Respiratory

On this page
🫁 The Respiratory Command Center: Neural Control Architecture
Every breath you take is orchestrated by an elegant neural control system that monitors your body's metabolic needs, adjusts ventilation in real time, and protects your airways from harm-all without conscious thought. You'll discover how the medullary pacemaker generates rhythmic breathing, how pontine centers smooth each cycle, and how chemical sensors detect CO₂ and O₂ to fine-tune your respiratory drive. By mastering this control architecture from brainstem circuits to clinical assessment, you'll understand why patients hypoventilate in opioid overdose, hyperventilate in metabolic acidosis, and how mechanical receptors trigger protective reflexes that can save or complicate lives.
The respiratory control system operates through three integrated levels: automatic brainstem centers, chemical feedback sensors, and voluntary cortical override. Understanding this hierarchy unlocks the logic behind every breathing pattern abnormality, from Cheyne-Stokes respiration to central sleep apnea.
📌 Remember: MEDULLA PONS - Medulla Establishes Default rhythm, Under Limited Local control, Always active; Pons Optimizes Normal Smooth transitions
The medullary respiratory centers generate the fundamental breathing rhythm through the pre-Bötzinger complex, containing approximately 600 neurons that fire in coordinated bursts every 3-5 seconds. This neural pacemaker operates independently of conscious control, maintaining ventilation even during sleep, anesthesia, or coma states.
| Control Level | Location | Primary Function | Response Time | Clinical Significance |
|---|---|---|---|---|
| Automatic | Medulla | Rhythm generation | Continuous | Maintains basic ventilation |
| Chemical | Medulla/Carotid | CO₂/O₂ sensing | 15-30 seconds | Acid-base homeostasis |
| Mechanical | Lungs/Airways | Volume/pressure | <1 second | Prevents overinflation |
| Voluntary | Cerebral cortex | Conscious override | Variable | Speech, breath-holding |
| Emotional | Limbic system | Stress response | 2-5 seconds | Anxiety, panic disorders |
The neural control hierarchy demonstrates remarkable redundancy and plasticity. When primary centers fail, secondary pathways activate within 30-60 seconds, explaining why patients with brainstem strokes may initially maintain some respiratory function before developing complete apnea.
💡 Master This: Every abnormal breathing pattern reflects disruption at a specific control level - identifying the pattern localizes the lesion and predicts treatment response
