Control of breathing US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Control of breathing. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Control of breathing US Medical PG Question 1: A research scientist attempts to understand the influence of carbon dioxide content in blood on its oxygen binding. The scientist adds carbon dioxide to dog blood and measures the uptake of oxygen in the blood versus oxygen pressure in the peripheral tissue. He notes in one dog that with the addition of carbon dioxide with a pressure of 90 mmHg, the oxygen pressure in the peripheral tissue rose from 26 to 33 mmHg. How can this phenomenon be explained?
- A. High partial pressure of CO2 in tissues decreases peripheral blood volume
- B. Binding of O2 to hemoglobin in lungs drives release of CO2 from hemoglobin
- C. High partial pressure of CO2 in tissues causes alkalemia, which is necessary for O2 unloading
- D. High partial pressure of CO2 in tissues facilitates O2 unloading in peripheral tissues (Correct Answer)
- E. The sum of the partial pressures of CO2 and O2 cannot exceed a known threshold in blood
Control of breathing Explanation: **High partial pressure of CO2 in tissues facilitates O2 unloading in peripheral tissues**
- An increase in **PCO2** leads to a decrease in pH (acidosis) in the tissues, which **decreases hemoglobin's affinity for oxygen**, promoting oxygen release.
- This phenomenon is known as the **Bohr effect**, where an acidic environment (from CO2) shifts the oxygen dissociation curve to the right, enhancing O2 unloading to meet tissue metabolic demands.
*High partial pressure of CO2 in tissues decreases peripheral blood volume*
- **Increased CO2** generally causes vasodilation in peripheral tissues, which would lead to an **increase**, not a decrease, in peripheral blood flow.
- Decreased blood volume is typically associated with conditions like hypovolemia or intense vasoconstriction, not elevated tissue CO2.
*Binding of O2 to hemoglobin in lungs drives release of CO2 from hemoglobin*
- This statement describes the **Haldane effect**, which occurs primarily in the lungs, where oxygen binding to hemoglobin facilitates the release of CO2.
- While true, it does not explain the **increased oxygen pressure in peripheral tissue** observed with added CO2, which is related to O2 unloading.
*High partial pressure of CO2 in tissues causes alkalemia, which is necessary for O2 unloading*
- High **PCO2** in tissues leads to the formation of carbonic acid and H+ ions, resulting in a **decrease in pH (acidosis)**, not alkalemia.
- **Acidosis** facilitates O2 unloading (Bohr effect), whereas alkalemia would increase hemoglobin's affinity for O2, inhibiting unloading.
*The sum of the partial pressures of CO2 and O2 cannot exceed a known threshold in blood*
- There is **no fixed threshold** for the sum of partial pressures of CO2 and O2 in the blood; these gases are independently regulated and their partial pressures fluctuate with metabolic activity.
- The partial pressure of a gas reflects its concentration and does not have an upper limit when considering the sum of different gases.
Control of breathing US Medical PG Question 2: A 24-year-old professional athlete is advised to train in the mountains to enhance his performance. After 5 months of training at an altitude of 1.5 km (5,000 feet), he is able to increase his running pace while competing at sea-level venues. Which of the following changes would produce the same effect on the oxygen-hemoglobin dissociation curve as this athlete's training did?
- A. Decreased 2,3-bisphosphoglycerate (Correct Answer)
- B. Increased carbon monoxide inhalation
- C. Decreased temperature
- D. Decreased pH
- E. Increased partial pressure of oxygen
Control of breathing Explanation: ***Decreased 2,3-bisphosphoglycerate***
- This is **NOT** the correct physiological adaptation from altitude training, making this question conceptually flawed.
- Altitude training causes **increased erythropoietin → polycythemia → increased total hemoglobin**, which increases oxygen-carrying capacity.
- 2,3-BPG is **initially increased** at altitude (right shift) to facilitate O2 release, and remains elevated or returns to normal with acclimatization, **not decreased**.
