Oxygen-hemoglobin dissociation curve US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Oxygen-hemoglobin dissociation curve. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Oxygen-hemoglobin dissociation curve US Medical PG Question 1: A 33-year-old woman is brought to the emergency department 30 minutes after being rescued from a fire in her apartment. She reports nausea, headache, and dizziness. Physical examination shows black discoloration of her oral mucosa. Pulse oximetry shows an oxygen saturation of 99% on room air. The substance most likely causing symptoms in this patient primarily produces toxicity by which of the following mechanisms?
- A. Inhibition of mitochondrial complex V
- B. Degradation of 2,3-bisphosphoglycerate
- C. Oxidation of Fe2+
- D. Rise in serum pH
- E. Competitive binding to heme (Correct Answer)
Oxygen-hemoglobin dissociation curve Explanation: ***Competitive binding to heme***
- The patient's symptoms (nausea, headache, dizziness, black oral mucosa) and history of being rescued from a fire strongly suggest **carbon monoxide (CO) poisoning** [1].
- **Carbon monoxide** primarily exerts its toxicity by competitively binding to the **heme iron** in hemoglobin with an affinity 200-250 times greater than oxygen, forming **carboxyhemoglobin (COHb)** and displacing oxygen [2].
*Inhibition of mitochondrial complex V*
- **Cyanide poisoning** inhibits **mitochondrial complex IV (cytochrome c oxidase)**, not complex V, leading to impaired cellular respiration.
- While both cyanide and CO poisoning can occur in fires, CO is more common due to incomplete combustion, and the specific presentation points toward CO.
*Degradation of 2,3-bisphosphoglycerate*
- **2,3-BPG** is an important regulator of oxygen affinity for hemoglobin, promoting oxygen release to tissues [2]. Its degradation would increase hemoglobin's affinity for oxygen, thus reducing oxygen unloading, but this is not the primary mechanism of toxicity for CO or common fire-related toxins.
- No common toxin directly causes widespread degradation of 2,3-BPG as its primary mechanism of acute toxicity or symptoms.
*Oxidation of Fe2+*
- The oxidation of **ferrous iron (Fe2+)** to **ferric iron (Fe3+)** in hemoglobin leads to the formation of **methemoglobin**, which cannot bind oxygen. This occurs in **methemoglobinemia** induced by certain drugs or toxins (e.g., nitrites, dapsone).
- While **methemoglobinemia** impairs oxygen transport, it does not explain the black oral mucosa or the strong association with fire smoke toxicity in the context of CO.
*Rise in serum pH*
- A rise in serum pH (alkalosis) is not a direct or primary mechanism of toxicity for common fire-related toxins like carbon monoxide or cyanide.
- Most severe forms of toxicity, including CO and cyanide poisoning, tend to cause **lactic acidosis** due to cellular hypoxia and anaerobic metabolism, leading to a
**decrease** in serum pH.
Oxygen-hemoglobin dissociation curve US Medical PG Question 2: A 2-day-old boy is examined on day of discharge from the newborn nursery. He was born at 39 weeks by vaginal delivery to a primigravid mother. The pregnancy and delivery were uncomplicated, and the baby has been stooling, urinating, and feeding normally. Both the patient’s mother and father have no known past medical history and are found to have normal hemoglobin electrophoresis results. Compared to adult hemoglobin, the infant’s predominant hemoglobin is most likely to exhibit which of the following properties?
- A. Decreased affinity for 2,3-bisphosphoglycerate (Correct Answer)
- B. More likely to form hexagonal crystals
- C. More likely to cause red blood cell sickling
- D. Lower affinity for binding oxygen
- E. Increased affinity for 2,3-bisphosphoglycerate
Oxygen-hemoglobin dissociation curve Explanation: ***Decreased affinity for 2,3-bisphosphoglycerate***
- The baby's predominant hemoglobin is **hemoglobin F (HbF)**, which has a **gamma globin subunit** instead of the beta globin subunit found in adult hemoglobin (HbA).
- The gamma subunit of HbF results in a **reduced binding affinity to 2,3-bisphosphoglycerate (2,3-BPG)**, which in turn leads to a **higher affinity for oxygen** and more efficient oxygen transfer from the mother to the fetus.