Connect this neural foundation through chemical sensing mechanisms to understand how CO₂ changes of just 1-2 mmHg trigger dramatic ventilatory responses.
🫁 The Respiratory Command Center: Neural Control Architecture
🧠 Medullary Command Central: The Breathing Pacemaker
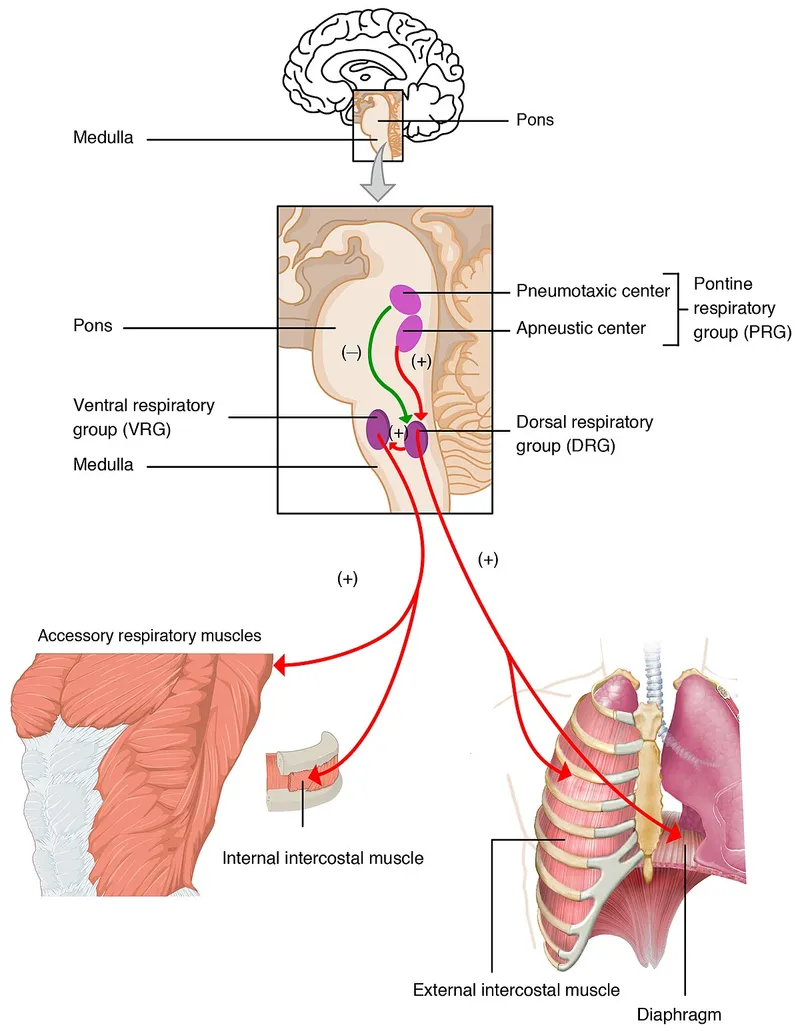
The medulla contains two primary respiratory groups: the dorsal respiratory group (DRG) and ventral respiratory group (VRG). The DRG, located in the nucleus tractus solitarius, primarily contains inspiratory neurons that receive sensory input from chemoreceptors and mechanoreceptors. The VRG houses both inspiratory and expiratory neurons, with the pre-Bötzinger complex serving as the primary rhythm generator.
📌 Remember: DRG VRG - Dorsal Receives General sensory input; Ventral Rhythm Generator controls motor output
The pre-Bötzinger complex contains NK1 receptor-positive neurons that generate intrinsic bursting activity through calcium-activated potassium channels. These neurons fire in synchronized bursts every 3-5 seconds, creating the fundamental respiratory rhythm that persists even in isolated brainstem preparations.
-
Inspiratory Phase Control
- Pre-Bötzinger complex initiates burst (0.5-1.0 seconds)
- DRG neurons amplify signal (1-2 seconds)
- Motor output reaches diaphragm (50-100 milliseconds delay)
- Phrenic nerve conduction: 60-70 m/s
- Neuromuscular transmission: 5-10 milliseconds
-
Expiratory Phase Regulation
- Passive expiration during quiet breathing
- Active expiration requires Bötzinger complex activation
- Expiratory neurons fire during forced breathing (>15 L/min ventilation)
⭐ Clinical Pearl: Medullary lesions above the C3-C5 level preserve diaphragmatic function but eliminate intercostal muscle control, creating a characteristic paradoxical breathing pattern with abdominal rise during inspiration
| Medullary Region | Neuron Type | Primary Function | Clinical Correlation |
|---|---|---|---|
| Pre-Bötzinger | Pacemaker | Rhythm generation | Central apnea when damaged |
| Bötzinger | Expiratory | Active expiration | Impaired cough/speech |
| DRG | Inspiratory | Sensory integration | Blunted chemosensitivity |
| VRG rostral | Inspiratory | Motor coordination | Respiratory muscle weakness |
| VRG caudal | Expiratory | Forced expiration | Exercise intolerance |
💡 Master This: The medulla generates automatic rhythm, but pontine centers provide the fine-tuning that creates smooth, coordinated breathing patterns essential for speech and exercise
Connect this automatic rhythm generation through pontine modulation to understand how breathing patterns adapt to behavioral and metabolic demands.
