Renal

On this page
🏗️ Nephron Architecture: The Kidney's Precision Engineering
Your kidneys filter 180 liters of plasma daily, yet you excrete only 1-2 liters of urine-a feat of molecular precision that maintains your body's chemical balance within razor-thin margins. This lesson takes you from the nephron's elegant architecture through its filtration barriers, transport machinery, and hormonal command systems to the countercurrent multiplier that concentrates urine and the acid-base mechanisms that defend pH. You'll build a unified framework connecting structure to function, then apply it to interpret clinical scenarios where these systems fail or adapt.
Nephron Structural Organization
- Renal Corpuscle (200 μm diameter)
- Glomerulus: 50 capillary loops with 300 m² surface area per kidney
- Bowman's capsule: 60 μm filtration barrier thickness
- Visceral layer: Podocytes with 25-60 nm filtration slits
- Parietal layer: Simple squamous epithelium
- Tubular System (15mm total length per nephron)
- Proximal tubule: 14mm length, 65% of total reabsorption
- Loop of Henle: 2-14mm depending on nephron type
- Thin descending: Water permeable, osmolality rises to 1200 mOsm/kg
- Thin ascending: NaCl permeable, water impermeable
- Thick ascending: Active Na-K-2Cl transport, 25% of filtered Na⁺
- Distal convoluted tubule: 5mm length, thiazide-sensitive transport
- Collecting duct: 20mm length, aldosterone-responsive fine-tuning
📌 Remember: GLOVE for nephron segments - Glomerulus, Loop of Henle, Other tubules (proximal/distal), Vasa recta, Efferent arteriole. Each segment handles specific percentages: Proximal (65%), Loop (25%), Distal (5-10%), with <1% final urine output.
Nephron Classification and Distribution
| Nephron Type | Percentage | Glomerular Location | Loop Length | Primary Function | Vasa Recta |
|---|---|---|---|---|---|
| Cortical | 85% | Outer cortex | Short (2mm) | Volume regulation | Minimal |
| Juxtamedullary | 15% | Corticomedullary junction | Long (14mm) | Concentration | Extensive |
| Superficial | 70% | Superficial cortex | Very short | Filtration | None |
| Midcortical | 15% | Mid cortex | Intermediate | Mixed function | Limited |
| Deep cortical | 15% | Deep cortex | Variable | Concentration support | Moderate |
Vascular Architecture Integration
- Afferent Arteriole (diameter 16-20 μm)
- Autoregulation maintains GFR 120-130 mL/min
- Myogenic response: 5-10 second constriction to pressure changes
- Tubuloglomerular feedback: 30-60 second response to distal delivery
- Glomerular Capillaries (7-10 μm diameter)
- Hydrostatic pressure: 60 mmHg (highest in body)
- Filtration fraction: 20% of renal plasma flow
- Surface area: 1.5-2.0 m² per kidney
- Efferent Arteriole (diameter 13-16 μm)
- Angiotensin II sensitivity: 10x greater than afferent
- Maintains filtration fraction during volume depletion
💡 Master This: The 3:1 pressure gradient (glomerular 60 mmHg vs peritubular 20 mmHg) drives both filtration and reabsorption. ACE inhibitors preferentially dilate efferent arterioles, reducing this gradient and explaining their renoprotective effects beyond blood pressure control.
Juxtaglomerular Apparatus: The Nephron's Control Center
- Macula Densa Cells (15-20 specialized cells)
- NaCl sensing via NKCC2 transporter activity
- Responds to 10-15% changes in distal NaCl delivery
- Releases adenosine and ATP for tubuloglomerular feedback
- Juxtaglomerular Cells (40-50 modified smooth muscle cells)
- Renin storage: 300-500 ng/mL in granules
- Baroreceptor function: Responds to 5-10 mmHg pressure changes
- β₁-adrenergic stimulation increases renin 3-5 fold
- Extraglomerular Mesangial Cells
- Lacis cells provide structural support
- Potential paracrine signaling between macula densa and JG cells
📌 Remember: BARN for JGA stimuli increasing renin release - Beta-adrenergic stimulation, Angiotensin II (low levels), Reduced NaCl delivery, No pressure (decreased perfusion pressure). Each stimulus can increase renin 2-10 fold within minutes.
The nephron's architectural precision enables 99% reabsorption efficiency while maintaining ±2% plasma osmolality control. This foundation supports understanding how 180 L/day filtration becomes 1.5 L/day urine output through coordinated tubular function.
🏗️ Nephron Architecture: The Kidney's Precision Engineering
⚡ Glomerular Filtration: The Kidney's Molecular Sieve
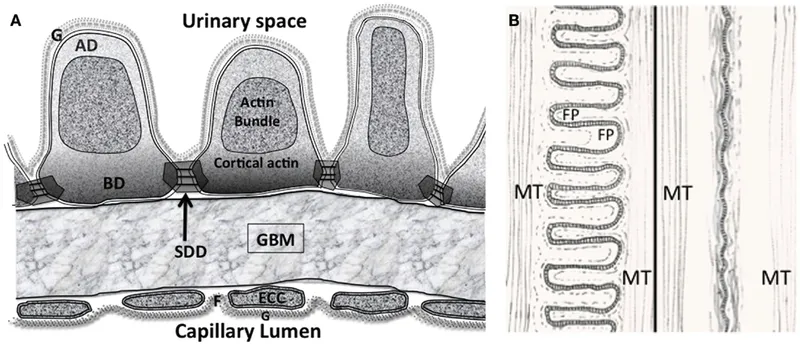
The Three-Layer Filtration Barrier
- Fenestrated Endothelium (70-100 nm pores)
- 4000-8000 fenestrae per cell
- Blocks cells and large proteins (>69 kDa)
- Glycocalyx layer: 200-400 nm thick, negative charge
- Filtration coefficient: >0.95 for molecules <40 kDa
- Glomerular Basement Membrane (300-350 nm thick)
- Type IV collagen network with 2-8 nm pores
- Heparan sulfate: Strong negative charge repels albumin
- Laminin and nidogen: Structural integrity
- Charge selectivity: 90% albumin exclusion despite 3.6 nm size
- Podocyte Slit Diaphragm (25-60 nm filtration slits)
- Nephrin and podocin: Primary filtration proteins
- 40 nm spacing between foot processes
- Final size barrier: 3.6 nm effective pore radius
⭐ Clinical Pearl: The filtration barrier's charge selectivity explains why albumin (3.6 nm, negative charge) is 99.5% excluded while neutral dextran of similar size passes freely. Loss of heparan sulfate in diabetes causes microalbuminuria (30-300 mg/day) before structural damage occurs.