*More likely to form hexagonal crystals*
- The formation of **hexagonal crystals** is characteristic of **hemoglobin C (HbC)** disease, a variant of adult hemoglobin, which is not predominant in a newborn.
- The parents have normal hemoglobin electrophoresis, ruling out the inheritance of significant hemoglobinopathies like HbC in a homozygous or compound heterozygous state.
*More likely to cause red blood cell sickling*
- **Red blood cell sickling** is a hallmark of **sickle cell anemia**, caused by hemoglobin S (HbS) which is an abnormal adult hemoglobin, not fetal hemoglobin.
- The parents have normal hemoglobin electrophoresis, meaning they are unlikely to carry the sickle cell trait, and the newborn's predominant HbF actually protects against sickling.
*Lower affinity for binding oxygen*
- HbF in newborns has a **higher affinity for oxygen** than adult hemoglobin (HbA) to facilitate efficient oxygen extraction from maternal blood across the placenta.
- A lower affinity for oxygen would be detrimental for a newborn as it would impair proper tissue oxygenation.
*Increased affinity for 2,3-bisphosphoglycerate*
- HbF has a **decreased affinity for 2,3-BPG**. An increased affinity for 2,3-BPG would lead to a reduction in oxygen binding affinity, which is the opposite of the physiological need in a newborn.
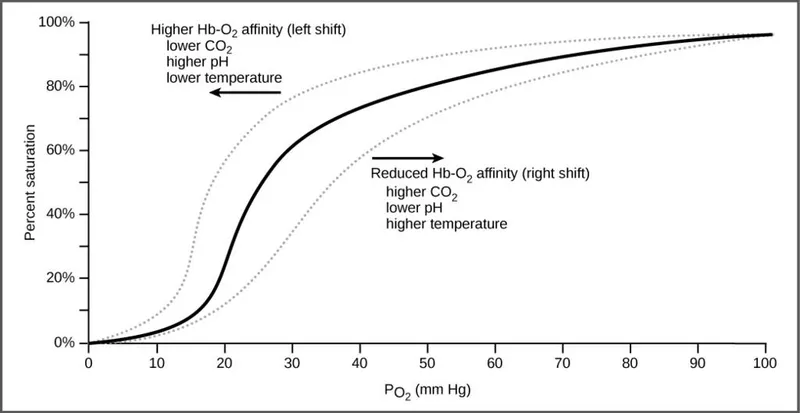
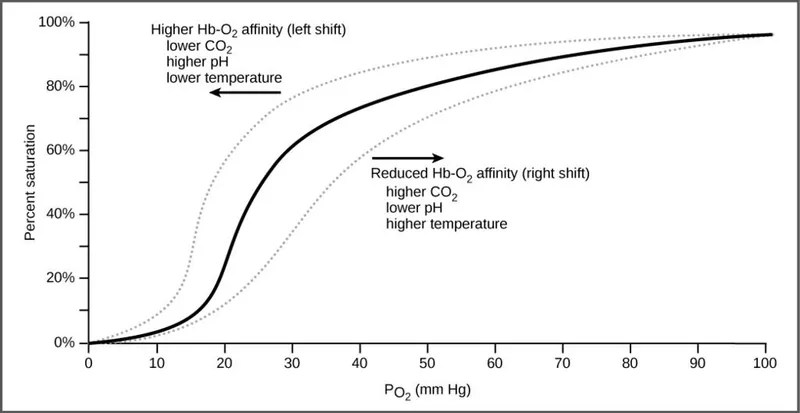
Oxygen-hemoglobin dissociation curve US Medical PG Question 3: An investigator is conducting a study on hematological factors that affect the affinity of hemoglobin for oxygen. An illustration of two graphs (A and B) that represent the affinity of hemoglobin for oxygen is shown. Which of the following best explains a shift from A to B?
- A. Decreased serum pCO2
- B. Increased serum pH
- C. Decreased serum 2,3-bisphosphoglycerate concentration
- D. Increased body temperature (Correct Answer)
- E. Increased hemoglobin γ-chain synthesis
Oxygen-hemoglobin dissociation curve Explanation: ***Increased body temperature***
- A shift from A to B represents a **rightward shift** of the oxygen-hemoglobin dissociation curve, indicating **decreased hemoglobin affinity for oxygen**.
- **Increased body temperature** (e.g., during exercise, fever) reduces hemoglobin's affinity for oxygen, facilitating **oxygen release to tissues**.