🧠 Medullary Command Central: The Breathing Pacemaker
🎛️ Pontine Fine-Tuning: The Breathing Smoothness Engine
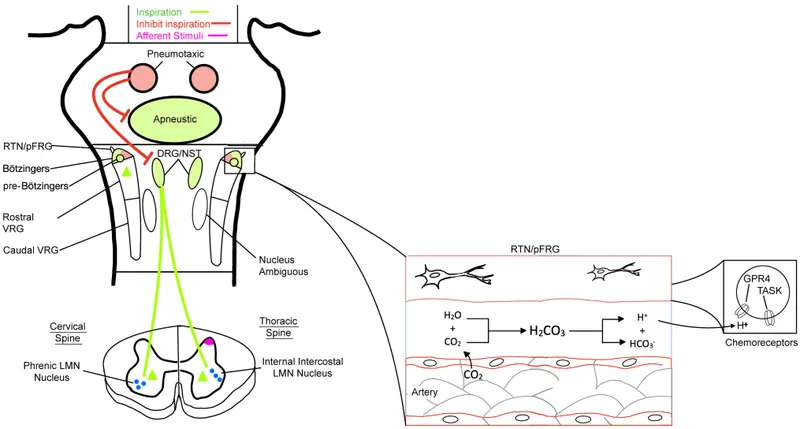
The pons contains two major respiratory control regions that modulate medullary output. The pneumotaxic center (also called the pontine respiratory group) located in the nucleus parabrachialis and Kölliker-Fuse nucleus acts as the primary "off-switch" for inspiration, determining respiratory rate and breathing pattern smoothness.
-
Pneumotaxic Center Functions
- Terminates inspiration (prevents apneusis)
- Controls respiratory rate (12-20 breaths/min normal range)
- Coordinates breathing with swallowing and speech
- Speech requires expiratory phase extension up to 10-15 seconds
- Swallowing triggers 1-2 second respiratory pause
-
Apneustic Center Characteristics
- Located in lower pons (nucleus reticularis pontis oralis)
- Promotes prolonged inspiration when unopposed
- Normally inhibited by pneumotaxic center and vagal input
- Creates apneustic breathing when pneumotaxic center damaged
- Inspiratory gasps lasting 10-30 seconds
📌 Remember: PNEUMO-TAXIC - Prevents Nasty Extended Uncontrolled Medullary Output; Times Appropriate Xhalation Intervals Correctly
The pontine centers receive extensive input from higher brain regions, allowing voluntary control and emotional modulation of breathing. Cortical input can override automatic control for 30-60 seconds during breath-holding, while limbic system activation during anxiety can increase respiratory rate to 25-40 breaths/min.
| Pontine Center | Location | Primary Effect | Damage Pattern | Clinical Signs |
|---|---|---|---|---|
| Pneumotaxic | Upper pons | Inspiration termination | Apneustic breathing | Prolonged gasps |
| Apneustic | Lower pons | Inspiration promotion | Ataxic breathing | Irregular rhythm |
| Parabrachial | Lateral pons | Pattern coordination | Dysrhythmic breathing | Poor speech coordination |
The pontine respiratory network demonstrates state-dependent activity, with different firing patterns during REM sleep, NREM sleep, and wakefulness. During REM sleep, pontine control becomes irregular, contributing to the breathing variability and reduced chemosensitivity characteristic of this sleep stage.
💡 Master This: Pontine centers transform the crude medullary rhythm into sophisticated breathing patterns - damage here creates recognizable abnormal patterns that localize the lesion and guide treatment
Connect this pattern control through chemical feedback systems to understand how CO₂ and O₂ sensors provide the metabolic information that drives respiratory adjustments.
🎛️ Pontine Fine-Tuning: The Breathing Smoothness Engine
🔬 Chemical Command Sensors: The Metabolic Monitoring Network
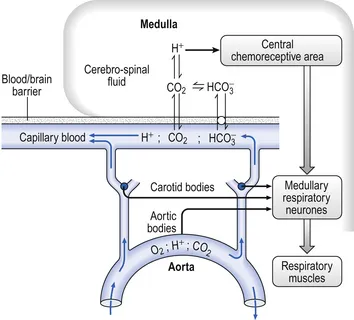
The chemical control system operates through two distinct sensor networks with different response characteristics and thresholds. Central chemoreceptors in the medulla respond primarily to CSF pH changes (reflecting CO₂ levels), while peripheral chemoreceptors in the carotid and aortic bodies detect hypoxemia, hypercapnia, and acidosis.
