal patient outcomes across all clinical scenarios.
🎯 Clinical Mastery Arsenal: The Expert's Toolkit
Practice Questions: V/Q mismatch
Test your understanding with these related questions
A 21-year-old man presents to his physician because he has been feeling increasingly tired and short of breath at work. He has previously had these symptoms but cannot recall the diagnosis he was given. Chart review reveals the following results: Oxygen tension in inspired air = 150 mmHg Alveolar carbon dioxide tension = 50 mmHg Arterial oxygen tension = 71 mmHg Respiratory exchange ratio = 0.80 Diffusion studies reveal normal diffusion distance. The patient is administered 100% oxygen but the patient's blood oxygen concentration does not improve. Which of the following conditions would best explain this patient's findings?