*Decreased serum pCO2*
- A **decrease in serum pCO2** leads to an **increase in pH** (alkalosis) and a **leftward shift** of the curve, meaning an increased affinity of hemoglobin for oxygen.
- This is part of the **Bohr effect**, where lower CO2 levels signal decreased tissue metabolic activity, thus reducing oxygen unloading.
*Increased serum pH*
- An **increase in serum pH** (alkalosis) causes a **leftward shift** of the oxygen-hemoglobin dissociation curve, signifying **increased hemoglobin affinity for oxygen**.
- This response is beneficial in the lungs, where higher pH promotes oxygen binding to hemoglobin.
*Decreased serum 2,3-bisphosphoglycerate concentration*
- A **decrease in 2,3-BPG** concentration leads to a **leftward shift** of the curve, representing **increased hemoglobin affinity for oxygen**.
- 2,3-BPG typically binds to deoxyhemoglobin, stabilizing its T-state and promoting oxygen release; thus, less 2,3-BPG means less release.
*Increased hemoglobin γ-chain synthesis*
- Increased **hemoglobin γ-chain synthesis** is characteristic of **fetal hemoglobin (HbF)**, which has a **higher affinity for oxygen** than adult hemoglobin (HbA).
- This would result in a **leftward shift** of the oxygen-hemoglobin dissociation curve, enhancing oxygen uptake by the fetus.
Oxygen-hemoglobin dissociation curve US Medical PG Question 4: A 34-year-old woman comes to a physician for a routine health maintenance examination. She moved to Denver 1 week ago after having lived in New York City all her life. She has no history of serious illness and takes no medications. Which of the following sets of changes is most likely on analysis of a blood sample obtained now compared to prior to her move?
Erythropoietin level | O2 saturation | Plasma volume
- A. ↑ unchanged unchanged
- B. ↑ ↓ ↓ (Correct Answer)
- C. Unchanged ↓ unchanged
- D. ↓ unchanged ↑
- E. Unchanged unchanged ↓
Oxygen-hemoglobin dissociation curve Explanation: ***↑ ↓ ↓***
- Moving to a high altitude like Denver (from sea level NYC) leads to **hypoxia**, which triggers increased **erythropoietin (EPO)** production to stimulate red blood cell formation.
- The immediate physiological response to high altitude is a **decrease in arterial PO2** and thus **oxygen saturation**, along with a **reduction in plasma volume** due to increased diuresis and fluid shifts.
*↑ unchanged unchanged*
- While **erythropoietin** would increase due to hypoxia at higher altitudes, **oxygen saturation** would decrease, not remain unchanged.
- **Plasma volume** also tends to decrease acutely at high altitudes, rather than staying unchanged.
*Unchanged ↓ unchanged*
- **Erythropoietin** would be expected to increase, not remain unchanged, as a compensatory mechanism to hypoxia.
- While **oxygen saturation** would decrease, **plasma volume** typically decreases acutely, not remaining unchanged.
*↓ unchanged ↑*
- **Erythropoietin** would increase, not decrease, in response to the lower atmospheric oxygen.
- Both **oxygen saturation** and **plasma volume** would decrease, not remain unchanged or increase, respectively.
*Unchanged unchanged ↓*
- **Erythropoietin** would increase, not remain unchanged, to stimulate red blood cell production in response to hypoxia.
- **Oxygen saturation** would decrease, not remain unchanged, at higher altitudes.
Oxygen-hemoglobin dissociation curve US Medical PG Question 5: A man returns home late at night to find his 15-year-old son and 40-year-old wife unconscious in the family room. He immediately summons emergency services. In the field, pulse oximetry shows oxygen saturation at 100% for both patients. 100%, yet they both appear cyanotic. Both patients are provided with 2L of oxygen by way of nasal cannula on the way to the hospital. An arterial blood gas is performed on the teenager and reveals pH of 7.35, PaCO2 of 31.8 mm Hg, PaO2 of 150 mm Hg, HCO3- of 20 mEq/L, SaO2 of 80%, and a COHb of 18%. What is the most likely cause of his condition?