- While decreased 2,3-BPG would cause a left shift (increased O2 affinity), this does NOT replicate altitude training adaptations.
*Increased carbon monoxide inhalation*
- Carbon monoxide binds hemoglobin with **200-250× higher affinity** than oxygen, forming carboxyhemoglobin.
- This **reduces oxygen-carrying capacity** and causes a left shift for remaining hemoglobin.
- This is harmful and does NOT replicate beneficial altitude adaptations.
*Decreased temperature*
- Decreases metabolic rate and causes a **left shift** (increased O2 affinity).
- Oxygen is held more tightly and released less readily to tissues.
- This does NOT replicate altitude training benefits.
*Decreased pH*
- Acidosis causes the **Bohr effect**: **right shift** (decreased O2 affinity).
- Facilitates O2 release to tissues during exercise.
- This is beneficial during exercise but does NOT replicate the chronic altitude adaptation of increased oxygen-carrying capacity.
*Increased partial pressure of oxygen*
- Higher PO2 increases hemoglobin saturation but does NOT shift the curve.
- This increases oxygen availability but does NOT replicate the physiological adaptation (polycythemia) from altitude training.
**Note:** This question is conceptually problematic as none of the options accurately replicate the primary altitude training adaptation (increased RBC mass/hemoglobin concentration).
Control of breathing US Medical PG Question 3: A 21-year-old man presents to his physician because he has been feeling increasingly tired and short of breath at work. He has previously had these symptoms but cannot recall the diagnosis he was given. Chart review reveals the following results:
Oxygen tension in inspired air = 150 mmHg
Alveolar carbon dioxide tension = 50 mmHg
Arterial oxygen tension = 71 mmHg
Respiratory exchange ratio = 0.80
Diffusion studies reveal normal diffusion distance. The patient is administered 100% oxygen but the patient's blood oxygen concentration does not improve. Which of the following conditions would best explain this patient's findings?
- A. Septal defect since birth (Correct Answer)
- B. Use of opioid medications
- C. Pulmonary fibrosis
- D. Pulmonary embolism
- E. Vacation at the top of a mountain
Control of breathing Explanation: ***Septal defect since birth***
- A congenital heart disease like a **septal defect** causes a right-to-left **shunt**, meaning deoxygenated blood bypasses the lungs and mixes with oxygenated blood.
- This type of shunt leads to **hypoxemia that is refractory to 100% oxygen** because the shunted blood will never pick up oxygen from the lungs.
*Use of opioid medications*
- Opioid use causes **respiratory depression**, leading to **hypoventilation** and increased arterial CO2 with decreased arterial O2.
- However, the hypoxemia from hypoventilation would typically improve significantly with **100% oxygen administration**, unlike in this case.
*Pulmonary fibrosis*
- **Pulmonary fibrosis** causes thickening of the alveolar-capillary membrane, leading to impaired gas exchange and **diffusion limitation**.
- While it causes hypoxemia, the diffusion studies are stated to be **normal**, and hypoxemia due to diffusion limitation often improves with supplemental oxygen.
*Pulmonary embolism*
- A **pulmonary embolism** leads to V/Q mismatch by blocking blood flow to a portion of the lung, causing ventilation with no perfusion.
- Hypoxemia from V/Q mismatch generally **responds well to supplemental oxygen**, as the non-affected lung areas can compensate, unlike the scenario described.
*Vacation at the top of a mountain*
- Being at a high altitude causes **hypobaric hypoxia**, meaning there is a reduced partial pressure of oxygen in the inspired air.
- This type of hypoxemia typically **improves with supplemental oxygen** as it increases the inspired oxygen tension, which is contrary to the patient's findings.
