Oxygen transport in blood US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Oxygen transport in blood. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Oxygen transport in blood US Medical PG Question 1: A research scientist attempts to understand the influence of carbon dioxide content in blood on its oxygen binding. The scientist adds carbon dioxide to dog blood and measures the uptake of oxygen in the blood versus oxygen pressure in the peripheral tissue. He notes in one dog that with the addition of carbon dioxide with a pressure of 90 mmHg, the oxygen pressure in the peripheral tissue rose from 26 to 33 mmHg. How can this phenomenon be explained?
- A. High partial pressure of CO2 in tissues decreases peripheral blood volume
- B. Binding of O2 to hemoglobin in lungs drives release of CO2 from hemoglobin
- C. High partial pressure of CO2 in tissues causes alkalemia, which is necessary for O2 unloading
- D. High partial pressure of CO2 in tissues facilitates O2 unloading in peripheral tissues (Correct Answer)
- E. The sum of the partial pressures of CO2 and O2 cannot exceed a known threshold in blood
Oxygen transport in blood Explanation: **High partial pressure of CO2 in tissues facilitates O2 unloading in peripheral tissues**
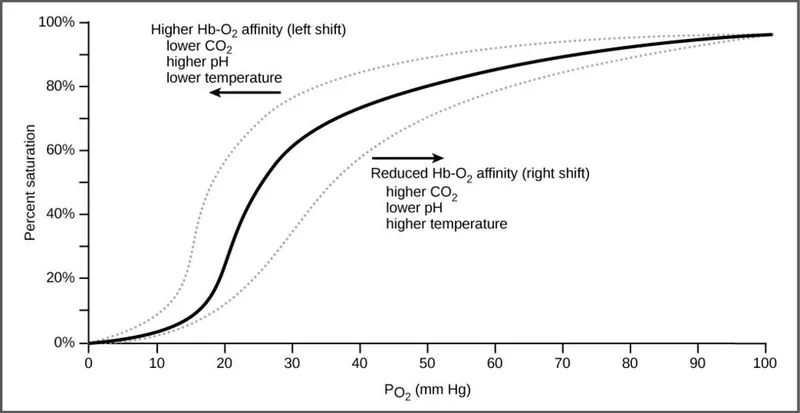
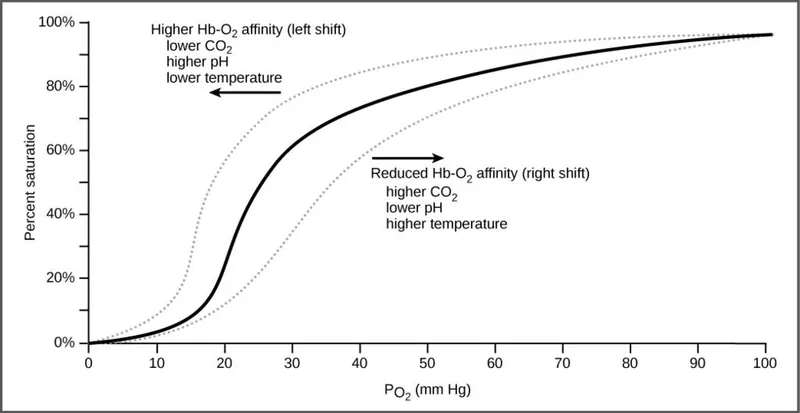
- An increase in **PCO2** leads to a decrease in pH (acidosis) in the tissues, which **decreases hemoglobin's affinity for oxygen**, promoting oxygen release.
- This phenomenon is known as the **Bohr effect**, where an acidic environment (from CO2) shifts the oxygen dissociation curve to the right, enhancing O2 unloading to meet tissue metabolic demands.
*High partial pressure of CO2 in tissues decreases peripheral blood volume*
- **Increased CO2** generally causes vasodilation in peripheral tissues, which would lead to an **increase**, not a decrease, in peripheral blood flow.
- Decreased blood volume is typically associated with conditions like hypovolemia or intense vasoconstriction, not elevated tissue CO2.
*Binding of O2 to hemoglobin in lungs drives release of CO2 from hemoglobin*
- This statement describes the **Haldane effect**, which occurs primarily in the lungs, where oxygen binding to hemoglobin facilitates the release of CO2.
- While true, it does not explain the **increased oxygen pressure in peripheral tissue** observed with added CO2, which is related to O2 unloading.
