Glomerular filtration

On this page
🔬 The Glomerular Engine: Precision Filtration Mastery
The kidney filters 180 liters of plasma daily through glomeruli that select molecules with nanometer precision, maintain filtration despite blood pressure swings, and serve as the clinical gateway to detecting renal disease. You'll master the three-layered filtration barrier that blocks proteins while passing waste, decode the autoregulation mechanisms that defend GFR when pressures fluctuate, apply Starling forces to predict filtration dynamics, and use clearance calculations to quantify kidney function in real patients.
📌 Remember: GFR-FLOW - Glomerular pressure (50 mmHg), Filtration coefficient (12.5 mL/min/mmHg), Resistance balance (afferent vs efferent), Forces (Starling's quartet), Load (oncotic 25 mmHg), Outflow pressure (10 mmHg), Water movement (180 L/day)
The glomerular filtration rate represents the kidney's primary functional measurement, with normal values ranging 90-120 mL/min/1.73m² in healthy adults. This rate depends on the intricate balance of hydrostatic and oncotic pressures across the glomerular capillary wall.
- Glomerular Capillary Pressure: 50 mmHg (driving force)
- Maintained by afferent arteriolar dilation
- Regulated by efferent arteriolar constriction
- Autoregulated between 80-180 mmHg systemic pressure
- Bowman's Space Pressure: 10 mmHg (opposing force)
- Increases with urinary obstruction
- Reflects downstream resistance
- Plasma Oncotic Pressure: 25 mmHg (opposing force)
- Rises along capillary length to 35 mmHg
- Determined by albumin concentration (4.0 g/dL)
| Parameter | Normal Value | Clinical Significance | Pathological Range | Impact on GFR |
|---|---|---|---|---|
| GFR | 90-120 mL/min/1.73m² | Kidney function baseline | <60 = CKD | Direct measure |
| Filtration Fraction | 20% | Efficiency indicator | >25% = efferent constriction | Indirect measure |
| Renal Blood Flow | 1200 mL/min | Perfusion adequacy | <800 = hypoperfusion | Proportional |
| Glomerular Pressure | 50 mmHg | Filtration driving force | <40 = reduced filtration | Linear relationship |
| Net Filtration Pressure | 15 mmHg | Effective gradient | <10 = filtration failure | Critical threshold |