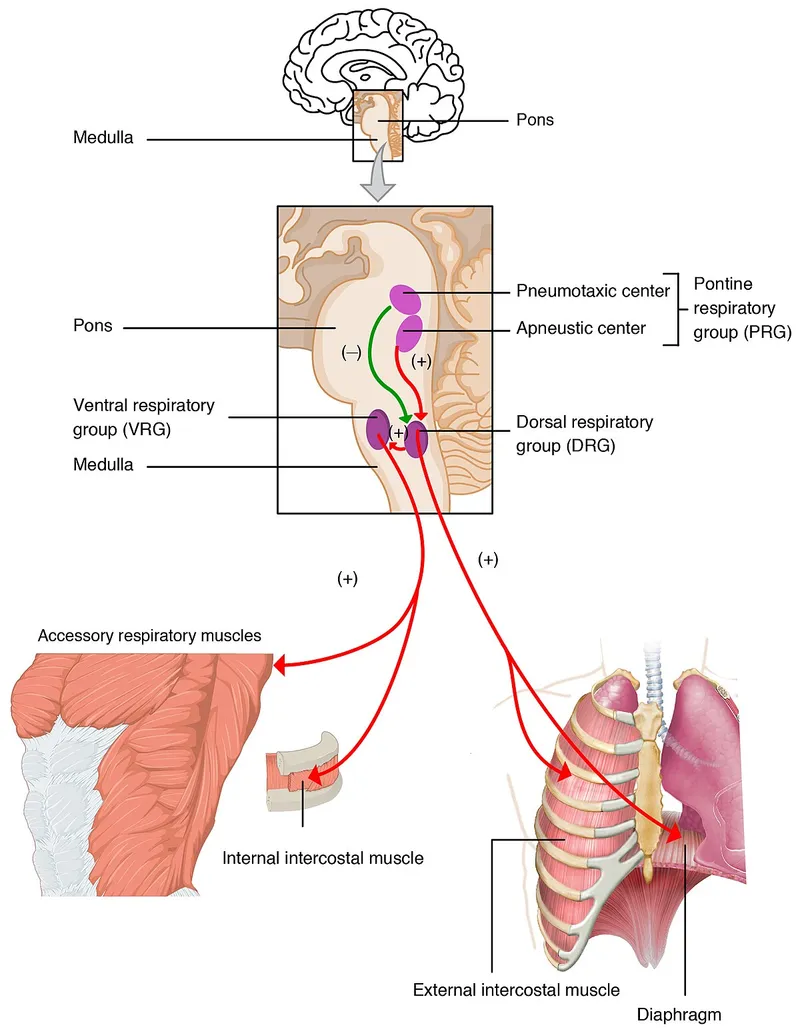
Control of breathing US Medical PG Question 4: A 55-year-old man is brought to the emergency department by ambulance from a long term nursing facility complaining of severe shortness of breath. He suffers from amyotrophic lateral sclerosis and lives at the nursing home full time. He has had the disease for 2 years and it has been getting harder to breath over the last month. He is placed on a rebreather mask and responds to questions while gasping for air. He denies cough or any other upper respiratory symptoms and denies a history of cardiovascular or respiratory disease. The blood pressure is 132/70 mm Hg, the heart rate is 98/min, the respiratory rate is 40/min, and the temperature is 37.6°C (99.7°F). During the physical exam, he begs to be placed in a sitting position. After he is repositioned his breathing improves a great deal. On physical examination, his respiratory movements are shallow and labored with paradoxical inward movement of his abdomen during inspiration. Auscultation of the chest reveals a lack of breath sounds in the lower lung bilaterally. At present, which of the following muscles is most important for inspiration in the patient?