*High partial pressure of CO2 in tissues causes alkalemia, which is necessary for O2 unloading*
- High **PCO2** in tissues leads to the formation of carbonic acid and H+ ions, resulting in a **decrease in pH (acidosis)**, not alkalemia.
- **Acidosis** facilitates O2 unloading (Bohr effect), whereas alkalemia would increase hemoglobin's affinity for O2, inhibiting unloading.
*The sum of the partial pressures of CO2 and O2 cannot exceed a known threshold in blood*
- There is **no fixed threshold** for the sum of partial pressures of CO2 and O2 in the blood; these gases are independently regulated and their partial pressures fluctuate with metabolic activity.
- The partial pressure of a gas reflects its concentration and does not have an upper limit when considering the sum of different gases.
Oxygen transport in blood US Medical PG Question 2: A 44-year-old male immigrant presents to his primary care physician for a new patient visit. The patient reports chronic fatigue but states that he otherwise feels well. His past medical history is not known, and he is not currently taking any medications. The patient admits to drinking 7 alcoholic beverages per day and smoking 1 pack of cigarettes per day. His temperature is 99.4°F (37.4°C), blood pressure is 157/98 mmHg, pulse is 99/min, respirations are 18/min, and oxygen saturation is 100% on room air. Physical exam demonstrates mild pallor but is otherwise not remarkable. Laboratory studies are ordered as seen below.
Hemoglobin: 9 g/dL
Hematocrit: 33%
Leukocyte count: 6,500/mm^3 with normal differential
Platelet count: 190,000/mm^3
Mean corpuscular volume (MCV): 60 femtoliters
Free iron: 272 mcg/dL
Total iron binding capacity (TIBC): 175 mcg/dL
Ferritin: 526 ng/mL
Reticulocyte count: 2.8%
Which of the following is the most likely diagnosis?
- A. Folate deficiency
- B. Beta-thalassemia (Correct Answer)
- C. Iron deficiency
- D. B12 deficiency
- E. Hemolytic anemia
Oxygen transport in blood Explanation: ***Beta-thalassemia***
- The patient presents with **microcytic anemia** (MCV 60 fL) and **elevated ferritin**, **high free iron**, and **low TIBC**, which are characteristic of thalassemia due to ineffective erythropoiesis and iron overload.
- A **reticulocyte count of 2.8%** (elevated for the degree of anemia) indicates the bone marrow is attempting to compensate, consistent with a hemolytic process like thalassemia.
*Folate deficiency*
- Folate deficiency typically causes **macrocytic anemia** (elevated MCV), which is not seen here; the patient has microcytic anemia.
- Alcohol abuse can cause folate deficiency, but the lab values for iron studies and MCV are inconsistent with this diagnosis.
*Iron deficiency*
- Iron deficiency anemia would present with **low ferritin**, **low free iron**, and **high TIBC**, which are opposite to the patient's lab results.
- Although the patient has microcytic anemia, the iron study profile rules out iron deficiency.
*B12 deficiency*
- Vitamin B12 deficiency also causes **macrocytic anemia** (elevated MCV), often with neurological symptoms, neither of which are observed in this patient.
- The patient's microcytic anemia and iron study results contradict a diagnosis of B12 deficiency.
*Hemolytic anemia*
- While beta-thalassemia is a form of hemolytic anemia, the term "hemolytic anemia" alone is too broad and does not specify the underlying cause, especially with the provided iron studies and MCV.
- Other common causes of hemolytic anemia, like autoimmune hemolytic anemia or G6PD deficiency, would require different diagnostic presentations or specific tests not consistent with the given lab values.
Oxygen transport in blood US Medical PG Question 3: A 40-year-old female volunteers for an invasive study to measure her cardiac function. She has no previous cardiovascular history and takes no medications. With the test subject at rest, the following data is collected using blood tests, intravascular probes, and a closed rebreathing circuit:
Blood hemoglobin concentration 14 g/dL
Arterial oxygen content 0.22 mL O2/mL
Arterial oxygen saturation 98%
Venous oxygen content 0.17 mL O2/mL
Venous oxygen saturation 78%
Oxygen consumption 250 mL/min
The patient's pulse is 75/min, respiratory rate is 14/ min, and blood pressure is 125/70 mm Hg. What is the cardiac output of this volunteer?
- A. Body surface area is required to calculate cardiac output.
- B. Stroke volume is required to calculate cardiac output.