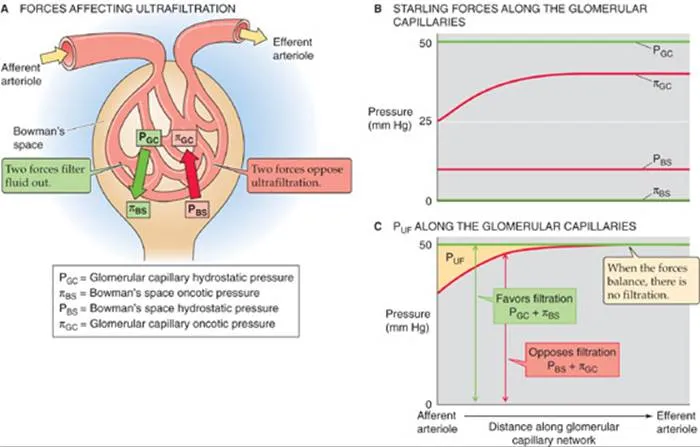
Starling Forces and Filtration Dynamics
| Location | Hydrostatic Pressure | Oncotic Pressure | Net Filtration Pressure |
|---|---|---|---|
| Afferent End | 60 mmHg | 28 mmHg | +32 mmHg |
| Mid-Capillary | 58 mmHg | 32 mmHg | +26 mmHg |
| Efferent End | 56 mmHg | 36 mmHg | +20 mmHg |
| Bowman's Space | 15 mmHg | 0 mmHg | Opposes filtration |
| Average Net | 57 mmHg | 32 mmHg | +10 mmHg |
- Kf (filtration coefficient): 12.5 mL/min/mmHg per kidney
- PGC (glomerular capillary pressure): 57 mmHg average
- PBS (Bowman's space pressure): 15 mmHg
- πGC (glomerular oncotic pressure): 32 mmHg average
- πBS (Bowman's space oncotic): 0 mmHg (protein-free)
💡 Master This: Filtration fraction (20%) determines oncotic pressure rise along the capillary. Higher filtration fractions (dehydration, efferent vasoconstriction) increase πGC from 28 to 36 mmHg, reducing net filtration pressure and providing autoregulation.
Autoregulation Mechanisms
- Myogenic Response (5-10 seconds)
- Afferent arteriole constriction to ↑ pressure
- Calcium channel activation at >80 mmHg
- Maintains GFR constant between 80-180 mmHg
- Effectiveness: 85% compensation for pressure changes
- Tubuloglomerular Feedback (30-60 seconds)
- Macula densa senses ↑ NaCl delivery
- Adenosine release causes afferent vasoconstriction
- ATP and adenosine reduce renin release
- Sensitivity: 10-15% change in distal NaCl delivery
📌 Remember: MAGIC for GFR regulation - Myogenic (fast, pressure), Adenosine (TGF), GFR maintained, Intrinsic mechanisms, Constant between 80-180 mmHg. Both mechanisms work within 80-180 mmHg range, beyond which GFR becomes pressure-dependent.

Clinical Filtration Markers
- Inulin Clearance (Gold Standard)
- Freely filtered, not reabsorbed or secreted
- Normal GFR: 120-130 mL/min/1.73m²
- Clearance formula: (U × V) / P
- Creatinine Clearance (Clinical Standard)
- 10-15% tubular secretion overestimates GFR
- Cockcroft-Gault: [(140-age) × weight] / (72 × SCr)
- MDRD: More accurate for GFR <60 mL/min
- Cystatin C (Emerging Marker)
- Not secreted, less muscle mass dependent
- Earlier detection of GFR decline
- Normal range: 0.5-1.0 mg/L
⭐ Clinical Pearl: Creatinine rises only after 50% GFR loss due to hyperfiltration in remaining nephrons and increased tubular secretion. Cystatin C detects 25-30% GFR reduction earlier, making it superior for early CKD detection.
The glomerular filtration barrier's triple-layer precision creates 180 L/day of protein-free ultrafiltrate, setting the stage for tubular processing that recovers 99% of filtered water and >95% of essential solutes through segment-specific transport mechanisms.