- A. Muscles of anterior abdominal wall
- B. Sternocleidomastoid muscles (Correct Answer)
- C. Internal intercostal muscles
- D. Trapezius muscle
- E. External intercostal muscles
Control of breathing Explanation: ***Sternocleidomastoid muscles***
- In advanced **amyotrophic lateral sclerosis (ALS)**, progressive motor neuron degeneration affects both the diaphragm and intercostal muscles
- The **paradoxical inward movement of the abdomen** during inspiration indicates severe diaphragmatic weakness or paralysis
- The **shallow respiratory movements** and **severe respiratory distress** (respiratory rate 40/min) suggest that both primary inspiratory muscle groups (diaphragm and external intercostals) are significantly compromised
- At this stage, **accessory muscles of inspiration**, particularly the **sternocleidomastoid muscles**, become critically important for maintaining ventilation by elevating the sternum and upper ribs
- The dramatic improvement when sitting upright (orthopnea relief) supports accessory muscle recruitment, as this position optimizes sternocleidomastoid mechanical advantage
- **Clinical pearl:** In neuromuscular respiratory failure, neck muscle recruitment (visible SCM contraction) is a key sign of impending respiratory failure requiring ventilatory support
*External intercostal muscles*
- The **external intercostal muscles** are normally primary muscles of inspiration that elevate the ribs
- However, in advanced ALS with **2 years of progressive disease** and worsening dyspnea over the past month, these muscles would also be significantly weakened by the neurodegenerative process
- The **lack of breath sounds in the lower lungs bilaterally** suggests poor chest wall expansion, indicating compromised intercostal function
- While they continue to contribute, they are insufficient to maintain adequate ventilation alone at this stage of disease
*Internal intercostal muscles*
- The **internal intercostal muscles** function primarily in **forced expiration** by depressing the ribs
- They do not play a significant role in inspiration
*Muscles of anterior abdominal wall*
- The **anterior abdominal wall muscles** (rectus abdominis, external/internal obliques, transversus abdominis) are **expiratory muscles** used in forced expiration and coughing
- The **paradoxical inward movement** of the abdomen during inspiration is a passive phenomenon resulting from diaphragmatic weakness—the negative intrathoracic pressure pulls the weakened diaphragm upward, which in turn draws the abdominal wall inward
- These muscles are not contributing to inspiration in this patient
*Trapezius muscle*
- The **trapezius** primarily functions in scapular movement and neck stabilization
- While it provides some mechanical stability for the shoulder girdle during accessory muscle breathing, it is not directly involved in rib cage elevation
- It plays a minor supportive role compared to the sternocleidomastoid in respiratory distress
Control of breathing US Medical PG Question 5: A 27-year-old woman develops progressive difficulty breathing after a long day of chores in a dusty house. These chores included brushing the family dog, vacuuming, dusting, and sweeping. She occasionally gets these episodes once or twice a year and has her medication on hand. Her symptoms are reversed by inhaling a β2-adrenergic receptor agonist. Which of the following chemical mediators is responsible for this patient’s breathing difficulties?
- A. Histamine
- B. Leukotrienes (Correct Answer)
- C. Serotonin
- D. Bradykinin
- E. Endorphins
Control of breathing Explanation: ***Correct: Leukotrienes***
- Leukotrienes, particularly **leukotriene C4, D4, and E4**, are potent **bronchoconstrictors** and are significantly involved in the pathogenesis of **asthma**.
- They also contribute to **bronchial hyperreactivity**, mucus secretion, and airway edema, all hallmarks of asthmatic exacerbations triggered by allergens and irritants.
*Incorrect: Histamine*
- Histamine is released by mast cells and basophils, causing **bronchoconstriction** and vasodilation, but its role in the **sustained and severe bronchospasm of asthma** is less prominent than leukotrienes.
- While it contributes to immediate hypersensitivity reactions, **antihistamines are generally not effective in treating asthma exacerbations**.
*Incorrect: Serotonin*
- Serotonin (5-hydroxytryptamine) is primarily involved in smooth muscle contraction and platelet aggregation, but it is **not a primary mediator of bronchoconstriction in human asthma**.
- Its effects on the airways are relatively minor compared to other mediators.
*Incorrect: Bradykinin*
- Bradykinin is a peptide that causes **vasodilation**, increased vascular permeability, and pain, and can induce **bronchoconstriction**, especially in sensitive individuals.