- C. 250 mL/min
- D. 5.0 L/min (Correct Answer)
- E. 50 L/min
Oxygen transport in blood Explanation: ***5.0 L/min***
- Cardiac output can be calculated using the **Fick principle**: Cardiac Output $(\text{CO}) = \frac{{\text{Oxygen Consumption}}}{{\text{Arterial } \text{O}_2 \text{ Content} - \text{Venous O}_2 \text{ Content}}}$.
- Given Oxygen Consumption = 250 mL/min, Arterial O$_2$ Content = 0.22 mL/mL, and Venous O$_2$ Content = 0.17 mL/mL. Thus, CO = $\frac{{250 \text{ mL/min}}}{{(0.22 - 0.17) \text{ mL } \text{O}_2/\text{mL blood}}} = \frac{{250 \text{ mL/min}}}{{0.05 \text{ mL } \text{O}_2/\text{mL blood}}} = 5000 \text{ mL/min } = 5.0 \text{ L/min}$.
*Body surface area is required to calculate cardiac output.*
- **Body surface area (BSA)** is used to calculate **cardiac index**, which is cardiac output normalized to body size, but not cardiac output directly.
- While a normal cardiac output might be compared to a patient's BSA for context, it is not a necessary component for calculating the absolute cardiac output.
*Stroke volume is required to calculate cardiac output.*
- Cardiac output can be calculated as **Stroke Volume (SV) x Heart Rate (HR)**. However, stroke volume is not provided directly in this question.
- The Fick principle allows for the calculation of cardiac output **without explicit knowledge of stroke volume** or heart rate, using oxygen consumption and arteriovenous oxygen difference.
*250 mL/min*
- 250 mL/min represents the **oxygen consumption**, not the cardiac output.
- Cardiac output is the volume of blood pumped by the heart per minute, which is influenced by both oxygen consumption and the difference in oxygen content between arterial and venous blood.
*50 L/min*
- A cardiac output of 50 L/min is an **extremely high and physiologically impossible** value for a resting individual.
- This value is 10 times higher than the calculated cardiac output and typically represents a calculation error.
Oxygen transport in blood US Medical PG Question 4: A 19-year-old male college student is brought to the emergency department by his girlfriend complaining of intense pain. They had been playing outside in the snow when the patient started to have severe hand and feet pain. He says the pain is 9 out of 10 and causing him to have trouble moving his fingers and toes. He also reports some difficulty “catching his breath.” He notes that he has been tiring easily for the past month but thought it was because he was studying and going out late. On physical examination, the patient appears uncomfortable. Bilateral conjunctivae are pale. His hands are swollen and tender to palpation. Cardiopulmonary examination is normal. Hemoglobin is 9.0 g/dL. An electrocardiogram shows mild sinus tachycardia. Hemoglobin electrophoresis is performed, which confirms sickle cell disease. The patient’s pain is managed, and he is discharged on hydroxyurea. Which of the following is the most likely to occur as a result of the new medication?
- A. Increase in hemoglobin with higher oxygen affinity
- B. Decrease in hemoglobin with higher oxygen affinity
- C. Increase in hemoglobin A
- D. Decrease in hemoglobin A
- E. Increase in fetal hemoglobin (Correct Answer)
Oxygen transport in blood Explanation: ***Increase in fetal hemoglobin***
- **Hydroxyurea** stimulates the production of **fetal hemoglobin (HbF)**, which reduces the polymerization of **hemoglobin S (HbS)** and sickling of red blood cells.
- Increased HbF improves red blood cell survival and reduces the frequency of **vaso-occlusive crises** and other complications in **sickle cell disease**.
*Increase in hemoglobin with higher oxygen affinity*
- This option is too vague and does not describe the specific mechanism of hydroxyurea.
- While **HbF** does have higher oxygen affinity than **HbA**, the therapeutic benefit comes specifically from **increasing HbF**, not from a general increase in hemoglobin with higher oxygen affinity.
- The key mechanism is **HbF preventing sickling**, not simply having higher oxygen affinity.
*Decrease in hemoglobin with higher oxygen affinity*
- Hydroxyurea aims to *increase* functional hemoglobin and reduce anemia, not decrease it.
- A *decrease* in total hemoglobin would be detrimental and is not a therapeutic effect of hydroxyurea.
*Increase in hemoglobin A*
- Patients with **sickle cell disease** produce little to no **hemoglobin A (HbA)**, as their beta-globin genes produce **hemoglobin S (HbS)**.