⚡ Glomerular Filtration: The Kidney's Molecular Sieve
🔧 Tubular Transport: The Nephron's Molecular Machinery
Proximal Tubule: The Bulk Reabsorber
- Segment S1 (First 2mm)
- Na⁺-glucose cotransporter (SGLT2): 90% glucose reabsorption
- Na⁺-amino acid cotransporters: 95% amino acid recovery
- Na⁺-phosphate cotransporter: 80% phosphate reabsorption
- Carbonic anhydrase: 80% bicarbonate reclamation
- Segment S2 (Middle 6mm)
- Na⁺-H⁺ exchanger (NHE3): 50% of total Na⁺ reabsorption
- Organic anion transporters (OAT1/3): PAH, furosemide secretion
- Organic cation transporters (OCT2): Creatinine, metformin secretion
- Segment S3 (Final 6mm)
- Glucose reabsorption completion via SGLT1
- Urea recycling: 50% reabsorption
- Ammonia production: pH-dependent glutamine metabolism
📌 Remember: GLAMP for proximal tubule reabsorption percentages - Glucose (100%), Lactate (100%), Amino acids (95%), Magnesium (25%), Phosphate (80%). The proximal tubule handles 65% of filtered sodium and 67% of filtered water through isosmotic reabsorption.
Loop of Henle: The Concentrating Engine
| Segment | Length | Permeability | Transport | Osmolality Change |
|---|---|---|---|---|
| Thin Descending | 2-10mm | Water only | Passive | 300→1200 mOsm |
| Thin Ascending | 1-2mm | NaCl only | Passive | 1200→600 mOsm |
| Thick Ascending | 1-2mm | Impermeable | NKCC2 active | 600→150 mOsm |
| Macula Densa | 0.1mm | NaCl sensing | NKCC2 | 150 mOsm |
- Aquaporin-1 (AQP1): High water permeability
- No active transport: Osmotic equilibration only
- Urea entry: UT-A2 transporters in deep nephrons
- Volume reduction: 67L to 15L per day
- Ascending Limb Transport
- NKCC2 transporter: 25% of filtered Na⁺ reabsorption
- ROMK channels: K⁺ recycling for continued transport
- ClC-Kb channels: Cl⁻ exit pathway
- Furosemide target: 20-30% natriuresis when blocked
⭐ Clinical Pearl: The thick ascending limb generates 200 mOsm/kg dilution capacity through NKCC2 activity. Loop diuretics blocking this transporter can increase Na⁺ excretion from <1% to 25% of filtered load, explaining their potent diuretic effect and ototoxicity (similar NKCC2 in inner ear).
Distal Convoluted Tubule: The Fine-Tuner
- Early DCT (3mm length)
- NCCT (thiazide-sensitive): 5-10% Na⁺ reabsorption
- Calcium reabsorption: TRPV5 and NCX1 transporters
- Magnesium reabsorption: TRPM6 channels (10% of filtered load)
- Late DCT (2mm length)
- ENaC channels: Aldosterone-sensitive Na⁺ transport
- Principal cells: 2-5% final Na⁺ reabsorption
- Intercalated cells: H⁺ and HCO₃⁻ transport for pH balance
💡 Master This: Thiazide diuretics block NCCT in early DCT, causing hypocalciuria (increased TRPV5 activity) and hyponatremia (enhanced ADH sensitivity). This explains why thiazides prevent kidney stones and can cause dilutional hyponatremia in elderly patients.
Collecting Duct: The Final Arbiter
- Principal Cells (65% of CD cells)
- ENaC: Aldosterone-regulated Na⁺ reabsorption
- AQP2: ADH-regulated water reabsorption
- ROMK: K⁺ secretion coupled to Na⁺ reabsorption
- Final regulation: 1-5% of filtered Na⁺
- Intercalated Cells (35% of CD cells)
- Type A: H⁺-ATPase for acid secretion
- Type B: HCO₃⁻ secretion for alkalosis correction
- Pendrin: Cl⁻/HCO₃⁻ exchange in type B cells
📌 Remember: PEAK for collecting duct regulation - Principal cells (Na⁺, K⁺, water), ENaC channels, Aldosterone control, K⁺ secretion linked to Na⁺ reabsorption. Amiloride blocks ENaC, causing hyperkalemia and volume retention.

Integrated Transport Efficiency
- Overall Reabsorption Rates
- Sodium: 99.5% (150 mEq filtered, <1 mEq excreted)
- Water: 99% (180L filtered, 1.5L excreted)
- Glucose: 100% (below 180 mg/dL plasma)
- Urea: 50% (recycled for concentration)
- Creatinine: 0% reabsorbed, 15% secreted
⭐ Clinical Pearl: Fractional excretion calculations reveal transport defects: FENa <1% indicates prerenal azotemia (intact tubular function), while FENa >2% suggests acute tubular necrosis with impaired reabsorption. FEUrea <35% provides additional prerenal confirmation.
The nephron's transport machinery achieves 99%+ efficiency through segment-specific specialization, transforming 180L of filtrate into 1.5L of concentrated urine while maintaining precise electrolyte balance and acid-base homeostasis.
🔧 Tubular Transport: The Nephron's Molecular Machinery
🔍 Hormonal Integration: The Nephron's Command Network
The RAAS: Volume Control Headquarters
- Renin Release Triggers
- Decreased perfusion pressure: <80 mmHg renal artery pressure
- Sympathetic stimulation: β₁-adrenergic activation
- Decreased NaCl delivery: <40 mEq/L at macula densa
- Prostaglandin E₂: PGE₂ enhances renin release 2-3 fold
- Angiotensin II Actions (Half-life: 1-2 minutes)
- Vasoconstriction: Efferent > afferent (maintains GFR)
- Aldosterone stimulation: 3-5 fold increase within 30 minutes
- ADH release: Synergistic water retention
- Proximal Na⁺ reabsorption: Direct tubular effect
📌 Remember: SAND for renin release stimuli - Sympathetic activation, Angiotensin II (low), NaCl delivery (decreased), Decreased perfusion pressure. Each stimulus can increase plasma renin activity from 1-3 ng/mL/hr to 10-50 ng/mL/hr within minutes.