- However, it is **not considered a primary or dominant mediator in typical allergic asthma exacerbations** as described in this patient.
*Incorrect: Endorphins*
- Endorphins are **endogenous opioid peptides** that primarily act as **neurotransmitters** and neuromodulators, involved in pain sensation and mood regulation.
- They have **no direct role in the acute pathophysiology of asthma** or bronchoconstriction.
Control of breathing US Medical PG Question 6: A 35-year-old woman volunteers for a study on respiratory physiology. Pressure probes A and B are placed as follows:
Probe A: between the parietal and visceral pleura
Probe B: within the cavity of an alveolus
The probes provide a pressure reading relative to atmospheric pressure. To obtain a baseline reading, she is asked to sit comfortably and breathe normally. Which of the following sets of values will most likely be seen at the end of inspiration?
- A. Probe A: -6 mm Hg; Probe B: 0 mm Hg (Correct Answer)
- B. Probe A: 0 mm Hg; Probe B: -1 mm Hg
- C. Probe A: -4 mm Hg; Probe B: 0 mm Hg
- D. Probe A: -4 mm Hg; Probe B: -1 mm Hg
- E. Probe A: -6 mm Hg; Probe B: -1 mm Hg
Control of breathing Explanation: ***Probe A: -6 mm Hg; Probe B: 0 mm Hg***
- At the **end of inspiration**, the **intrapleural pressure (Probe A)** is at its most negative, typically around -6 to -8 cm H2O (equivalent to -4 to -6 mmHg), reflecting the maximum expansion of the thoracic cavity.
- At the **end of inspiration**, just before exhalation begins, there is **no airflow**, so the **intrapulmonary pressure (Probe B)** equalizes with atmospheric pressure, resulting in a 0 mm Hg reading.
*Probe A: 0 mm Hg; Probe B: -1 mm Hg*
- An **intrapleural pressure of 0 mm Hg** would indicate a **pneumothorax** since it should always be negative to prevent lung collapse.
- An **intrapulmonary pressure of -1 mm Hg** would indicate that **inspiration is still ongoing**, as air would be flowing into the lungs.
*Probe A: -4 mm Hg; Probe B: 0 mm Hg*
- While an **intrapulmonary pressure of 0 mm Hg** is correct at the end of inspiration, an **intrapleural pressure of -4 mm Hg** is typical for the **end of expiration (Functional Residual Capacity)** during quiet breathing, not the end of inspiration.
- The **intrapleural pressure becomes more negative** during inspiration due to increased thoracic volume, so -4 mm Hg would be insufficient.
*Probe A: -4 mm Hg; Probe B: -1 mm Hg*
- An **intrapleural pressure of -4 mm Hg** is the normal pressure at the **end of expiration**, not the end of inspiration, where it becomes more negative.
- An **intrapulmonary pressure of -1 mm Hg** indicates that **inspiration is still in progress**, not at its end, as air would still be flowing into the lungs.
*Probe A: -6 mm Hg; Probe B: -1 mm Hg*
- While an **intrapleural pressure of -6 mm Hg** is consistent with the end of inspiration, an **intrapulmonary pressure of -1 mm Hg** means that **airflow is still occurring into the lungs**.
- At the **very end of inspiration**, just before the start of exhalation, airflow momentarily ceases, and intrapulmonary pressure becomes zero relative to the atmosphere.