- Hydroxyurea does not induce the production of **HbA**; its mechanism of action is through the upregulation of **HbF**.
*Decrease in hemoglobin A*
- Since patients with **sickle cell disease** already have an absence or very low levels of **hemoglobin A (HbA)**, a further decrease is not a relevant therapeutic effect.
- Hydroxyurea's action is to increase **fetal hemoglobin (HbF)**, which acts as a protective factor against sickling.
Oxygen transport in blood US Medical PG Question 5: A 48-year-old man is brought to the emergency department 20 minutes after being rescued from a house fire. He reports headache, metallic taste, abdominal pain, and nausea. He appears confused and agitated. His pulse is 125/min, respirations are 33/min, and blood pressure is 100/65 mm Hg. Pulse oximetry on room air shows an oxygen saturation of 98%. Physical examination shows a bright red color of the skin. His breath smells of bitter almonds. Hyperbaric oxygen therapy and appropriate pharmacotherapy are initiated. The expected beneficial effect of this drug is most likely due to which of the following mechanisms?
- A. Synthesis of 2,3-bisphosphoglycerate
- B. Formation of methemoglobin (Correct Answer)
- C. Inhibition of cytochrome c oxidase
- D. Dissociation of carboxyhemoglobin
- E. Reduction of ferric iron
Oxygen transport in blood Explanation: ***Formation of methemoglobin***
- This patient's symptoms (headache, confusion, bright red skin, bitter almond breath, high pulse oximetry despite severe symptoms) are classic for **cyanide poisoning**.
- Many antidotes for cyanide poisoning, such as **nitrites**, work by forming **methemoglobin**, which has a higher affinity for cyanide than cytochrome c oxidase, thus detaching cyanide from the enzyme and allowing cellular respiration to resume.
*Synthesis of 2,3-bisphosphoglycerate*
- **2,3-bisphosphoglycerate (2,3-BPG)** helps regulate oxygen release from hemoglobin in red blood cells.
- While important for oxygen delivery, increasing 2,3-BPG is not a direct therapeutic mechanism for **cyanide poisoning**.
*Inhibition of cytochrome c oxidase*
- **Cyanide** itself inhibits cytochrome c oxidase, leading to cellular hypoxia despite adequate oxygen supply.
- The therapeutic goal is to reverse this inhibition, not to further inhibit the enzyme.
*Dissociation of carboxyhemoglobin*
- **Carbon monoxide poisoning**, not cyanide poisoning, causes carboxyhemoglobin formation and presents with cherry-red skin, but there is no foul-smelling breath.
- Dissociating carboxyhemoglobin is relevant for carbon monoxide poisoning, not cyanide poisoning.
*Reduction of ferric iron*
- Reducing ferric iron (Fe3+) back to ferrous iron (Fe2+) would reverse **methemoglobinemia**, which is often a side effect of some cyanide antidotes.
- The therapeutic strategy for cyanide poisoning involves *inducing* methemoglobinemia to sequester cyanide.
Oxygen transport in blood US Medical PG Question 6: Which factor most strongly influences coronary blood flow during exercise?
- A. Endothelin release
- B. Metabolic demand (Correct Answer)
- C. Myogenic response
- D. Neural regulation
- E. Baroreceptor reflex
Oxygen transport in blood Explanation: **Metabolic demand**
- During exercise, increased **myocardial activity** leads to a higher demand for oxygen and nutrients, prompting a significant increase in coronary blood flow.
- Local release of **metabolites** such as adenosine, nitric oxide, and hydrogen ions causes powerful vasodilation of coronary arteries, closely matching blood supply to demand.
*Endothelin release*
- **Endothelin** is a potent vasoconstrictor and plays a role in regulating vascular tone, but its primary influence is not the immediate or strongest factor dictating increased coronary flow during exercise.
- While it can modulate flow, metabolic changes are the dominant driver for the rapid and substantial increases needed during exertion.
*Myogenic response*
- The **myogenic response** is an intrinsic property of vascular smooth muscle cells to contract when stretched (due to increased pressure) and relax when pressure decreases, helping to maintain relatively constant blood flow.
- This mechanism primarily contributes to **autoregulation** and flow stability, but it does not account for the massive increase in flow required by the heart during exercise.
*Neural regulation*
- **Neural regulation**, primarily sympathetic stimulation, increases heart rate and contractility, which indirectly increases metabolic demand.