Aldosterone: The Sodium Saver
| Parameter | Baseline | Aldosterone Effect | Time Course | Clinical Significance |
|---|---|---|---|---|
| ENaC Expression | 100% | ↑300-500% | 2-6 hours | Na⁺ retention |
| ROMK Channels | 100% | ↑200-300% | 1-3 hours | K⁺ loss |
| Na-K-ATPase | 100% | ↑150-200% | 6-12 hours | Sustained effect |
| Urinary Na⁺ | 150 mEq/day | ↓10-20 mEq/day | 24-48 hours | Volume expansion |
| Urinary K⁺ | 90 mEq/day | ↑150-200 mEq/day | 12-24 hours | Hypokalemia risk |
- Nuclear translocation: 30-60 minutes
- Gene transcription: Serum and glucocorticoid kinase (SGK1)
- Protein synthesis: ENaC subunits and Na-K-ATPase
- Maximal effect: 24-48 hours for full response
- Aldosterone Escape Mechanism
- ANP release: Volume expansion triggers natriuretic peptides
- Pressure natriuresis: ↑ perfusion pressure enhances Na⁺ excretion
- Prevents severe volume overload despite continued aldosterone
⭐ Clinical Pearl: Primary aldosteronism causes hypertension with hypokalemia (<3.5 mEq/L) and metabolic alkalosis. Aldosterone:renin ratio >20 with aldosterone >15 ng/dL suggests autonomous aldosterone production, requiring salt loading tests for confirmation.

ADH: The Water Conservator
- Osmotic Regulation (Primary pathway)
- Osmoreceptors: Hypothalamic neurons sensitive to 1-2% osmolality changes
- Threshold: 280-285 mOsm/kg for ADH release
- Sensitivity: 2.5 pg/mL ADH increase per mOsm/kg rise
- Maximum concentration: Urine osmolality 1200 mOsm/kg
- Volume Regulation (Secondary pathway)
- Baroreceptors: Carotid sinus and aortic arch
- Threshold: 5-10% volume depletion for ADH release
- High-pressure receptors: Left atrial stretch receptors
- Sensitivity: Exponential ADH rise with >15% volume loss
💡 Master This: ADH has dual thresholds: osmotic (285 mOsm/kg) and volume (10% depletion). Volume stimuli override osmotic control, explaining why hyponatremia develops in heart failure and cirrhosis despite low plasma osmolality.
Parathyroid Hormone: The Calcium Controller
- PTH Actions on Kidney (Within 15 minutes)
- Distal tubule: ↑ Ca²⁺ reabsorption via TRPV5 channels
- Proximal tubule: ↓ phosphate reabsorption via NPT2a inhibition
- 1α-hydroxylase: ↑ calcitriol production (1,25(OH)₂D₃)
- 24-hydroxylase: ↓ calcitriol degradation
- Integrated Calcium Homeostasis
- Normal ionized Ca²⁺: 4.5-5.5 mg/dL (1.1-1.4 mmol/L)
- PTH response: 4-fold increase with 10% Ca²⁺ decrease
- Phosphate handling: Inverse relationship maintains Ca × PO₄ product
📌 Remember: CHAMP for PTH effects - Calcium reabsorption (↑), Hydroxylase activation (1α), Absorption intestinal (via calcitriol), Mobilization from bone, Phosphate excretion (↑). PTH increases urinary phosphate while decreasing urinary calcium.
Atrial Natriuretic Peptide: The Volume Brake
- ANP Release Triggers
- Atrial stretch: >15% increase in atrial pressure
- Volume expansion: >10% increase in blood volume
- Plasma ANP: Rises from 25 pg/mL to 200+ pg/mL
- Renal Effects (Peak at 15-30 minutes)
- GFR increase: ↑ afferent dilation, ↓ mesangial contraction
- Natriuresis: Inner medullary collecting duct Na⁺ transport inhibition
- Diuresis: Reduced ADH sensitivity
- RAAS suppression: ↓ renin release, ↓ aldosterone production
⭐ Clinical Pearl: BNP (brain natriuretic peptide) serves as a volume overload biomarker. BNP >400 pg/mL indicates heart failure with 95% sensitivity, while BNP <100 pg/mL excludes heart failure with 95% specificity in dyspneic patients.
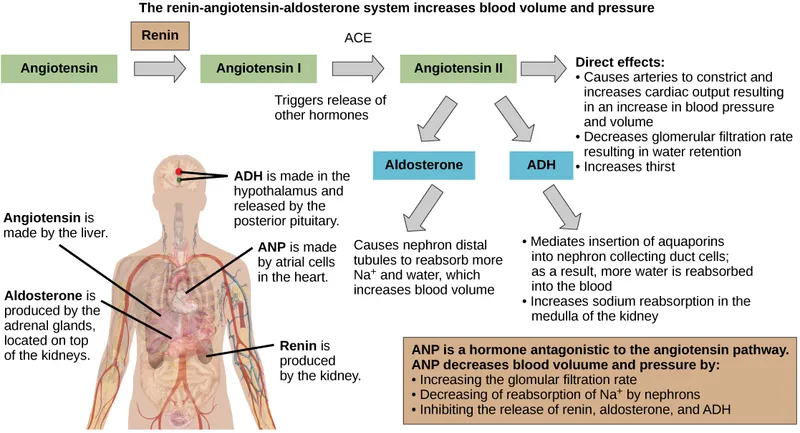
Integrated Hormonal Responses
- Volume Depletion Response (Coordinated activation)
- Renin-Angiotensin: Primary volume restoration
- ADH: Water conservation
- Sympathetic: Immediate vasoconstriction
- Aldosterone: Sustained Na⁺ retention
- Volume Overload Response (Coordinated suppression)
- ANP/BNP: Natriuresis and diuresis
- RAAS suppression: ↓ renin, ↓ aldosterone
- Pressure natriuresis: Mechanical Na⁺ excretion
The nephron's hormonal integration network achieves ±2% osmolality control and ±5% volume regulation through coordinated multi-hormone responses, enabling homeostasis across wide physiological variations in intake and output.