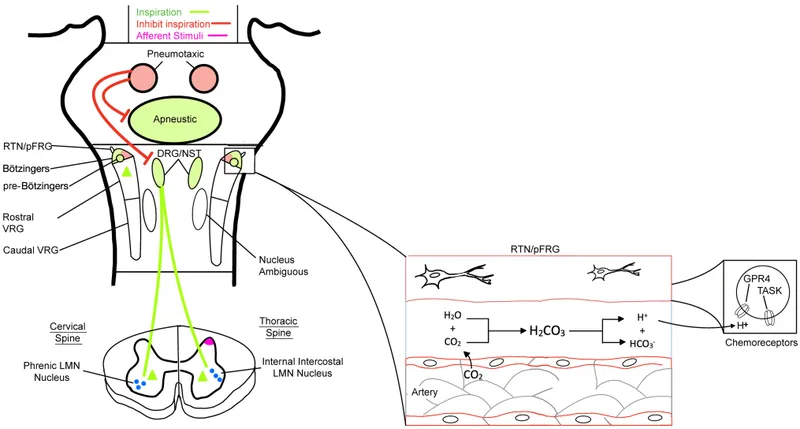
Control of breathing US Medical PG Question 7: A 55-year-old man presents with an unremitting cough and swelling of the lower limbs for the past 2 weeks. He says he has had a chronic cough for years, however, he feels it is getting worse. He reports a 30-pack-year smoking history. Physical examination reveals mild central cyanosis and expiratory wheezes throughout the chest. Oxygen therapy is ordered immediately but, soon after administering it, his respiratory rate starts to slow down and he becomes drowsy. Dysfunction of which of the following receptors most likely led to this patient’s current condition?
- A. Pleural pain receptors
- B. Central chemoreceptors
- C. Airway stretch receptors
- D. Pulmonary stretch receptors
- E. Peripheral chemoreceptors (Correct Answer)
Control of breathing Explanation: ***Peripheral chemoreceptors***
- In patients with chronic obstructive pulmonary disease (COPD) like this patient, the **central chemoreceptors** become desensitized to chronically elevated CO2 levels. Their primary respiratory drive then shifts to the **peripheral chemoreceptors** (carotid and aortic bodies), which are sensitive to **hypoxia**.
- Administering high-flow oxygen **eliminates the hypoxic stimulus** sensed by these normally functioning peripheral chemoreceptors, removing the hypoxic drive to breathe and leading to **hypoventilation, CO2 retention, respiratory acidosis**, and drowsiness (CO2 narcosis).
*Pleural pain receptors*
- These receptors are primarily involved in sensing pain associated with **pleural inflammation** or injury, contributing to the sensation of pain with breathing.
- They do not play a role in regulating the primary ventilatory drive in response to blood gas changes.
*Central chemoreceptors*
- These receptors are located in the **medulla** and are primarily sensitive to changes in **arterial PCO2** and pH (via H+ ions in CSF).
- In chronic respiratory diseases with CO2 retention, they become **desensitized** to elevated CO2, shifting the main respiratory drive to the peripheral chemoreceptors' response to hypoxia.
*Airway stretch receptors*
- These receptors, including **slowly adapting stretch receptors** and **rapidly adapting irritant receptors**, are located in the airways and respond to lung inflation and irritants.
- They are involved in the Hering-Breuer reflex and cough reflex but are not the primary drivers of ventilation in response to hypoxemia.
*Pulmonary stretch receptors*
- These receptors are located in the **bronchial smooth muscle** and respond to lung distension, contributing to the **Hering-Breuer reflex** which inhibits inspiration to prevent overinflation.
- While important for lung mechanics, they do not directly sense blood gas levels to drive ventilation in the context of hypoxia or hypercapnia.