- However, direct neural effects on coronary arteries can be complex (both vasodilation and vasoconstriction depending on receptor type), and the overriding control during exercise is typically metabolic.
Oxygen transport in blood US Medical PG Question 7: A 63-year-old man presents to the clinic with fever accompanied by shortness of breath. The symptoms developed a week ago and have been progressively worsening over the last 2 days. He reports his cough is productive of thick, yellow sputum. He was diagnosed with chronic obstructive pulmonary disease 3 years ago and has been on treatment ever since. He quit smoking 10 years ago but occasionally experiences shortness of breath along with chest tightness that improves with the use of an inhaler. However, this time the symptoms seem to be more severe and unrelenting. His temperature is 38.6°C (101.4°F), the respirations are 21/min, the blood pressure is 100/60 mm Hg, and the pulse is 105/min. Auscultation reveals bilateral crackles and expiratory wheezes. His oxygen saturation is 95% on room air. According to this patient’s history, which of the following should be the next step in the management of this patient?
- A. Chest X-ray (Correct Answer)
- B. Arterial blood gases
- C. Bronchoprovocation test
- D. Bronchoscopy
- E. CT scan
Oxygen transport in blood Explanation: ***Chest X-ray***
- A **chest X-ray** is a crucial initial step to evaluate for **pneumonia** or other acute pulmonary processes, given the fever, productive cough, and worsening respiratory symptoms in a patient with COPD [1].
- It can identify infiltrates, effusions, or other anatomical changes that explain the patient's acute decompensation [1].
*Arterial blood gases*
- While important for assessing **respiratory failure** and guiding ventilator management, **ABGs** are usually performed after initial imaging to quantify gas exchange abnormalities once an etiology is suspected [1].
- The patient's **oxygen saturation of 95% on room air** does not immediately suggest severe hypoxemia, although hypercapnia could still be present.
*Bronchoprovocation test*
- A **bronchoprovocation test** is used to diagnose **asthma** or assess **airway hyperresponsiveness** in stable patients.
- It is contraindicated in acute exacerbations due to the risk of worsening bronchoconstriction.
*Bronchoscopy*
- **Bronchoscopy** is an invasive procedure typically reserved for cases of suspicion of **tumor**, **foreign body aspiration**, or non-resolving infiltrates and would not be the immediate next step for fever and productive cough.
- It is not indicated for the initial diagnosis of community-acquired pneumonia or COPD exacerbation.
*CT scan*
- A **CT scan** provides more detailed imaging but is usually reserved for cases where the chest X-ray is inconclusive or to look for specific pathologies like **pulmonary embolism** or **bronchiectasis**.
- It's not the initial imaging choice for suspected **pneumonia** due to cost, radiation exposure, and the adequacy of X-ray for this purpose [1].
Oxygen transport in blood US Medical PG Question 8: A 50-year-old man presents to the urgent care clinic for 3 hours of worsening cough, shortness of breath, and dyspnea. He works as a long-haul truck driver, and he informs you that he recently returned to the west coast from a trip to Arkansas. His medical history is significant for gout, hypertension, hypercholesterolemia, diabetes mellitus type 2, chronic obstructive pulmonary disease (COPD), and mild intellectual disability. He currently smokes 1 pack of cigarettes/day, drinks a 6-pack of beer/day, and he endorses a past history of injection drug use but currently denies any illicit drug use. The vital signs include: temperature 36.7°C (98.0°F), blood pressure 126/74 mm Hg, heart rate 87/min, and respiratory rate 23/min. His physical examination shows mild, bilateral, coarse rhonchi, but otherwise clear lungs on auscultation, grade 2/6 holosystolic murmur, and a benign abdominal physical examination. He states that he ran out of his albuterol inhaler 6 days ago and has been meaning to follow-up with his primary care physician (PCP) for a refill. Complete blood count (CBC) and complete metabolic panel are within normal limits. He also has a D-dimer result within normal limits. Which of the following is the most appropriate next step in evaluation?
- A. Chest computed tomography (CT) with contrast
- B. Chest radiographs (Correct Answer)
- C. Pulmonary function tests
- D. Sputum gram stain and culture
- E. Arterial blood gas
Oxygen transport in blood Explanation: ***Chest radiographs***
- A **chest X-ray** is the most appropriate initial imaging study for evaluating acute respiratory symptoms in a patient with a history of COPD and recent exacerbating factors (running out of albuterol). It can help identify common causes like **pneumonia**, **pneumothorax**, or **acute exacerbation of COPD**.