- A. Ischemic hypoxia
- B. Methemoglobinemia
- C. Diffusion-limited hypoxia
- D. Carbon monoxide poisoning (Correct Answer)
- E. Anemic hypoxia
Oxygen-hemoglobin dissociation curve Explanation: ***Carbon monoxide poisoning***
- The combination of **cyanosis**, **normal pulse oximetry readings (due to inaccurate readings for CO)**, and a **high COHb level (18%)** is highly indicative of carbon monoxide poisoning.
- Exposure to carbon monoxide forms **carboxyhemoglobin (COHb)**, which has a higher affinity for hemoglobin than oxygen, leading to **tissue hypoxia** despite normal PaO2.
*Ischemic hypoxia*
- This type of hypoxia occurs when there is **reduced blood flow** to a tissue, often due to conditions like **heart failure, shock**, or **arterial occlusion**.
- There is no clinical or lab evidence in the scenario to suggest reduced blood flow as the primary cause of the patient's symptoms.
*Methemoglobinemia*
- While methemoglobinemia can also cause **cyanosis** and an **oxygen saturation gap** (discrepancy between SaO2 and pulse oximetry), it is characterized by a high level of **methemoglobin (MetHb)**.
- The patient's COHb level is elevated at 18%, but there's no information suggesting elevated MetHb, distinguishing it from carbon monoxide poisoning.
*Diffusion-limited hypoxia*
- This occurs when the **diffusion of oxygen from the alveoli to the blood is impaired**, as seen in conditions like **pulmonary fibrosis** or **severe emphysema**.
- The patient's PaO2 of 150 mmHg is very high, indicating excellent oxygen loading in the lungs, which rules out a diffusion limitation.
*Anemic hypoxia*
- Anemic hypoxia results from a **decreased oxygen-carrying capacity of the blood** due to a **low hemoglobin concentration**.
- The scenario does not provide information about the patient's hemoglobin level, and the primary issue is the inability of hemoglobin to release oxygen due to CO binding, not a lack of hemoglobin itself.
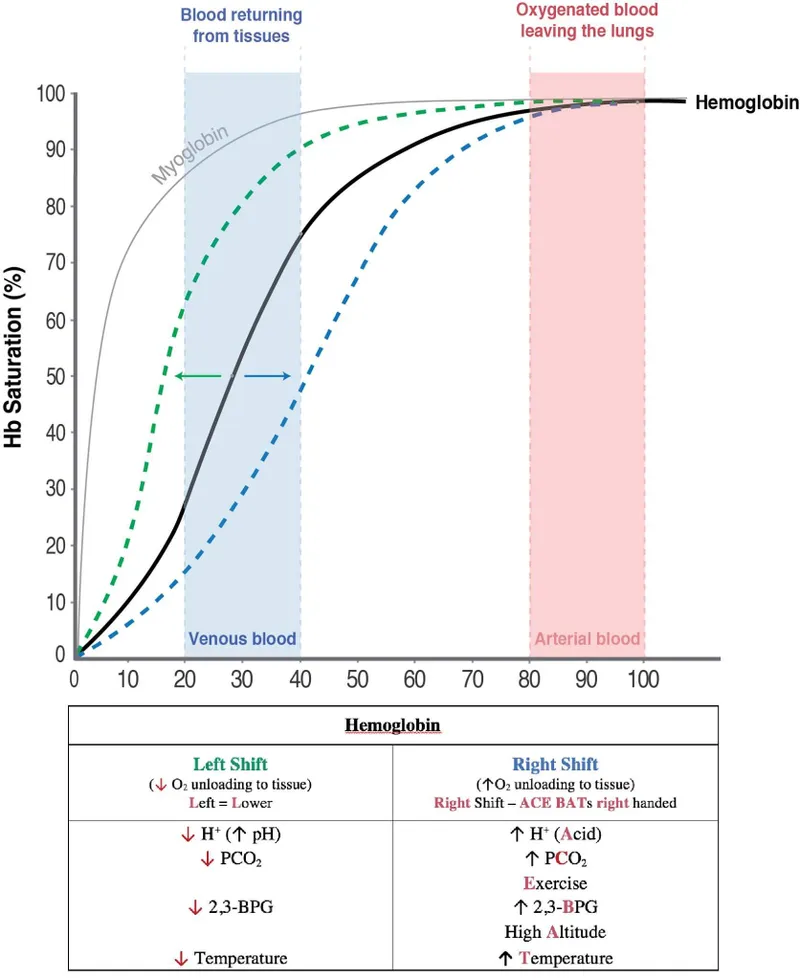
Oxygen-hemoglobin dissociation curve US Medical PG Question 6: A 67-year-old man presents to the surgical clinic with swelling of his right leg, fever, and chills for 2 days. The maximum recorded temperature was 38.3°C (101.0°F) at home. His right leg is red and swollen from the dorsum of the foot to the thigh with an ill-defined edge. Venous stasis ulcers are present in both of his limbs, but those on the right have a yellow discharge. His vitals include the following: blood pressure is 120/78 mm Hg, heart rate is 94/min, temperature is 38.3°C (101.0°F), and respiratory rate is 16/min. On physical examination, there is tenderness and warmth compared with his normal leg. Dorsalis pedis pulses are present on both of the ankles. What is the most likely cause of the right shift of the hemoglobin dissociation curve for his condition?