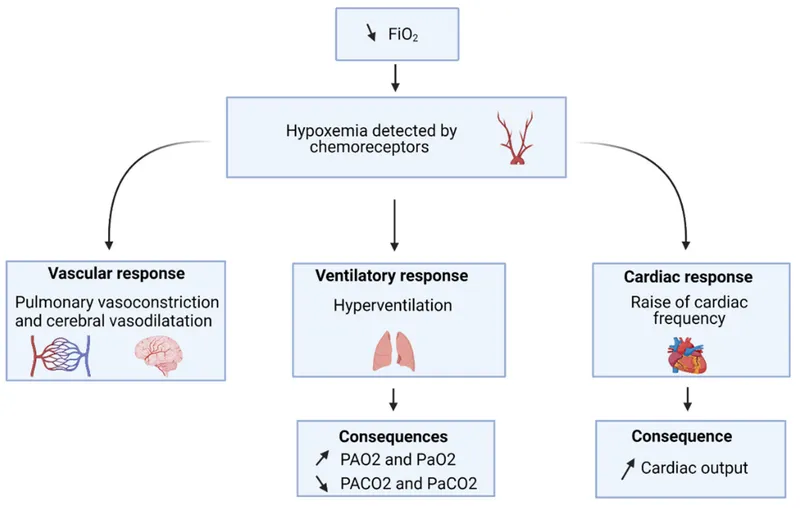
Control of breathing US Medical PG Question 8: A 34-year-old woman comes to a physician for a routine health maintenance examination. She moved to Denver 1 week ago after having lived in New York City all her life. She has no history of serious illness and takes no medications. Which of the following sets of changes is most likely on analysis of a blood sample obtained now compared to prior to her move?
Erythropoietin level | O2 saturation | Plasma volume
- A. ↑ unchanged unchanged
- B. ↑ ↓ ↓ (Correct Answer)
- C. Unchanged ↓ unchanged
- D. ↓ unchanged ↑
- E. Unchanged unchanged ↓
Control of breathing Explanation: ***↑ ↓ ↓***
- Moving to a high altitude like Denver (from sea level NYC) leads to **hypoxia**, which triggers increased **erythropoietin (EPO)** production to stimulate red blood cell formation.
- The immediate physiological response to high altitude is a **decrease in arterial PO2** and thus **oxygen saturation**, along with a **reduction in plasma volume** due to increased diuresis and fluid shifts.
*↑ unchanged unchanged*
- While **erythropoietin** would increase due to hypoxia at higher altitudes, **oxygen saturation** would decrease, not remain unchanged.
- **Plasma volume** also tends to decrease acutely at high altitudes, rather than staying unchanged.
*Unchanged ↓ unchanged*
- **Erythropoietin** would be expected to increase, not remain unchanged, as a compensatory mechanism to hypoxia.
- While **oxygen saturation** would decrease, **plasma volume** typically decreases acutely, not remaining unchanged.
*↓ unchanged ↑*
- **Erythropoietin** would increase, not decrease, in response to the lower atmospheric oxygen.
- Both **oxygen saturation** and **plasma volume** would decrease, not remain unchanged or increase, respectively.
*Unchanged unchanged ↓*
- **Erythropoietin** would increase, not remain unchanged, to stimulate red blood cell production in response to hypoxia.
- **Oxygen saturation** would decrease, not remain unchanged, at higher altitudes.
Control of breathing US Medical PG Question 9: A researcher is studying proteins that contribute to intestinal epithelial permeability. He has isolated intestinal tissue from several mice. After processing the tissue into its individual components, he uses a Western blot analysis to identify a protein that forms part of a multi-protein complex at the apical aspect of epithelial cells. The complex is known to provide a diffusion barrier between the apical and basolateral aspects of epithelial cells. Which of the following proteins is this researcher most likely investigating?
- A. Integrin
- B. Connexon
- C. Desmoglein
- D. E-cadherin
- E. Claudin (Correct Answer)
Control of breathing Explanation: ***Claudin***
- **Claudins** are integral membrane proteins that are primary components of **tight junctions** (zonulae occludentes), which form a diffusion barrier at the **apical aspect** of epithelial cells.
- They regulate **paracellular permeability**, crucial for maintaining the integrity of the intestinal epithelial barrier.
*Integrin*
- **Integrins** are transmembrane receptors that mediate cell-extracellular matrix (ECM) adhesion and cell-cell adhesion, but they are not the primary components of tight junction diffusion barriers.
- They are involved in cell signaling and structural support, rather than forming a direct paracellular seal.