💡 Master This: Net filtration pressure = (Glomerular hydrostatic 50 - Bowman's space 10) - (Plasma oncotic 25 - Bowman's oncotic 0) = 15 mmHg. This 15 mmHg gradient drives 180 liters of daily filtration.
The filtration coefficient (Kf) of 12.5 mL/min/mmHg represents the kidney's hydraulic permeability, incorporating both surface area (1.5 m² per kidney) and membrane permeability. Understanding this relationship predicts how diseases affecting glomerular structure impact overall kidney function.
🔬 The Glomerular Engine: Precision Filtration Mastery
⚡ Filtration Barrier Architecture: The Molecular Gatekeeper
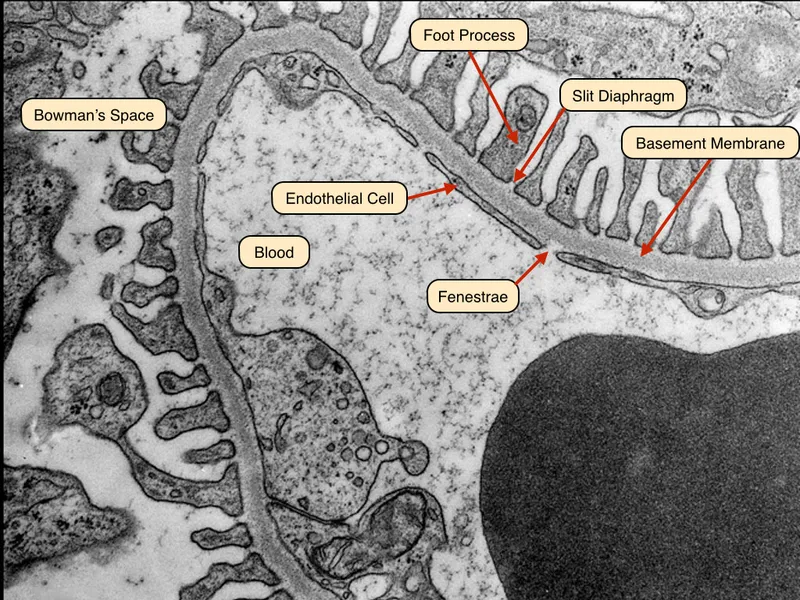
📌 Remember: FENCE - Fenestrated endothelium (70-100 nm pores), Electronegative charges, Nephrin slit diaphragms (4 nm), Collagen IV matrix, Epithelial foot processes (podocytes)
The filtration barrier components work synergistically to achieve molecular selectivity:
- Fenestrated Endothelium (First Layer)
- Pore size: 70-100 nm diameter
- Blocks cellular elements and large proteins
- Allows free passage of molecules <70 kDa
- Maintains negative surface charge via glycocalyx
- Glomerular Basement Membrane (Second Layer)
- Thickness: 300-350 nm in adults
- Type IV collagen and laminin matrix
- Negative charge from heparan sulfate
- Size cutoff: 3-4 nm (approximately 70 kDa)
- Podocyte Slit Diaphragm (Third Layer)
- Slit width: 4 nm between foot processes
- Nephrin and podocin proteins create selective barrier
- Final checkpoint for protein retention
- Maintains 99.5% albumin retention
| Molecule | Molecular Weight (kDa) | Filtration Coefficient | Clinical Significance | Normal Urine Level |
|---|---|---|---|---|
| Water | 0.018 | 1.0 | Free filtration | Variable |
| Glucose | 0.18 | 1.0 | Freely filtered, reabsorbed | <0.1 g/day |
| Creatinine | 0.113 | 1.0 | Ideal filtration marker | 1-2 g/day |
| Albumin | 66 | 0.005 | Proteinuria marker | <30 mg/day |
| IgG | 150 | 0.001 | Glomerular damage indicator | <10 mg/day |
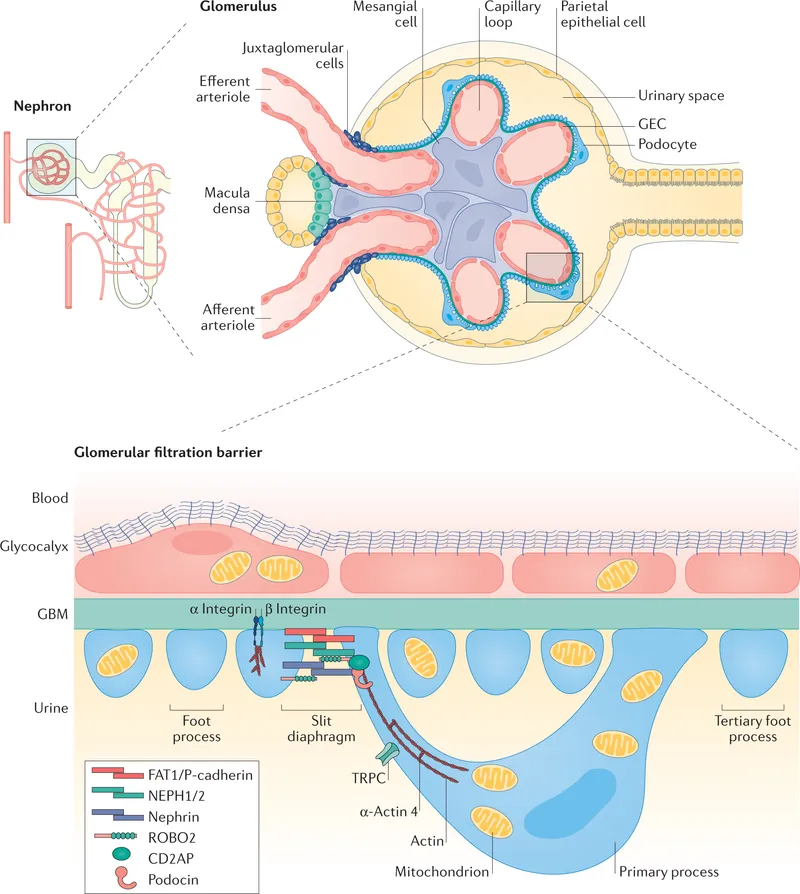
The charge selectivity of the barrier preferentially retains negatively charged proteins. Albumin (pI 4.7) carries negative charge at physiological pH, enhancing its retention despite borderline size (3.6 nm Stokes radius).