- The patient's presentation with worsening cough, shortness of breath, and dyspnea, particularly in the context of running out of his albuterol inhaler, suggests a primary pulmonary issue that a chest X-ray can quickly assess.
*Chest computed tomography (CT) with contrast*
- A **chest CT with contrast** is more detailed but not the initial diagnostic study in this scenario, especially with a normal D-dimer ruling out pulmonary embolism as a high probability.
- It exposes the patient to **higher radiation** and risks associated with contrast, making it less suitable as a first-line investigation unless the chest X-ray is inconclusive or more specific findings are suspected.
*Pulmonary function tests*
- **Pulmonary function tests (PFTs)** are used to diagnose and monitor chronic lung conditions like COPD, but they are generally not performed in an acute urgent care setting for patients presenting with acute respiratory distress.
- PFTs require patient cooperation and are designed to assess baseline lung function, not to identify the **acute cause** of respiratory decompensation.
*Sputum gram stain and culture*
- A **sputum gram stain and culture** might be considered if there's strong suspicion of a bacterial infection (e.g., fever, purulent sputum), but the patient's current symptoms are more aligned with a COPD exacerbation or other acute pulmonary issue.
- Without clear signs of bacterial infection, this test is **not the most immediate or appropriate first step** in evaluating acute dyspnea, as it requires time for results and may delay more crucial diagnostic steps.
*Arterial blood gas*
- An **arterial blood gas (ABG)** can provide information on oxygenation, ventilation, and acid-base status, which is useful in assessing the severity of respiratory failure.
- However, it's typically ordered after an initial clinical and imaging assessment to quantify the physiological impact of the respiratory distress, rather than being the **very first diagnostic step** to identify the cause.
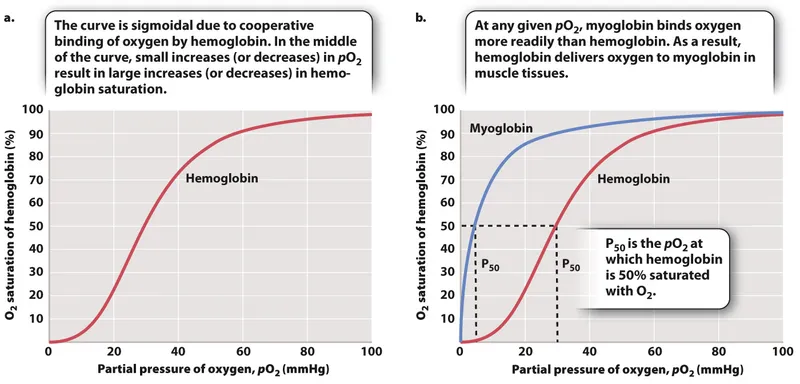
Oxygen transport in blood US Medical PG Question 9: An investigator is conducting a study on hematological factors that affect the affinity of hemoglobin for oxygen. An illustration of two graphs (A and B) that represent the affinity of hemoglobin for oxygen is shown. Which of the following best explains a shift from A to B?
- A. Decreased serum pCO2
- B. Increased serum pH
- C. Decreased serum 2,3-bisphosphoglycerate concentration
- D. Increased body temperature (Correct Answer)
- E. Increased hemoglobin γ-chain synthesis
Oxygen transport in blood Explanation: ***Increased body temperature***
- A shift from A to B represents a **rightward shift** of the oxygen-hemoglobin dissociation curve, indicating **decreased hemoglobin affinity for oxygen**.
- **Increased body temperature** (e.g., during exercise, fever) reduces hemoglobin's affinity for oxygen, facilitating **oxygen release to tissues**.
*Decreased serum pCO2*
- A **decrease in serum pCO2** leads to an **increase in pH** (alkalosis) and a **leftward shift** of the curve, meaning an increased affinity of hemoglobin for oxygen.
- This is part of the **Bohr effect**, where lower CO2 levels signal decreased tissue metabolic activity, thus reducing oxygen unloading.
*Increased serum pH*
- An **increase in serum pH** (alkalosis) causes a **leftward shift** of the oxygen-hemoglobin dissociation curve, signifying **increased hemoglobin affinity for oxygen**.
- This response is beneficial in the lungs, where higher pH promotes oxygen binding to hemoglobin.