*Connexon*
- A **connexon** is a protein assembly that forms a **gap junction**, allowing direct communication and passage of small molecules between adjacent cells.
- Gap junctions facilitate intercellular communication, but do not primarily contribute to sealing the paracellular space as a diffusion barrier.
*Desmoglein*
- **Desmoglein** is a cadherin family protein found in **desmosomes** (maculae adherens), which are cell-cell adhesion complexes that provide strong mechanical attachments between cells.
- Desmosomes resist shearing forces and provide structural integrity but do not regulate paracellular permeability as tight junctions do.
*E-cadherin*
- **E-cadherin** is a crucial component of **adherens junctions** (zonula adherens), which provide cell-cell adhesion and help establish and maintain cell polarity.
- While important for epithelial integrity, E-cadherin primarily links cells to the actin cytoskeleton and is not directly responsible for forming the selective diffusion barrier itself.
Control of breathing US Medical PG Question 10: A 30-year-old woman presents to the emergency department with breathlessness for the last hour. She is unable to provide any history due to her dyspnea. Her vitals include: respiratory rate 20/min, pulse 100/min, and blood pressure 144/84 mm Hg. On physical examination, she is visibly obese, and her breathing is labored. There are decreased breath sounds and hyperresonance to percussion across all lung fields bilaterally. An arterial blood gas is drawn, and the patient is placed on inhaled oxygen. Laboratory findings reveal:
pH 7.34
pO2 63 mm Hg
pCO2 50 mm Hg
HCO3 22 mEq/L
Her alveolar partial pressure of oxygen is 70 mm Hg. Which of the following is the most likely etiology of this patient’s symptoms?
- A. Right to left shunt
- B. Alveolar hypoventilation (Correct Answer)
- C. Ventricular septal defect
- D. Impaired gas diffusion
- E. Ventilation/perfusion mismatch
Control of breathing Explanation: ***Alveolar hypoventilation***
- The patient exhibits features of **obesity** and **labored breathing** with decreased breath sounds and hyperresonance, along with arterial blood gas results showing **respiratory acidosis** (pH 7.34, pCO2 50 mmHg) and **hypoxia** (pO2 63 mmHg).
- The calculated A-a gradient (Alveolar O2 - arterial O2) is low (70 mmHg - 63 mmHg = 7 mmHg), indicating that the problem is primarily with **overall ventilation** rather than a defect in gas exchange across the alveolar-capillary membrane.
*Right to left shunt*
- A right-to-left shunt would cause a **large A-a gradient**, as deoxygenated blood bypasses the lungs and mixes with oxygenated blood.
- While it causes **hypoxemia**, it would not typically be associated with hypercapnia unless very severe, and the A-a gradient calculation here does not support a significant shunt.
*Ventricular septal defect*
- A ventricular septal defect is a **structural heart abnormality** that can cause a left-to-right shunt initially, leading to pulmonary hypertension and eventually a right-to-left shunt (Eisenmenger syndrome).
- While it can cause hypoxemia due to shunting, it would not primarily manifest with increased pCO2 or the specific lung physical exam findings of decreased breath sounds and hyperresonance in the absence of other cardiac signs.
*Impaired gas diffusion*
- Impaired gas diffusion would lead to a **large A-a gradient** and **hypoxemia**, but typically not significant hypercapnia unless the impairment is extremely severe.
- Conditions like **pulmonary fibrosis** or **emphysema** cause impaired diffusion, but the patient's presentation and particularly the low A-a gradient do not support this.
*Ventilation/perfusion mismatch*
- A V/Q mismatch also causes a **large A-a gradient** and **hypoxemia**, as some areas of the lung are either poorly ventilated or poorly perfused.
- While it can cause hypercapnia in severe cases, the primary issue indicated by the low A-a gradient here is one of overall inadequate ventilation, not selective areas of ventilation-perfusion imbalance.
More Control of breathing US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.