💡 Master This: Loss of negative charge in the GBM (diabetic nephropathy) allows albumin passage before structural damage occurs, explaining why microalbuminuria precedes GFR decline by 5-10 years in diabetes.
Understanding barrier selectivity predicts proteinuria patterns: selective proteinuria (albumin predominant) suggests charge loss, while non-selective proteinuria indicates structural barrier disruption with filtration coefficient changes affecting molecules >40 kDa.
⚡ Filtration Barrier Architecture: The Molecular Gatekeeper
🎛️ Autoregulation Command Center: Pressure Independence Mastery
📌 Remember: AUTO-PILOT - Afferent constriction, Uniform GFR maintenance, Tubuloglomerular feedback, Optimal pressure range (80-180 mmHg), Pressure-independent flow, Intrinsic mechanisms, Limit protection, Oscillation dampening, Time constant (5-10 seconds)
The autoregulation system maintains renal blood flow at 1200 mL/min and GFR at 120 mL/min across the autoregulatory range:
- Myogenic Mechanism (Immediate Response)
- Response time: 1-3 seconds
- Pressure threshold: >80 mmHg activation
- Mechanism: Stretch-activated calcium channels
- Effect: Afferent arteriolar constriction with pressure ↑
- Efficiency: Maintains 85% flow stability
- Tubuloglomerular Feedback (Delayed Response)
- Response time: 5-10 seconds
- Sensor: Macula densa NaCl detection
- Threshold: >40 mEq/L NaCl delivery
- Mediator: Adenosine and ATP release
- Effect: Afferent constriction via A1 receptors
| Blood Pressure Range | Autoregulation Status | GFR Maintenance | Primary Mechanism | Clinical Significance |
|---|---|---|---|---|
| <80 mmHg | Below threshold | Pressure-dependent decline | None active | Risk of acute kidney injury |
| 80-180 mmHg | Active autoregulation | ±10% variation | Myogenic + TGF | Normal kidney protection |
| >180 mmHg | Overwhelmed system | Pressure-dependent increase | Breakthrough | Glomerular damage risk |
| Chronic HTN | Reset autoregulation | Shifted curve right | Adaptive changes | Requires higher pressures |
| ACE inhibitor use | Impaired autoregulation | Enhanced pressure sensitivity | Efferent dilation | Monitor for AKI |
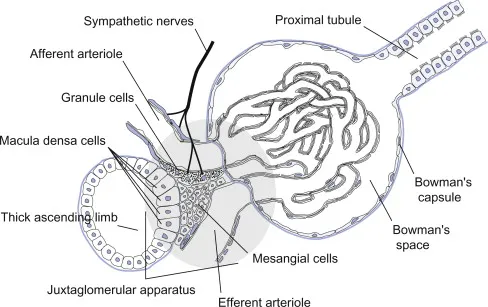
The tubuloglomerular feedback system creates oscillations in single nephron GFR with period of 30-40 seconds, representing the delay time for fluid transit from glomerulus to macula densa plus the response time for adenosine-mediated vasoconstriction.
💡 Master This: TGF sensitivity increases with volume depletion and decreases with volume expansion, explaining why dehydrated patients show exaggerated GFR responses to ACE inhibitors that block efferent arteriolar tone.
Autoregulation efficiency decreases with age, dropping from 90% effectiveness in young adults to 60% in patients >70 years, contributing to increased susceptibility to pressure-related kidney injury in elderly patients.
