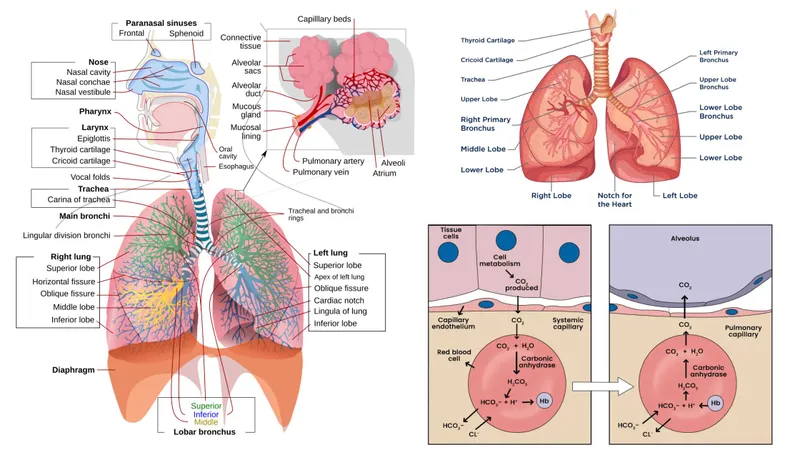
Chloride shift mechanism US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Chloride shift mechanism. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Chloride shift mechanism US Medical PG Question 1: During a clinical study examining the diffusion of gas between the alveolar compartment and the pulmonary capillary blood, men between the ages of 20 and 50 years are evaluated while they hold a sitting position. After inhaling a water-soluble gas that rapidly combines with hemoglobin, the concentration of the gas in the participant's exhaled air is measured and the diffusion capacity is calculated. Assuming that the concentration of the inhaled gas remains the same, which of the following is most likely to increase the flow of the gas across the alveolar membrane?
- A. Deep exhalation
- B. Entering a cold chamber
- C. Treadmill exercise (Correct Answer)
- D. Standing straight
- E. Assuming a hunched position
Chloride shift mechanism Explanation: ***Correct: Treadmill exercise***
- **Treadmill exercise** increases cardiac output and pulmonary blood flow, which in turn recruits and distends more **pulmonary capillaries**. This increases the **surface area** available for gas exchange and reduces the diffusion distance, thereby enhancing the flow of gas across the alveolar membrane.
- Exercise also typically leads to deeper and more frequent breaths, increasing the **ventilation-perfusion matching** and overall efficiency of gas exchange.
- According to Fick's law of diffusion (Vgas = A/T × D × ΔP), increasing the surface area (A) directly increases gas flow.
*Incorrect: Deep exhalation*
- **Deep exhalation** would empty the lungs more completely, potentially leading to alveolar collapse in some regions and thus **decreasing the alveolar surface area** available for gas exchange.
- This would also reduce the **driving pressure** for gas diffusion by lowering the alveolar concentration of the inhaled gas.
*Incorrect: Entering a cold chamber*
- Exposure to a **cold chamber** can cause **bronchoconstriction** in some individuals, particularly those with reactive airways, which would increase airway resistance and potentially reduce alveolar ventilation.
- While metabolic rate may slightly increase in the cold, the primary effect on the lungs is unlikely to promote increased gas diffusion in a healthy individual.
*Incorrect: Standing straight*
- **Standing straight** is a normal physiological posture and does not significantly alter the **pulmonary capillary recruitment** or the alveolar surface area in a way that would dramatically increase gas flow compared to a seated position.
- There might be minor gravitational effects on blood flow distribution, but these are generally less impactful than dynamic changes like exercise.
*Incorrect: Assuming a hunched position*
- **Assuming a hunched position** can restrict chest wall expansion and diaphragm movement, leading to **reduced tidal volume** and overall alveolar ventilation.
- This posture, by reducing lung volumes and potentially compressing the lungs, would likely **decrease the effective surface area** for gas exchange and therefore reduce gas flow.
Chloride shift mechanism US Medical PG Question 2: A 58-year-old man presents to the emergency department with a chief complaint of ringing in his ears that started several hours previously that has progressed to confusion. The patient denies any history of medical problems except for bilateral knee arthritis. He was recently seen by an orthopedic surgeon to evaluate his bilateral knee arthritis but has opted to not undergo knee replacement and prefers medical management. His wife noted that prior to them going on a hike today, he seemed confused and not himself. They decided to stay home, and roughly 14 hours later, he was no longer making any sense. Physical exam is notable for a confused man. The patient's vitals are being performed and his labs are being drawn. Which of the following is most likely to be seen on blood gas analysis?