*Decreased serum 2,3-bisphosphoglycerate concentration*
- A **decrease in 2,3-BPG** concentration leads to a **leftward shift** of the curve, representing **increased hemoglobin affinity for oxygen**.
- 2,3-BPG typically binds to deoxyhemoglobin, stabilizing its T-state and promoting oxygen release; thus, less 2,3-BPG means less release.
*Increased hemoglobin γ-chain synthesis*
- Increased **hemoglobin γ-chain synthesis** is characteristic of **fetal hemoglobin (HbF)**, which has a **higher affinity for oxygen** than adult hemoglobin (HbA).
- This would result in a **leftward shift** of the oxygen-hemoglobin dissociation curve, enhancing oxygen uptake by the fetus.
Oxygen transport in blood US Medical PG Question 10: A 32-year-old female with Crohn's disease diagnosed in her early 20s comes to your office for a follow-up appointment. She is complaining of headaches and fatigue. Which of the following arterial blood gas findings might you expect?
- A. High PaO2, normal O2 saturation (SaO2), normal O2 content (CaO2)
- B. Low PaO2, low O2 saturation (SaO2), low O2 content (CaO2)
- C. Normal PaO2, normal O2 saturation (SaO2), normal O2 content (CaO2)
- D. Normal PaO2, normal O2 saturation (SaO2), low O2 content (CaO2) (Correct Answer)
- E. Low PaO2, normal O2 saturation (SaO2), normal O2 content (CaO2)
Oxygen transport in blood Explanation: ***Normal PaO2, normal O2 saturation (SaO2), low O2 content (CaO2)***
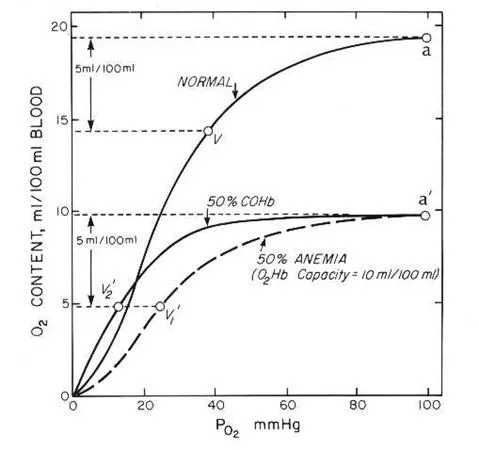
- Patients with **Crohn's disease** are prone to developing **iron deficiency anemia** due to chronic inflammation, malabsorption, and blood loss, leading to reduced hemoglobin levels.
- While PaO2 and SaO2 measure oxygen *tension* and *percentage saturation* of available hemoglobin, respectively, **O2 content (CaO2)** directly reflects the *total amount* of oxygen delivered to tissues, which is primarily dependent on hemoglobin concentration. Therefore, with anemia, CaO2 will be low despite normal PaO2 and SaO2 because there is less hemoglobin to carry oxygen.
*High PaO2, normal O2 saturation (SaO2), normal O2 content (CaO2)*
- High PaO2 would indicate **hyperoxygenation**, which is not an expected complication of Crohn's disease or its associated anemia.
- Normal O2 content is inconsistent with the presence of anemia, which significantly reduces the body's total oxygen-carrying capacity.
*Low PaO2, low O2 saturation (SaO2), low O2 content (CaO2)*
- Low PaO2 and SaO2 suggest a primary **respiratory problem** or severe hypoxemia, which is not directly linked to Crohn's disease or the typical presentation of iron deficiency anemia.
- While low O2 content is correct for anemia, the accompanying low PaO2 and SaO2 indicate a different underlying pathology for oxygen transport issues.
*Normal PaO2, normal O2 saturation (SaO2), normal O2 content (CaO2)*
- This finding would indicate **normal oxygenation** and oxygen-carrying capacity, which is contrary to the clinical scenario of a patient with Crohn's likely complicated by anemia.
- The patient's symptoms of headaches and fatigue are consistent with poor tissue oxygenation, which would not occur if all these parameters were normal.
*Low PaO2, normal O2 saturation (SaO2), normal O2 content (CaO2)*
- A low PaO2 with a normal SaO2 is physiologically unlikely unless there is a **left shift of the oxygen dissociation curve** with adequate hemoglobin, which doesn't fit the expected anemic state.
- Normal O2 content would rule out the presence of anemia as a cause for the symptoms, which is a common complication in Crohn's disease.
More Oxygen transport in blood US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.