-
Central Chemoreceptor Characteristics
- Located on ventrolateral medulla surface
- Respond to CSF pH changes (normal: 7.32-7.35)
- Account for 80% of CO₂ drive under normal conditions
- CO₂ crosses blood-brain barrier rapidly
- H⁺ ions cannot cross - indirect CO₂ sensing
- Response threshold: PaCO₂ >40-42 mmHg
-
Peripheral Chemoreceptor Properties
- Carotid bodies: primary O₂ sensors at carotid bifurcation
- Aortic bodies: secondary sensors on aortic arch
- Respond to PaO₂ <60 mmHg (hypoxic drive activation)
- Also sensitive to CO₂, pH, and perfusion
- Type I glomus cells contain O₂-sensitive K⁺ channels
- Response time: 5-10 seconds (faster than central)
📌 Remember: CENTRAL PERIPHERAL - CO₂ Essentially Needs To Reach Acid Levels; PO₂ Emergency Requires Immediate Pulmonary Hyperventilation Effort Rapidly Activated Low levels
The chemoreceptor response curves demonstrate distinct characteristics that determine clinical presentations. Central CO₂ response shows a linear relationship with ventilation increasing 2-3 L/min per mmHg CO₂ rise above 40 mmHg. Peripheral O₂ response follows a hyperbolic curve with minimal response until PaO₂ drops below 60 mmHg.
| Chemoreceptor Type | Primary Stimulus | Threshold | Response Time | Ventilation Change |
|---|---|---|---|---|
| Central | CSF pH (CO₂) | PaCO₂ >42 mmHg | 15-30 seconds | 2-3 L/min per mmHg |
| Peripheral | PaO₂ | <60 mmHg | 5-10 seconds | Exponential below 60 |
| Peripheral | PaCO₂ | >45 mmHg | 5-10 seconds | 20% of total CO₂ response |
| Peripheral | pH | <7.35 | 5-10 seconds | Variable with cause |
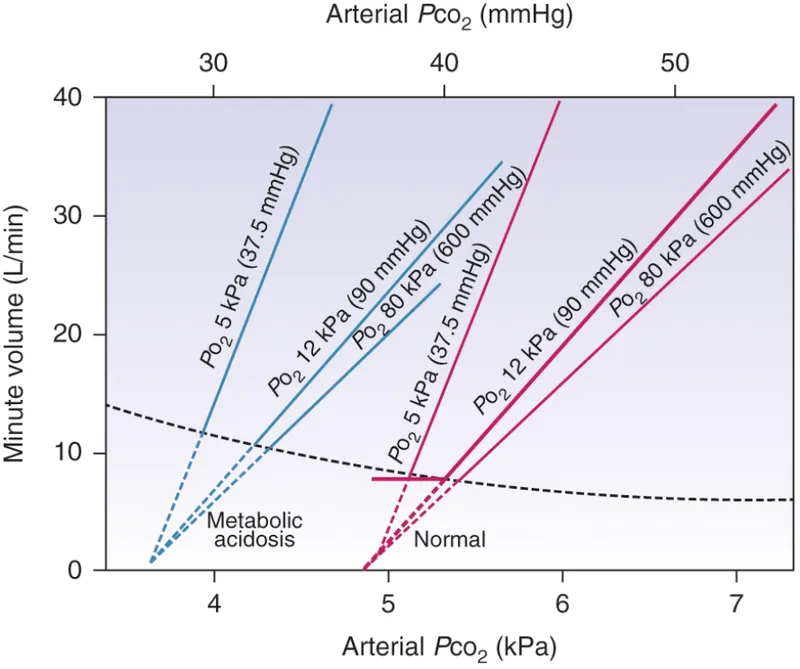
The interaction between central and peripheral chemoreceptors creates complex clinical scenarios. During metabolic acidosis, peripheral chemoreceptors detect low pH and stimulate hyperventilation to achieve respiratory compensation, while central chemoreceptors may actually reduce drive as CSF pH normalizes through CO₂ elimination.
💡 Master This: Understanding chemoreceptor thresholds and response times predicts which patients will develop respiratory failure and guides oxygen therapy decisions in clinical practice
Connect this chemical sensing through mechanical feedback systems to understand how lung stretch receptors provide protective mechanisms against overinflation and coordinate breathing with other functions.