- A. pH: 7.30, PaCO2: 15 mmHg, HCO3-: 16 mEq/L (Correct Answer)
- B. pH: 7.37, PaCO2: 41 mmHg, HCO3-: 12 mEq/L
- C. pH: 7.41, PaCO2: 65 mmHg, HCO3-: 34 mEq/L
- D. pH: 7.47, PaCO2: 11 mmHg, HCO3-: 24 mEq/L
- E. pH: 7.31, PaCO2: 31 mmHg, HCO3-: 15 mEq/L
Chloride shift mechanism Explanation: ***pH: 7.30, PaCO2: 15 mmHg, HCO3-: 16 mEq/L***
- This blood gas analysis shows a **low pH** (acidemia), **low PaCO2** (hypocapnia), and **low HCO3-** (bicarbonate). This pattern is consistent with a **primary metabolic acidosis** with a **compensatory respiratory alkalosis**.
- In this clinical scenario, the patient likely has **salicylate toxicity** (aspirin poisoning). Salicylate toxicity initially causes respiratory alkalosis due to direct stimulation of the respiratory center, followed by a high anion gap metabolic acidosis as salicylates interfere with cellular metabolism. This specific ABG reflects a mixed disorder where metabolic acidosis is predominant and respiratory compensation is attempting to raise the pH. The **tinnitus** and **confusion** are classic symptoms of salicylate toxicity.
*pH: 7.37, PaCO2: 41 mmHg, HCO3-: 12 mEq/L*
- This blood gas shows a **normal pH**, **normal PaCO2**, and **low HCO3-**. This suggests a **compensated metabolic acidosis**, where the body has fully compensated to bring the pH back to normal.
- While the patient likely has metabolic acidosis from salicylate toxicity, full compensation to a normal pH is less characteristic of an acute, severe presentation with significant neurological symptoms.
*pH: 7.41, PaCO2: 65 mmHg, HCO3-: 34 mEq/L*
- This blood gas shows a **normal pH**, **high PaCO2**, and **high HCO3-**. This indicates a **compensated respiratory acidosis**, where the kidneys have compensated for chronic CO2 retention.
- This pattern is not consistent with salicylate toxicity, which typically causes **respiratory alkalosis** early on, and later **metabolic acidosis**.
*pH: 7.47, PaCO2: 11 mmHg, HCO3-: 24 mEq/L*
- This blood gas analysis shows a **high pH** (alkalemia), **very low PaCO2** (severe hypocapnia), and a **normal HCO3-**. This indicates a **primary respiratory alkalosis** with no significant metabolic compensation.
- While salicylate toxicity can cause respiratory alkalosis, severe confusion and the progression of symptoms suggest a more advanced stage, usually involving a metabolic acidosis component, making a pure, uncompensated respiratory alkalosis less likely.
*pH: 7.31, PaCO2: 31 mmHg, HCO3-: 15 mEq/L*
- This blood gas shows a **low pH**, **low PaCO2**, and **low HCO3-**. This also indicates a **metabolic acidosis** with **respiratory compensation**.
- However, compared to pH 7.30, PaCO2 15 mmHg, and HCO3- 16 mEq/L, this option shows slightly **less severe respiratory compensation** (PaCO2 is higher), which is less typical for the profound respiratory stimulation seen in severe salicylate poisoning. The chosen correct option demonstrates a more characteristic and maximal respiratory compensation for the degree of metabolic acidosis.
Chloride shift mechanism US Medical PG Question 3: A 17-year-old female is brought to the emergency room by her parents shortly after a suicide attempt by aspirin overdose. Which of the following acid/base changes will occur FIRST in this patient?
- A. Metabolic alkalosis
- B. Respiratory acidosis
- C. Anion gap metabolic acidosis
- D. Respiratory alkalosis (Correct Answer)
- E. Non-anion gap metabolic acidosis
Chloride shift mechanism Explanation: ***Respiratory alkalosis***
- **Aspirin overdose** initially causes direct stimulation of the **respiratory center in the medulla**, leading to **hyperventilation**.
- This increased rate and depth of breathing blows off CO2, resulting in a primary **respiratory alkalosis**.
*Metabolic alkalosis*
- This is an unlikely primary event in aspirin overdose, which typically causes acidosis.
- While aspirin can cause electrolyte disturbances, a direct metabolic alkalosis as the *first* change is not characteristic.
*Respiratory acidosis*
- Respiratory depression, leading to respiratory acidosis, can occur in *severe* and *late-stage* aspirin overdose due to central nervous system depression.
- However, the initial effect is stimulation of respiration, causing alkalosis.
*Anion gap metabolic acidosis*
- This is a significant acid-base disturbance that *does* occur in aspirin overdose, but it develops *later*.
- Salicylates uncouple oxidative phosphorylation and impair cellular metabolism, leading to the accumulation of organic acids (e.g., lactic acid), causing a high anion gap metabolic acidosis.
*Non-anion gap metabolic acidosis*
- This type of acidosis is characterized by a preservation of the anion gap and is often associated with conditions like diarrhea or renal tubular acidosis.
- It is not the expected initial or primary acid-base disturbance in aspirin overdose.