- A. Decrease in temperature
- B. Increase in CO2 production
- C. Increase in pH
- D. Increase in temperature (Correct Answer)
- E. Decrease in 2,3-DPG
Oxygen-hemoglobin dissociation curve Explanation: ***Increase in temperature***
- The patient presents with **fever (38.3°C)**, which is explicitly mentioned multiple times in the clinical scenario and represents a **systemic response** to infection.
- **Increased temperature** directly causes a **right shift** in the oxygen-hemoglobin dissociation curve by **decreasing hemoglobin's affinity for oxygen**.
- This facilitates oxygen release to metabolically active tissues, particularly important in areas of infection and inflammation.
- While multiple factors can cause right shifts during infection, the **fever is the most prominently featured clinical finding** in this case and represents a measurable systemic change.
*Decrease in temperature*
- A **decrease in temperature** causes a **left shift** in the oxygen-hemoglobin dissociation curve, **increasing hemoglobin's affinity for oxygen**.
- This would impair oxygen release to tissues, which is counterproductive during infection when tissues require increased oxygen delivery.
*Increase in CO2 production*
- While **increased CO2 production** does occur during infection due to increased tissue metabolism and does cause a **right shift** via the **Bohr effect** (CO2 + H2O → H2CO3 → H+ + HCO3-, leading to decreased pH), this is not the primary factor being highlighted in this clinical presentation.
- The Bohr effect (acidosis from increased CO2 and metabolic acids) is an important physiological response, but the question emphasizes the **fever** as the key feature of this patient's condition.
- In the context of this question asking about "his condition," the **temperature elevation is the most direct and measurable systemic change** presented.
*Increase in pH*
- An **increase in pH** (alkalosis) causes a **left shift** in the oxygen-hemoglobin dissociation curve, **increasing hemoglobin's oxygen affinity**.
- This would hinder oxygen delivery to tissues, which is not beneficial during infection when tissue oxygen demand is elevated.
*Decrease in 2,3-DPG*
- A **decrease in 2,3-bisphosphoglycerate (2,3-DPG)** causes a **left shift** in the oxygen-hemoglobin dissociation curve.
- This increases hemoglobin's affinity for oxygen, making oxygen release to tissues more difficult.
- During infection, 2,3-DPG levels typically remain stable or may increase slightly, not decrease.
Oxygen-hemoglobin dissociation curve US Medical PG Question 7: A male infant is born at 27 weeks following premature rupture of membranes and a precipitous labor to a G4P3 female. Given the speed of delivery steroids are not given. Shortly after delivery he develops respiratory distress and the decision is made to administer surfactant replacement therapy. While the components of the surfactant used in surfactant therapy may vary based on institution, what is the main component of pulmonary surfactant produced by type II pneumocytes?
- A. Cholesterol
- B. Protein S
- C. Surfactant-associated proteins
- D. Phospholipids (Correct Answer)
- E. Zinc finger protein
Oxygen-hemoglobin dissociation curve Explanation: ***Phospholipids***
- The main component of **pulmonary surfactant** produced by **type II pneumocytes** is **dipalmitoylphosphatidylcholine (DPPC)**, a type of **phospholipid**.
- These **phospholipids** reduce **alveolar surface tension**, preventing alveolar collapse at the end of expiration.
*Cholesterol*
- While **cholesterol** is present in biological membranes, it is a minor component of pulmonary surfactant and does not primarily determine its function.
- Its role is mainly in regulating the fluidity of the **surfactant film**, rather than reducing surface tension.