🔍 Hormonal Integration: The Nephron's Command Network
⚖️ Acid-Base Mastery: The Nephron's pH Control Center
Proximal Tubule: The Bicarbonate Reclaimer
- Bicarbonate Reabsorption Mechanism
- Na⁺-H⁺ exchanger (NHE3): Primary H⁺ secretion pathway
- Carbonic anhydrase IV: Luminal CO₂ formation
- Carbonic anhydrase II: Intracellular H⁺ generation
- NBC1 cotransporter: Basolateral HCO₃⁻ exit (1 Na⁺ : 3 HCO₃⁻)
- Quantitative Bicarbonate Handling
- Filtered load: 4320 mEq/day (180L × 24 mEq/L)
- Proximal reabsorption: 80% (3456 mEq/day)
- Threshold: 26-28 mEq/L plasma HCO₃⁻
- Tm HCO₃⁻: 2.8 mEq/min/1.73m² maximum reabsorption
📌 Remember: CHAMP for proximal HCO₃⁻ reabsorption - Carbonic anhydrase (IV and II), H⁺ secretion (NHE3), Anhydrase converts CO₂, Membrane NBC1 exit, Proximal handles 80%. Acetazolamide blocks carbonic anhydrase, causing 15-20% bicarbonate loss.
Distal Acid Secretion: The Fine-Tuning System
| Cell Type | Location | Primary Function | H⁺ Secretion Rate | Buffer System |
|---|---|---|---|---|
| α-Intercalated | Collecting Duct | Acid secretion | 50-100 nmol/min | NH₄⁺, HPO₄²⁻ |
| β-Intercalated | Collecting Duct | Base secretion | Variable | HCO₃⁻ |
| DCT Cells | Distal Tubule | HCO₃⁻ reclaim | 20-30 nmol/min | Residual HCO₃⁻ |
| Principal Cells | Collecting Duct | K⁺ secretion | Minimal | None |
- H⁺-ATPase: Primary active H⁺ secretion
- H⁺-K⁺-ATPase: K⁺-sparing acid secretion
- AE1 exchanger: Basolateral Cl⁻/HCO₃⁻ exchange
- Carbonic anhydrase II: Intracellular H⁺ generation
- β-Intercalated Cell Mechanisms
- Pendrin (SLC26A4): Apical Cl⁻/HCO₃⁻ exchange
- AE4 exchanger: Basolateral Cl⁻/HCO₃⁻ exchange
- Activated during metabolic alkalosis
⭐ Clinical Pearl: Type 1 RTA (distal) results from H⁺-ATPase defects in α-intercalated cells, causing inability to acidify urine below pH 5.5. Nephrolithiasis and nephrocalcinosis develop from hypercalciuria and hypocitraturia in alkaline urine.

Ammonia Production: The Acid Buffer Factory
- Proximal Tubule Ammonia Genesis
- Glutamine metabolism: Primary substrate (2-4 mmol/day/kidney)
- Glutaminase: Glutamine → glutamate + NH₃
- Glutamate dehydrogenase: Glutamate → α-ketoglutarate + NH₃
- pH regulation: Acidosis increases glutaminase activity 3-5 fold
- Ammonia Transport and Trapping
- NH₃ diffusion: Lipophilic, crosses cell membranes
- NH₄⁺ trapping: Protonated form trapped in acidic urine
- Rh glycoproteins: NH₃ transporters in collecting duct
- Countercurrent multiplication: Medullary NH₃ concentration
💡 Master This: Ammonia excretion represents net acid excretion because each NH₄⁺ in urine removes one H⁺ from the body. Normal ammonia excretion is 30-50 mEq/day, increasing to 200-300 mEq/day during chronic acidosis through adaptive glutaminase upregulation.
Titratable Acid: The Phosphate Buffer System
- Phosphate Buffer Chemistry
- pKa: 6.8 (optimal for urine pH 4.5-8.0)
- HPO₄²⁻ + H⁺ → H₂PO₄⁻: Titratable acid formation
- Normal excretion: 10-30 mEq/day
- Limited by filtered phosphate load (1200 mg/day)
- Other Titratable Acids
- Creatinine: pKa 2.6, minimal buffering
- Uric acid: pKa 5.8, 5-10 mEq/day
- Organic acids: Lactate, ketoacids during pathologic states
📌 Remember: PANT for renal acid excretion - Phosphate (titratable), Ammonia (major), Net acid excretion formula, Titratable + NH₄⁺ - HCO₃⁻. Net acid excretion = (NH₄⁺ + titratable acid) - HCO₃⁻ in urine.