🎛️ Autoregulation Command Center: Pressure Independence Mastery
🧪 Starling Forces Dynamics: The Pressure Equation Mastery
📌 Remember: PUSH-PULL - Plasma hydrostatic (50 mmHg favors filtration), Urinary hydrostatic (10 mmHg opposes), Serum oncotic (25-35 mmHg opposes), Hydrostatic gradient (40 mmHg), Protein-free filtrate, Ultrafiltration coefficient (12.5), Linear pressure relationship, Length-dependent changes
The Starling equation: GFR = Kf × [(PGC - PBS) - (πGC - πBS)]
Where normal values create the filtration driving force:
- Glomerular Capillary Hydrostatic Pressure (PGC): 50 mmHg
- Determined by afferent/efferent arteriolar resistance ratio
- Maintained constant along capillary length
- Primary determinant of filtration rate
- Autoregulated between 80-180 mmHg systemic pressure
- Bowman's Space Hydrostatic Pressure (PBS): 10 mmHg
- Reflects tubular and urinary tract resistance
- Increases with obstruction (stones, BPH)
- Relatively constant in health
- Glomerular Capillary Oncotic Pressure (πGC): 25-35 mmHg
- Rises from 25 mmHg (afferent) to 35 mmHg (efferent)
- Increases with filtration fraction
- Determined by plasma protein concentration
- Bowman's Space Oncotic Pressure (πBS): 0 mmHg
- Normally protein-free ultrafiltrate
- Increases with proteinuria (glomerular disease)
| Location | Hydrostatic Pressure | Oncotic Pressure | Net Filtration Pressure | Filtration Status |
|---|---|---|---|---|
| Afferent end | 50 mmHg | 25 mmHg | 15 mmHg | Active filtration |
| Mid-capillary | 50 mmHg | 30 mmHg | 10 mmHg | Continued filtration |
| Efferent end | 50 mmHg | 35 mmHg | 5 mmHg | Minimal filtration |
| Filtration equilibrium | 50 mmHg | 40 mmHg | 0 mmHg | No net filtration |
| Post-equilibrium | 50 mmHg | >40 mmHg | Negative | Theoretical reabsorption |
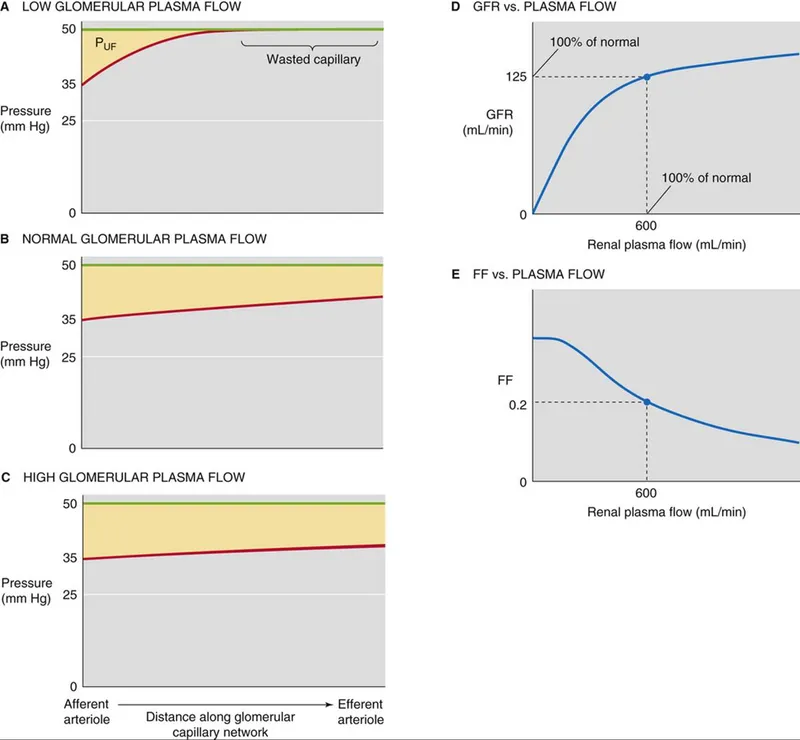
The filtration fraction (FF = GFR/RPF = 20%) determines how quickly oncotic pressure rises. Higher FF (efferent constriction) accelerates equilibrium, while lower FF (afferent dilation) maintains filtration pressure longer.
- Normal Filtration Fraction: 20% (GFR 120/RPF 600)
- High FF (>25%): Efferent constriction (ACE inhibitors reverse)
- Low FF (<15%): Afferent constriction or increased RPF
- FF and Equilibrium: Higher FF → earlier equilibrium → limited GFR
💡 Master This: Efferent arteriolar constriction (angiotensin II) increases both glomerular pressure and filtration fraction, creating a biphasic GFR response: initial increase from higher pressure, then plateau from earlier filtration equilibrium.
Understanding Starling forces predicts drug effects: ACE inhibitors preferentially dilate efferent arterioles, reducing both glomerular pressure (-5 to -10 mmHg) and filtration fraction, explaining the 10-20% GFR decline that indicates appropriate renoprotection.