*Protein S*
- **Protein S** is a **vitamin K-dependent plasma protein** that functions as a **natural anticoagulant**; it is not a component of pulmonary surfactant.
- Its deficiency is associated with **thrombotic disorders**.
*Surfactant-associated proteins*
- **Surfactant-associated proteins (SPs)**, such as SP-A, SP-B, SP-C, and SP-D, are crucial for the **function and regulation** of pulmonary surfactant.
- However, they constitute a much smaller proportion by mass compared to **phospholipids**, which are the main structural and functional components.
*Zinc finger protein*
- **Zinc finger proteins** are a diverse class of proteins that bind to DNA, RNA, or other proteins and are involved in various cellular processes, including **gene regulation**.
- They are not a structural or functional component of **pulmonary surfactant**.
Oxygen-hemoglobin dissociation curve US Medical PG Question 8: A 30-year-old woman presents to clinic for a routine checkup. She reports that she is in good health but that she felt short of breath on her hiking and skiing trip to Colorado the week prior. She explains that this was the first time she has gone that high into the mountains and was slightly concerned for the first few days because she felt chronically short of breath. She reports a history of childhood asthma, but this experience did not feel the same. She was on the verge of seeking medical attention, but it resolved three days later, and she has felt fine ever since. What other listed physiological change results in a physiologic alteration similar to that which occurred in this patient?
- A. Increase in partial pressure of water in air
- B. Increase in blood pH
- C. Increase in concentration of dissolved carbon dioxide in blood
- D. Increase in concentration of 2,3-bisphosphoglycerate in blood (Correct Answer)
- E. Decreased body temperature
Oxygen-hemoglobin dissociation curve Explanation: ***Increase in concentration of 2,3-bisphosphoglycerate in blood***
- At high altitude, the body **increases 2,3-BPG production** as a key acclimatization mechanism over several days, which is why the patient's symptoms resolved after 3 days.
- Increased 2,3-BPG shifts the **oxygen-hemoglobin dissociation curve to the right**, decreasing hemoglobin's affinity for oxygen and facilitating **oxygen unloading at tissues**.
- This is one of the primary chronic adaptations to high altitude hypoxia, along with increased erythropoietin production.
*Increase in partial pressure of water in air*
- An increase in the partial pressure of water vapor in the air would decrease the partial pressure of inspired oxygen (PiO2 = FiO2 × (Patm - PH2O)).
- This would worsen hypoxia rather than represent an adaptive response to altitude.
*Decreased body temperature*
- Decreased body temperature shifts the oxygen-hemoglobin dissociation curve to the **left**, increasing hemoglobin's affinity for oxygen.
- This would **impair oxygen unloading** at tissues, which is the opposite effect of increased 2,3-BPG.
- This is not a physiological response to high altitude.
*Increase in blood pH*
- An increase in blood pH (respiratory alkalosis) does occur acutely at high altitude due to hyperventilation in response to hypoxia.
- However, this shifts the curve to the **left** (Bohr effect), increasing oxygen affinity, which is the **opposite effect** of increased 2,3-BPG.
- While this occurs as an immediate response, the body compensates through renal bicarbonate excretion and increased 2,3-BPG production to maintain tissue oxygen delivery.
*Increase in concentration of dissolved carbon dioxide in blood*
- At high altitude, hyperventilation leads to **decreased CO2** (hypocapnia), not increased CO2.
- Increased CO2 would cause acidosis and shift the curve to the right (decreasing oxygen affinity), but this does not occur at high altitude.
- The opposite physiological change (decreased CO2) actually occurs.
Oxygen-hemoglobin dissociation curve US Medical PG Question 9: A scientist is working on creating synthetic hemoglobin that can be used to replace blood loss in humans. She therefore starts to study the behavior of this artificial hemoglobin in terms of its ability to bind oxygen. She begins by measuring the affinity between this synthetic hemoglobin and oxygen in a purified system before introducing modifications to this system. Specifically, she reduces the level of carbon dioxide in the system to mimic conditions within the lungs and plots an affinity curve. Which of the following should be observed in this artificial hemoglobin if it mimics the behavior of normal hemoglobin?