Integrated Acid-Base Responses
- Metabolic Acidosis Compensation
- Acute (hours): ↑ H⁺ secretion, ↑ NH₃ production
- Chronic (days): ↑ glutaminase, ↑ H⁺-ATPase expression
- Maximal response: Net acid excretion 300-500 mEq/day
- Metabolic Alkalosis Compensation
- ↓ H⁺ secretion, ↑ HCO₃⁻ excretion
- β-intercalated cell activation
- Limitation: Volume depletion maintains alkalosis
⭐ Clinical Pearl: Urine anion gap estimates unmeasured NH₄⁺ excretion: UAG = (Na⁺ + K⁺) - Cl⁻. Negative UAG (<-20 mEq/L) indicates appropriate NH₄⁺ excretion in normal anion gap acidosis, while positive UAG suggests renal tubular acidosis.
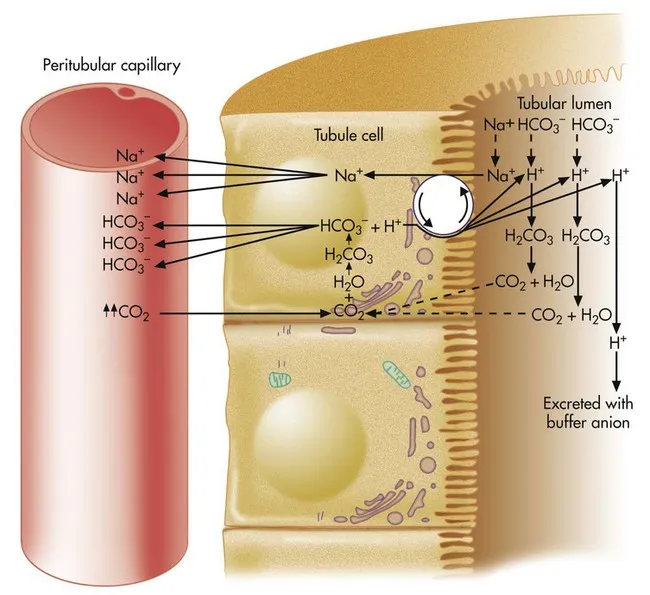
Clinical Acid-Base Integration
- Renal Tubular Acidosis Classification
- Type 1 (Distal): H⁺-ATPase defect, urine pH >5.5
- Type 2 (Proximal): HCO₃⁻ wasting, urine pH <5.5
- Type 4 (Hyperkalemic): Aldosterone deficiency, ↓ NH₄⁺ excretion
- Compensation Time Courses
- Respiratory: Minutes to hours
- Renal: Hours to days for full compensation
- Chronic adaptation: Weeks for maximal capacity
The nephron's acid-base control system maintains pH 7.40 ± 0.02 through coordinated bicarbonate reclamation (4320 mEq/day), ammonia production (30-300 mEq/day), and titratable acid excretion (10-30 mEq/day), providing definitive pH correction across wide physiological and pathological variations.
⚖️ Acid-Base Mastery: The Nephron's pH Control Center
🔗 Countercurrent Systems: The Kidney's Concentration Powerhouse
Loop of Henle: The Gradient Generator
- Descending Limb Characteristics
- Aquaporin-1: High water permeability (>100 μm/s)
- No active transport: Passive osmotic equilibration
- Urea permeability: UT-A2 in deep nephrons only
- Volume reduction: 67L/day to 15L/day
- Ascending Limb Transport
- Thick segment: NKCC2 active transport (25% filtered Na⁺)
- Thin segment: Passive NaCl efflux in deep nephrons
- Water impermeability: No aquaporins present
- Diluting capacity: Creates 150 mOsm/kg tubular fluid
📌 Remember: WILD for loop characteristics - Water permeable (descending), Impermeable to water (ascending), Loop creates gradient, Dilution in ascending limb. The single effect of 200 mOsm/kg becomes 900 mOsm/kg gradient through countercurrent multiplication.
Medullary Osmotic Gradient Architecture
| Medullary Depth | Osmolality | Primary Solutes | Contribution |
|---|---|---|---|
| Outer Cortex | 300 mOsm/kg | NaCl, glucose | Baseline |
| Outer Medulla | 600 mOsm/kg | NaCl (70%), urea (30%) | NKCC2 transport |
| Inner Medulla | 900 mOsm/kg | NaCl (50%), urea (50%) | Urea recycling |
| Papillary Tip | 1200 mOsm/kg | Urea (60%), NaCl (40%) | Maximum concentration |
- Active transport: NKCC2 in thick ascending limb
- Passive transport: NaCl efflux in thin ascending limb
- Urea recycling: UT-A1/A3 in inner medullary collecting duct
- Vasa recta: Countercurrent exchange preserves gradient
⭐ Clinical Pearl: Loop diuretics (furosemide) block NKCC2, reducing medullary osmolality by 30-40% and impairing concentrating ability for 24-48 hours after single dose. Chronic loop diuretic use causes persistent concentrating defects through medullary washout.
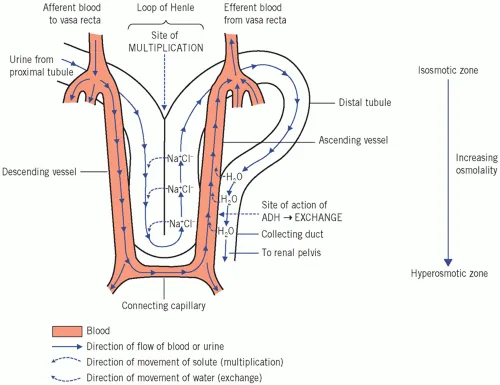
Vasa Recta: The Gradient Preserver
- Anatomical Organization
- Descending vasa recta: Arterial supply to medulla
- Ascending vasa recta: Venous drainage from medulla
- Hairpin configuration: Parallel to loops of Henle
- Blood flow: 1-2% of total renal blood flow
- Countercurrent Exchange Function
- Solute equilibration: NaCl and urea exchange
- Water movement: Osmotic equilibration along gradient
- Gradient preservation: Minimal net solute removal
- Oxygen delivery: Maintains medullary metabolism
💡 Master This: Vasa recta blood flow is critical for gradient maintenance. Increased flow (vasodilators, volume expansion) causes medullary washout, while decreased flow (vasoconstrictors, dehydration) preserves but may cause medullary hypoxia.