🧪 Starling Forces Dynamics: The Pressure Equation Mastery
🔄 Clearance Calculations: The Quantitative Precision Engine
📌 Remember: CLEAR-CUT - Clearance = UV/P formula, Liters per minute units, Exact GFR measurement (inulin), Approximation methods (creatinine), Renal plasma flow (PAH), Creatinine overestimation (10-20%), Urine collection accuracy, Timed collections essential
The fundamental clearance equation: Clearance = (Urine concentration × Urine flow rate) / Plasma concentration
C = UV/P where:
- C = Clearance (mL/min)
- U = Urine concentration (mg/dL)
- V = Urine flow rate (mL/min)
- P = Plasma concentration (mg/dL)
- Inulin Clearance (Gold Standard GFR)
- Freely filtered, not reabsorbed or secreted
- Clearance = 120 mL/min = actual GFR
- Requires continuous infusion
- 100% accuracy for GFR measurement
- Creatinine Clearance (Clinical Standard)
- Overestimates GFR by 10-20% (tubular secretion)
- Normal: 90-120 mL/min/1.73m²
- 24-hour urine collection required
- Formula: (Urine creatinine × Volume) / (Plasma creatinine × 1440 minutes)
- PAH Clearance (Renal Plasma Flow)
- 90% extraction ratio at low concentrations
- Effective RPF = 650 mL/min
- True RPF = 720 mL/min (correcting for 10% non-extracted)
- Measures tubular secretory function
| Substance | Normal Clearance | Filtration | Reabsorption | Secretion | Clinical Use |
|---|---|---|---|---|---|
| Inulin | 120 mL/min | 100% | 0% | 0% | True GFR measurement |
| Creatinine | 130 mL/min | 100% | 0% | 10-20% | Clinical GFR estimate |
| Urea | 60 mL/min | 100% | 50% | 0% | Concentration ability |
| Glucose | 0 mL/min | 100% | 100% | 0% | Reabsorption capacity |
| PAH | 650 mL/min | 100% | 0% | 90% | Renal plasma flow |
Estimated GFR equations eliminate urine collection requirements:
- Cockcroft-Gault: (140-age) × weight / (72 × creatinine) × 0.85 (if female)
- MDRD: Complex equation using creatinine, age, race, gender
- CKD-EPI: Most accurate for GFR >60 mL/min/1.73m²
- Accuracy: ±30% of measured clearance in 90% of patients
💡 Master This: Creatinine clearance overestimates GFR because 10-20% of urinary creatinine comes from tubular secretion via OCT2 and MATE1 transporters, blocked by cimetidine and trimethoprim, causing artificial GFR decline.
Understanding clearance principles predicts drug dosing: medications with renal clearance >30% require dose adjustment when GFR falls below 60 mL/min/1.73m², with linear dose reduction proportional to GFR decline.
🔄 Clearance Calculations: The Quantitative Precision Engine
🎯 Clinical Integration Matrix: Pathophysiology Pattern Recognition
📌 Remember: RAPID-DX - Ratio analysis (BUN/Cr), Acute vs chronic timeline, Pre/intrinsic/post classification, Imaging for obstruction, Drug history review, Dipstick proteinuria, X-ray for size/stones
Pre-Renal Azotemia (Functional GFR Reduction):
- BUN/Creatinine ratio: >20:1 (normal 10-15:1)
- Fractional excretion of sodium (FENa): <1%
- Urine osmolality: >500 mOsm/kg
- Response to volume: GFR improves >25% with fluid
- Mechanism: Preserved tubular function with reduced perfusion
Intrinsic Renal Disease (Structural GFR Loss):
- BUN/Creatinine ratio: 10-15:1 (proportional rise)
- FENa: >2% (tubular dysfunction)
- Proteinuria: >300 mg/day (glomerular disease)
- Hematuria: RBC casts (glomerulonephritis)
- Recovery: Limited, depends on reversibility
Post-Renal Obstruction (Mechanical GFR Blockade):
- Hydronephrosis: Bilateral or solitary kidney
- Bladder distension: >400 mL residual volume
- Creatinine response: Rapid improvement with relief
- Timeline: Hours to days for reversibility
- Imaging: Ultrasound 95% sensitive for obstruction
| Parameter | Pre-Renal | Intrinsic Renal | Post-Renal | Clinical Significance |