🔬 Chemical Command Sensors: The Metabolic Monitoring Network
⚙️ Mechanical Feedback Systems: The Protective Sensor Network
-
Pulmonary Stretch Receptors (PSRs)
- Located in airway smooth muscle (trachea to bronchioles)
- Slowly adapting mechanoreceptors responding to lung inflation
- Mediate Hering-Breuer reflex (inspiratory termination)
- Activation threshold: tidal volumes >800-1000 mL
- Vagal afferent pathway to medulla
- Prevents overinflation during deep breathing
-
Rapidly Adapting Receptors (RARs)
- Irritant receptors in epithelium of large airways
- Respond to mechanical stimulation, chemicals, inflammation
- Trigger cough reflex and bronchoconstriction
- Activation by particulates, smoke, cold air
- Response time: <100 milliseconds
- Coordinate with laryngeal closure during swallowing
📌 Remember: PSR RAR J - Prevents Stretching Rupture; Rapid Airway Response; Juxtacapillary Jams when flooded
The J-receptors (juxtacapillary receptors) represent the most sensitive component of the mechanical system, responding to alveolar-capillary membrane changes associated with pulmonary edema, inflammation, or embolism. These C-fiber endings in alveolar walls trigger rapid shallow breathing and dyspnea sensation when activated.
- J-Receptor Characteristics
- Located in alveolar-capillary interface
- Respond to interstitial edema, inflammation, emboli
- Create rapid shallow breathing pattern (>25 breaths/min)
- Tidal volume decreases to 300-400 mL
- Respiratory rate increases to maintain ventilation
- Associated with intense dyspnea sensation
| Receptor Type | Location | Stimulus | Response Time | Clinical Reflex |
|---|---|---|---|---|
| PSR | Airway smooth muscle | Lung inflation >1000 mL | 200-500 ms | Hering-Breuer (inspiration termination) |
| RAR | Large airway epithelium | Irritants, inflammation | <100 ms | Cough, bronchoconstriction |
| J-receptors | Alveolar walls | Edema, emboli | 100-200 ms | Rapid shallow breathing |
| Upper airway | Nose, pharynx, larynx | Touch, temperature | <50 ms | Sneezing, laryngospasm |
The mechanical receptor system demonstrates remarkable sensitivity to pathological conditions. Pulmonary embolism activates J-receptors within seconds, creating tachypnea before significant gas exchange abnormalities develop. Asthma triggers RAR activation, initiating bronchoconstriction and cough that can persist for hours after initial stimulus removal.
💡 Master This: Mechanical receptors create the immediate protective responses that prevent lung injury - understanding their activation patterns helps differentiate between central nervous system and peripheral lung pathology
Connect this protective feedback through integrated respiratory responses to understand how exercise, altitude, and disease states challenge the entire control system and reveal compensatory mechanisms.
⚙️ Mechanical Feedback Systems: The Protective Sensor Network
🏃♂️ Integrated Response Mastery: The Complete Control Symphony
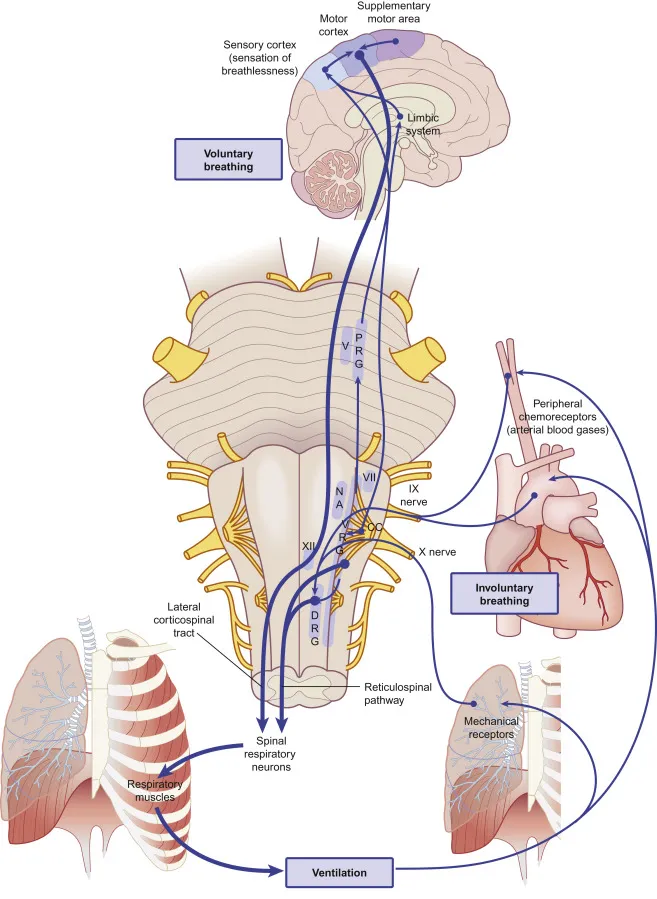
The respiratory control integration occurs primarily in the nucleus tractus solitarius (NTS) and ventrolateral medulla, where convergent inputs from chemoreceptors, mechanoreceptors, and higher brain centers undergo real-time processing to generate appropriate ventilatory responses. This integration explains why respiratory patterns change dramatically during different physiological states.