Urea Recycling: The Concentration Amplifier
- Urea Handling Along Nephron
- Proximal tubule: 50% reabsorption via UT-A2
- Descending limb: Variable permeability (UT-A2 in long loops)
- Ascending limb: Impermeable to urea
- Collecting duct: ADH-regulated UT-A1 and UT-A3
- Inner Medullary Urea Cycling
- UT-A1: ADH-stimulated urea efflux from collecting duct
- UT-A3: Basolateral urea efflux from collecting duct
- UT-A2: Urea entry into descending limb
- Net effect: Urea trapping in medullary interstitium
📌 Remember: AUDIT for urea recycling - ADH stimulates UT-A1, Urea trapped in medulla, Descending limb UT-A2 entry, Inner medulla concentration, Trapping creates 60% of papillary osmolality. Protein restriction reduces urea and impairs concentrating ability.
ADH and Collecting Duct Integration
- ADH Mechanism in Collecting Duct
- V2 receptor: Gs-protein coupled, ↑ cAMP
- Protein kinase A: Phosphorylates regulatory proteins
- AQP2 insertion: Apical membrane water channels
- Time course: 15-30 minutes for maximal effect
- Quantitative Water Reabsorption
- No ADH: <5% water reabsorption, urine 50-100 mOsm/kg
- Maximal ADH: >95% water reabsorption, urine 1200 mOsm/kg
- Variable response: Physiological ADH levels 2-12 pg/mL
⭐ Clinical Pearl: Diabetes insipidus demonstrates countercurrent system integrity. Central DI (ADH deficiency) responds to desmopressin with urine osmolality >750 mOsm/kg, while nephrogenic DI (collecting duct resistance) shows minimal response, indicating intact medullary gradient but impaired water reabsorption.
Integrated Concentration and Dilution
- Maximum Dilution (Water loading)
- ADH suppression: <1 pg/mL plasma levels
- Free water clearance: Up to 20 L/day
- Minimum osmolality: 50-100 mOsm/kg
- Mechanism: Ascending limb dilution without collecting duct water reabsorption
- Maximum Concentration (Water deprivation)
- ADH stimulation: >10 pg/mL plasma levels
- Free water reabsorption: >99% of filtered water
- Maximum osmolality: 1200 mOsm/kg
- Mechanism: Medullary gradient plus collecting duct water reabsorption
💡 Master This: Free water clearance calculation: CH₂O = V - Cosm, where Cosm = (Uosm × V) / Posm. Positive values indicate dilute urine (water excretion), negative values indicate concentrated urine (water conservation).
Clinical Concentrating Defects
- Medullary Washout Causes
- Loop diuretics: NKCC2 blockade
- Volume expansion: ↑ vasa recta flow
- Protein restriction: ↓ urea availability
- Chronic kidney disease: ↓ functional nephrons
- Collecting Duct Defects
- Central DI: ADH deficiency
- Nephrogenic DI: V2 receptor or AQP2 mutations
- Lithium toxicity: AQP2 downregulation
- Hypercalcemia: ADH resistance
The kidney's countercurrent systems achieve 4-fold urine concentration through architectural precision, creating osmotic gradients that enable water conservation during dehydration and dilute urine production during water excess, maintaining plasma osmolality within ±2% despite 10-fold variations in water intake.
🔗 Countercurrent Systems: The Kidney's Concentration Powerhouse
🎯 Clinical Integration: The Nephron Mastery Toolkit
Rapid Nephron Assessment Framework
- Essential Clinical Calculations
- FENa = [(UNa × PCr) / (PNa × UCr)] × 100
- FEUrea = [(UUrea × PCr) / (PUrea × UCr)] × 100
- Creatinine Clearance = [(140-age) × weight] / (72 × SCr)
- Protein excretion = 24-hour urine or spot protein/creatinine ratio
📌 Remember: RIFLE criteria for AKI staging - Risk (SCr ↑1.5x, UO <0.5 mL/kg/hr × 6hr), Injury (SCr ↑2x, UO <0.5 × 12hr), Failure (SCr ↑3x, UO <0.3 × 24hr), Loss (>4 weeks), End-stage (>3 months).