|---|---|---|---|---|
| BUN/Cr Ratio | >20:1 | 10-15:1 | Variable | Distinguishes functional vs structural |
| FENa | <1% | >2% | Variable | Tubular function assessment |
| Urine Osmolality | >500 mOsm/kg | <350 mOsm/kg | Variable | Concentrating ability |
| Proteinuria | Minimal | Often >300 mg/day | Minimal | Glomerular integrity |
| Response to Volume | Rapid improvement | No improvement | No improvement | Functional reserve |
Chronic Kidney Disease Staging based on GFR:
- Stage 1: GFR >90 with kidney damage
- Stage 2: GFR 60-89 with kidney damage
- Stage 3a: GFR 45-59 (moderate decrease)
- Stage 3b: GFR 30-44 (moderate-severe decrease)
- Stage 4: GFR 15-29 (severe decrease)
- Stage 5: GFR <15 or dialysis (kidney failure)
💡 Master This: GFR decline >25% from baseline within 48 hours defines acute kidney injury regardless of absolute value, while sustained GFR <60 mL/min/1.73m² for >3 months defines chronic kidney disease requiring nephrology referral.
Drug-Induced GFR Changes follow predictable patterns: ACE inhibitors cause 10-20% decline (acceptable), NSAIDs reduce GFR via afferent constriction (reversible), while aminoglycosides cause tubular necrosis with FENa >2% and granular casts.
🎯 Clinical Integration Matrix: Pathophysiology Pattern Recognition
🏆 GFR Mastery Arsenal: Clinical Command Tools
📌 Remember: MASTER-GFR - Measurement accuracy (±10%), Assessment timeline (<24 hours), Staging precision (CKD 1-5), Trend monitoring (>25% change), Etiology classification (pre/intrinsic/post), Referral thresholds (GFR <30), Guideline adherence (KDIGO), Follow-up intervals, Risk stratification
Essential Clinical Thresholds:
- Normal GFR: >90 mL/min/1.73m² (age-adjusted)
- Nephrology referral: GFR <30 mL/min/1.73m²
- Drug dosing adjustment: GFR <60 mL/min/1.73m²
- Contrast precautions: GFR <45 mL/min/1.73m²
- Dialysis consideration: GFR <15 mL/min/1.73m²
- Transplant evaluation: GFR <20 mL/min/1.73m²
Rapid Assessment Protocol:
- Step 1: Calculate eGFR using CKD-EPI equation
- Step 2: Assess BUN/Creatinine ratio for pre-renal component
- Step 3: Check urinalysis for proteinuria and cellular elements
- Step 4: Review medication list for nephrotoxic agents
- Step 5: Determine acute vs chronic based on baseline values
- Step 6: Apply appropriate monitoring interval
| GFR Range | Monitoring Frequency | Key Actions | Medication Adjustments | Specialist Referral |
|---|---|---|---|---|
| >90 | Annual | Routine screening | None required | Not indicated |
| 60-89 | Every 6 months | Risk factor modification | Monitor nephrotoxic drugs | Consider if declining |
| 45-59 | Every 3 months | CKD education, bone health | Dose adjust >50% renal excretion | Recommended |
| 30-44 | Every 3 months | Anemia screening, acidosis | Dose adjust >30% renal excretion | Required |
| 15-29 | Monthly | Dialysis education | Avoid nephrotoxic drugs | Urgent referral |
| <15 | Weekly | Renal replacement therapy | Dialysis dosing protocols | Immediate referral |
Clinical Decision Algorithm:
💡 Master This: Creatinine doubling represents 50% GFR loss, tripling indicates 67% loss, and quadrupling means 75% loss - use these relationships for rapid mental calculations of functional kidney reserve during acute illness.
Understanding GFR mastery transforms clinical practice through systematic assessment, appropriate referral timing, and evidence-based interventions that optimize patient outcomes while preventing progression to end-stage renal disease.
🏆 GFR Mastery Arsenal: Clinical Command Tools
Practice Questions: Glomerular filtration
Test your understanding with these related questions
Which region of the nephron reabsorbs the highest percentage of filtered bicarbonate?