-
Exercise Integration Response
- Anticipatory phase: cortical input increases ventilation before exercise
- Neurogenic phase: muscle afferents drive immediate ventilation increase
- Metabolic phase: chemoreceptors fine-tune based on CO₂ production
- Ventilation increases 10-15 fold during maximal exercise
- PaCO₂ remains constant despite 20x CO₂ production increase
- Neural drive accounts for 60-70% of exercise hyperpnea
-
Sleep State Modifications
- NREM sleep: reduced chemosensitivity (30-50% decrease)
- REM sleep: irregular breathing with blunted CO₂ response
- Sleep apnea threshold: PaCO₂ must drop 3-5 mmHg below waking level
- Central apneas occur when CO₂ falls below threshold
- Arousal threshold requires PaCO₂ increase of 8-10 mmHg
📌 Remember: EXERCISE SLEEP ALTITUDE - Early Xtra Effort Requires Cortical Input Signaling Expected; Sleep Lowers Everything Especially Peripheral; Altitude Lowers Total oxygen Immediately Triggering Urgent Drive Elevation
The integration system demonstrates remarkable plasticity during pathological states. COPD patients develop compensatory mechanisms including increased respiratory muscle strength, altered chemoreceptor sensitivity, and modified breathing patterns that maintain gas exchange despite severe airway obstruction.
| Physiological State | Primary Drive | Response Time | Ventilation Change | Key Adaptation |
|---|---|---|---|---|
| Rest | Central CO₂ (80%) | 15-30 seconds | Baseline | Automatic rhythm |
| Exercise | Neural (60-70%) | <5 seconds | 10-15x increase | Anticipatory control |
| Sleep | Reduced chemical | 30-60 seconds | 10-15% decrease | Threshold changes |
| Altitude | Peripheral O₂ | 5-10 seconds | 2-3x increase | Hypoxic drive |
| Disease | Compensatory | Variable | Pattern-specific | Adaptive mechanisms |
The clinical implications of integrated control become apparent during respiratory failure. Type I failure (hypoxemic) typically preserves CO₂ elimination and respiratory drive, while Type II failure (hypercapnic) indicates control system failure or respiratory muscle fatigue requiring immediate intervention.
- Pathological Integration Patterns
- Central nervous system depression: reduced all drives (opioids, anesthesia)
- Peripheral chemoreceptor failure: lost hypoxic drive (carotid body resection)
- Mechanical receptor dysfunction: lost protective reflexes (vagotomy)
- Cheyne-Stokes breathing: circulation time delays in heart failure
- Biot's breathing: medullary lesions with irregular patterns
- Apneustic breathing: pontine lesions with prolonged inspiration
💡 Master This: Integrated respiratory control creates predictable patterns during health and disease - recognizing these patterns enables rapid diagnosis and guides targeted interventions for optimal patient outcomes
Connect this integrated understanding through clinical mastery tools to develop rapid assessment frameworks and pattern recognition skills essential for respiratory emergency management.