- A. No shift in the curve and increased oxygen binding
- B. Left-shifted curve and decreased oxygen binding
- C. Right-shifted curve and decreased oxygen binding
- D. Right-shifted curve and increased oxygen binding
- E. Left-shifted curve and increased oxygen binding (Correct Answer)
Oxygen-hemoglobin dissociation curve Explanation: ***Left-shifted curve and increased oxygen binding***
- A **left shift** in the oxygen-hemoglobin dissociation curve indicates an **increased affinity** of hemoglobin for oxygen. This occurs in the lungs where **low CO2** (and thus higher pH) and lower temperature promote oxygen binding.
- Reduced carbon dioxide levels mimic the conditions in the **lungs**, promoting oxygen loading onto hemoglobin for transport to tissues.
*No shift in the curve and increased oxygen binding*
- A **lack of shift** in the curve suggests that the synthetic hemoglobin's affinity for oxygen is not changing in response to the altered CO2 levels, which would be abnormal.
- While increased oxygen binding is the goal in the lungs, it should be accompanied by a **left shift** reflecting the higher affinity.
*Left-shifted curve and decreased oxygen binding*
- A **left shift** indicates **increased oxygen affinity**, which would lead to **increased oxygen binding**, not decreased. These two concepts are contradictory.
- This option incorrectly couples a left shift (higher affinity) with decreased binding.
*Right-shifted curve and decreased oxygen binding*
- A **right shift** indicates **decreased oxygen affinity**, meaning hemoglobin releases oxygen more readily. This occurs in tissues where CO2 levels are high, not in the lungs where CO2 is low.
- Under conditions of low CO2, a right shift and decreased binding would be an inappropriate physiological response for oxygen loading in the lungs.
*Right-shifted curve and increased oxygen binding*
- A **right shift** signifies **decreased oxygen affinity**, which would naturally lead to **decreased oxygen binding**, not increased. These are conflicting outcomes.
- This combination of effects would impair oxygen loading in the lungs, making it unsuitable for a blood substitute.
Oxygen-hemoglobin dissociation curve US Medical PG Question 10: A 24-year-old professional athlete is advised to train in the mountains to enhance his performance. After 5 months of training at an altitude of 1.5 km (5,000 feet), he is able to increase his running pace while competing at sea-level venues. Which of the following changes would produce the same effect on the oxygen-hemoglobin dissociation curve as this athlete's training did?
- A. Decreased 2,3-bisphosphoglycerate (Correct Answer)
- B. Increased carbon monoxide inhalation
- C. Decreased temperature
- D. Decreased pH
- E. Increased partial pressure of oxygen
Oxygen-hemoglobin dissociation curve Explanation: ***Decreased 2,3-bisphosphoglycerate***
- This is **NOT** the correct physiological adaptation from altitude training, making this question conceptually flawed.
- Altitude training causes **increased erythropoietin → polycythemia → increased total hemoglobin**, which increases oxygen-carrying capacity.
- 2,3-BPG is **initially increased** at altitude (right shift) to facilitate O2 release, and remains elevated or returns to normal with acclimatization, **not decreased**.
- While decreased 2,3-BPG would cause a left shift (increased O2 affinity), this does NOT replicate altitude training adaptations.
*Increased carbon monoxide inhalation*
- Carbon monoxide binds hemoglobin with **200-250× higher affinity** than oxygen, forming carboxyhemoglobin.
- This **reduces oxygen-carrying capacity** and causes a left shift for remaining hemoglobin.
- This is harmful and does NOT replicate beneficial altitude adaptations.
*Decreased temperature*
- Decreases metabolic rate and causes a **left shift** (increased O2 affinity).
- Oxygen is held more tightly and released less readily to tissues.
- This does NOT replicate altitude training benefits.
*Decreased pH*
- Acidosis causes the **Bohr effect**: **right shift** (decreased O2 affinity).
- Facilitates O2 release to tissues during exercise.
- This is beneficial during exercise but does NOT replicate the chronic altitude adaptation of increased oxygen-carrying capacity.
*Increased partial pressure of oxygen*
- Higher PO2 increases hemoglobin saturation but does NOT shift the curve.
- This increases oxygen availability but does NOT replicate the physiological adaptation (polycythemia) from altitude training.
**Note:** This question is conceptually problematic as none of the options accurately replicate the primary altitude training adaptation (increased RBC mass/hemoglobin concentration).
More Oxygen-hemoglobin dissociation curve US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.