Nephron Segment-Specific Disorders
| Segment | Primary Disorders | Key Features | Diagnostic Tests | Treatment Targets |
|---|---|---|---|---|
| Glomerulus | Nephritic/Nephrotic | Proteinuria, hematuria | Biopsy, complement | Immunosuppression |
| Proximal Tubule | Fanconi Syndrome | Glucosuria, phosphaturia | Urine amino acids | Phosphate, bicarbonate |
| Loop of Henle | Bartter Syndrome | Hypokalemia, alkalosis | Genetic testing | K+, Mg2+ replacement |
| Distal Tubule | Gitelman Syndrome | Hypokalemia, hypomagnesemia | Thiazide response | Mg2+, K+ supplementation |
| Collecting Duct | Liddle Syndrome | Hypertension, hypokalemia | Amiloride response | ENaC blockers |
Diuretic Mechanisms and Clinical Applications
- Loop Diuretics (Furosemide, Bumetanide)
- Target: NKCC2 in thick ascending limb
- Natriuresis: 20-25% of filtered sodium
- Clinical use: Heart failure, pulmonary edema
- Side effects: Hypokalemia, ototoxicity, hyperuricemia
- Thiazide Diuretics (Hydrochlorothiazide, Chlorthalidone)
- Target: NCCT in distal convoluted tubule
- Natriuresis: 5-10% of filtered sodium
- Clinical use: Hypertension, heart failure
- Side effects: Hyponatremia, hyperglycemia, hypercalcemia
- Potassium-Sparing Diuretics
- Amiloride/Triamterene: ENaC blockers
- Spironolactone: Aldosterone receptor antagonist
- Clinical use: Hyperaldosteronism, heart failure
- Side effects: Hyperkalemia, gynecomastia (spironolactone)
💡 Master This: Diuretic resistance develops through compensatory mechanisms: ↑ distal Na⁺ reabsorption, ↑ aldosterone**, volume depletion. Combination therapy (loop + thiazide) achieves sequential nephron blockade for refractory edema.
Diabetic Nephropathy: Integrated Nephron Dysfunction
- Early Changes (Years 1-5)
- Glomerular hyperfiltration: GFR >140 mL/min/1.73m²
- Microalbuminuria: 30-300 mg/day
- Glomerular hypertrophy: ↑ mesangial matrix
- Tubular hypertrophy: ↑ proximal tubule transport**
- Progressive Changes (Years 5-15)
- Macroalbuminuria: >300 mg/day
- GFR decline: 2-20 mL/min/year
- Glomerulosclerosis: Kimmelstiel-Wilson nodules
- Tubulointerstitial fibrosis: ↓ concentrating ability**
- End-Stage Changes (Years 15-25)
- GFR <15 mL/min/1.73m²
- Nephrotic syndrome: >3.5 g/day proteinuria
- Global sclerosis: >50% glomeruli affected
- Dialysis requirement: Uremic complications
⭐ Clinical Pearl: SGLT2 inhibitors reduce diabetic nephropathy progression by 30-40% through multiple mechanisms: ↓ glomerular hyperfiltration, ↓ albuminuria, ↓ inflammation. Empagliflozin and canagliflozin show renoprotection independent of glycemic control.
Hypertensive Nephrosclerosis: Vascular-Tubular Integration
- Pathophysiology Cascade
- Afferent arteriosclerosis: ↓ GFR, ↑ filtration fraction
- Glomerular ischemia: ↓ single-nephron GFR
- Tubular atrophy: ↓ concentrating ability
- Interstitial fibrosis: Progressive nephron loss
- Clinical Progression
- Stage 1: Normal GFR, minimal proteinuria
- Stage 2: Mild GFR reduction (60-89 mL/min)
- Stage 3: Moderate GFR reduction (30-59 mL/min)
- Stage 4: Severe GFR reduction (15-29 mL/min)
Acute Tubular Necrosis: Transport Failure
- Ischemic ATN (85% of cases)
- S3 segment vulnerability: High oxygen consumption
- Loss of brush border: ↓ reabsorptive capacity
- Tubular obstruction: Cast formation
- Recovery: Regeneration over 7-21 days
- Nephrotoxic ATN (15% of cases)
- Aminoglycosides: Proximal tubule accumulation
- Contrast agents: Osmotic and toxic effects
- Cisplatin: DNA damage in tubular cells
- Prevention: Volume expansion, dose adjustment
📌 Remember: MUDPILES for high anion gap metabolic acidosis - Methanol, Uremia, Diabetic ketoacidosis, Propylene glycol, Isoniazid, Lactic acidosis, Ethylene glycol, Salicylates. Uremic acidosis results from ↓ ammonia production and ↓ acid excretion.
CKD Progression and Intervention Points
- CKD Stage-Specific Interventions
- Stage 1-2 (GFR >60): BP control, diabetes management, proteinuria reduction
- Stage 3 (GFR 30-59): Mineral metabolism, anemia screening, cardiovascular risk
- Stage 4 (GFR 15-29): Dialysis planning, vascular access, transplant evaluation
- Stage 5 (GFR <15): Renal replacement therapy, uremic complications
- Evidence-Based Targets
- Blood pressure: <130/80 mmHg (or <120/80 with proteinuria)
- Proteinuria: <1 g/day target with ACE inhibitors/ARBs
- Hemoglobin: 10-12 g/dL with ESA therapy
- Phosphorus: 2.7-4.6 mg/dL with binders
Rapid Clinical Decision Framework
- AKI Evaluation (<6 hours)
- Volume status: Physical exam, CVP, BUN/Cr ratio
- Urinalysis: Casts, proteinuria, specific gravity
- FENa/FEUrea: Prerenal vs intrinsic differentiation
- Imaging: Obstruction evaluation if indicated
- CKD Management (Ongoing)
- GFR monitoring: Every 3-6 months based on stage
- Proteinuria: ACE inhibitor titration to maximum tolerated
- Complications: Anemia, bone disease, cardiovascular risk
- Progression: >25% GFR decline or >5 mL/min/year concerning
The nephron mastery toolkit enables rapid assessment, accurate diagnosis, and targeted therapy for renal disorders, transforming complex pathophysiology into practical clinical expertise that improves patient outcomes across the spectrum of kidney disease.
🎯 Clinical Integration: The Nephron Mastery Toolkit
Practice Questions: Renal
Test your understanding with these related questions
Which region of the nephron reabsorbs the highest percentage of filtered bicarbonate?