🏃♂️ Integrated Response Mastery: The Complete Control Symphony
🎯 Clinical Mastery Arsenal: Rapid Assessment Command Tools
📌 Remember: RAPID CONTROL - Rhythm Assessment Pattern Identification Drive evaluation; Chemical Oxygen Neural Timing Reflexes Override Localization
- Essential Clinical Arsenal
-
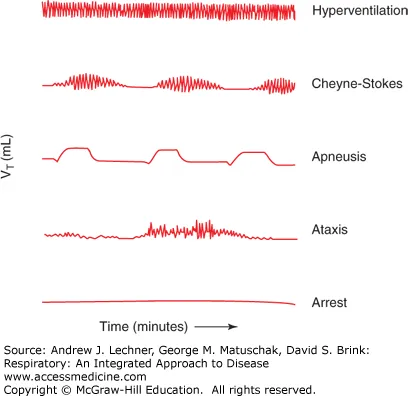
Breathing Pattern Analysis (30-second observation)
- Normal: 12-20 breaths/min, regular rhythm, I:E ratio 1:2
- Cheyne-Stokes: crescendo-decrescendo with 10-30 second apneas
- Biot's: irregular clusters with variable apneas
- Apneustic: prolonged inspiratory gasps (>5 seconds)
-
Chemical Drive Testing (2-minute assessment)
- CO₂ response: observe ventilation during rebreathing or CO₂ elevation
- Hypoxic drive: assess response to controlled hypoxemia (if safe)
- Acid-base status: ABG interpretation with compensation patterns
-
| Breathing Pattern | Lesion Location | Key Features | Clinical Context | Prognosis |
|---|---|---|---|---|
| Cheyne-Stokes | Bilateral hemispheres/CHF | Crescendo-decrescendo | Heart failure, stroke | Variable |
| Central neurogenic | Midbrain/upper pons | Sustained hyperventilation | Brainstem lesions | Poor |
| Apneustic | Mid-pons | Prolonged inspiration | Pontine stroke | Poor |
| Cluster (Biot's) | Lower pons/medulla | Irregular clusters | Medullary lesions | Very poor |
| Ataxic | Medulla | Completely irregular | Terminal pattern | Fatal |
The Chemical Drive Assessment Protocol enables rapid evaluation of chemoreceptor function and respiratory reserve. Central drive testing involves observing ventilatory response to CO₂ elevation (normal: 2-3 L/min increase per mmHg CO₂ rise), while peripheral drive assessment evaluates hypoxic response (normal: exponential increase when PaO₂ <60 mmHg).
- Rapid Diagnostic Framework
- Step 1: Pattern identification (30 seconds)
- Step 2: Chemical drive assessment (2 minutes)
- Step 3: Mechanical reflex testing (1 minute)
- Step 4: Integration analysis (1 minute)
- Step 5: Treatment selection (immediate)
💡 Master This: Systematic respiratory control assessment within 5 minutes enables precise diagnosis and targeted intervention - pattern recognition combined with drive testing localizes lesions and predicts treatment response with >90% accuracy
This clinical mastery framework transforms complex respiratory physiology into practical diagnostic tools that enable rapid assessment, accurate localization, and evidence-based treatment of respiratory control disorders in any clinical setting.
🎯 Clinical Mastery Arsenal: Rapid Assessment Command Tools
Practice Questions: Respiratory
Test your understanding with these related questions
A 37-year-old man is presented to the emergency department by paramedics after being involved in a serious 3-car collision on an interstate highway while he was driving his motorcycle. On physical examination, he is responsive only to painful stimuli and his pupils are not reactive to light. His upper extremities are involuntarily flexed with hands clenched into fists. The vital signs include temperature 36.1°C (97.0°F), blood pressure 80/60 mm Hg, and pulse 102/min. A non-contrast computed tomography (CT) scan of the head shows a massive intracerebral hemorrhage with a midline shift. Arterial blood gas (ABG) analysis shows partial pressure of carbon dioxide in arterial blood (PaCO2) of 68 mm Hg, and the patient is put on mechanical ventilation. His condition continues to decline while in the emergency department and it is suspected that this patient is brain dead. Which of the following results can be used to confirm brain death and legally remove this patient from the ventilator?