Chloride shift mechanism US Medical PG Question 4: A histological examination of the carotid body reveals glomus cells containing dense-core vesicles. These cells function primarily as chemoreceptors for which of the following?
- A. Partial pressure of oxygen (Correct Answer)
- B. Blood pH
- C. Temperature
- D. Blood glucose levels
Chloride shift mechanism Explanation: ***Partial pressure of oxygen***
- Carotid body **glomus cells** are highly specialized **chemoreceptors** that primarily sense changes in the **partial pressure of oxygen (PO2)** in arterial blood.
- When PO2 decreases (e.g., hypoxia), these cells are activated and stimulate the respiratory and cardiovascular systems to increase oxygen uptake.
*Blood pH*
- While carotid body chemoreceptors can sense large changes in blood pH, their primary and most sensitive role is in detecting changes in **PO2**.
- Central chemoreceptors in the brainstem are more crucial for routine regulation of respiration in response to changes in **pH and PCO2**.
*Temperature*
- **Thermoreceptors** located in the skin, hypothalamus, and other internal organs are responsible for sensing body temperature, not the carotid body.
- The carotid body's main function is related to blood gas homeostasis, not temperature regulation.
*Blood glucose levels*
- Blood glucose levels are regulated by specialized cells in the **pancreas** (islets of Langerhans) that secrete hormones like insulin and glucagon.
- The carotid body is not directly involved in sensing or regulating glucose homeostasis.
Chloride shift mechanism US Medical PG Question 5: A 50-year-old woman presents to the ED 6 hours after ingesting three bottles of baby aspirin. She complains of nausea, vomiting, dizziness, and tinnitus. Her blood pressure is 135/80 mmHg, pulse is 110/min, respirations are 32/min, temperature is 100.1 deg F (37.8 deg C), and oxygen saturation is 99% on room air. Arterial blood gas at room air shows, PCO2 11 mmHg, and PO2 129 mmHg. Blood salicylate level is 55 mg/dL. Management should involve which of the following acid-base principles?
- A. Serum neutralization, urine alkalization
- B. Serum alkalization, urine alkalization (Correct Answer)
- C. Serum neutralization, urine acidification
- D. Serum acidification, urine acidification
- E. Serum acidification, urine alkalization
Chloride shift mechanism Explanation: ***Serum alkalization, urine alkalization***
- Managing **aspirin overdose** involves **aggressive serum alkalization** to promote the shift of salicylic acid from the cells into the bloodstream, where it remains ionized and cannot freely diffuse into the CNS. This also reduces its toxicity by increasing the proportion of the ionized form.
- Subsequently, **urine alkalization** with **sodium bicarbonate** is used to trap the ionized salicylate in the renal tubules, preventing reabsorption and enhancing its excretion.
*Serum neutralization, urine alkalization*
- This option is flawed because the goal is not to "neutralize" the serum pH to a neutral 7.0 but rather to raise it above normal towards an alkaline state (typically pH 7.45-7.55) to enhance salicylate elimination.
- While urine alkalization is correct, the idea of serum neutralization is incorrect and could lead to inadequate treatment.
*Serum neutralization, urine acidification*
- This approach is entirely incorrect for **salicylate toxicity** as **acidifying the urine** would promote the reabsorption of salicylic acid from the renal tubules, worsening toxicity.
- Serum neutralization, as mentioned, is not the correct term or goal for managing **aspirin overdose**.
*Serum acidification, urine acidification*
- This strategy would be **dangerous** in the context of **salicylate overdose** as it would significantly increase the proportion of **non-ionized salicylic acid**, allowing it to more readily cross cell membranes, including the blood-brain barrier, thereby increasing systemic and central nervous system toxicity.
- It would also drastically reduce elimination.
*Serum acidification, urine alkalization*
- **Serum acidification** is contraindicated in **salicylate poisoning** as it drives salicylate into the tissues, exacerbating its toxicity, particularly in the central nervous system.
- While urine alkalization is correct for enhancing elimination, combining it with serum acidification would counteract its benefits and worsen patient outcomes.
Chloride shift mechanism US Medical PG Question 6: A 70-year-old woman is brought to the emergency department due to worsening lethargy. She lives with her husband who says she has had severe diarrhea for the past few days. Examination shows a blood pressure of 85/60 mm Hg, pulse of 100/min, and temperature of 37.8°C (100.0°F). The patient is stuporous, while her skin appears dry and lacks turgor. Laboratory tests reveal:
Serum electrolytes
Sodium 144 mEq/L
Potassium 3.5 mEq/L
Chloride 115 mEq/L
Bicarbonate 19 mEq/L
Serum pH 7.3
PaO2 80 mm Hg
Pco2 38 mm Hg
This patient has which of the following acid-base disturbances?
- A. Chronic respiratory acidosis
- B. Anion gap metabolic acidosis with respiratory compensation
- C. Anion gap metabolic acidosis
- D. Non-anion gap metabolic acidosis with respiratory compensation (Correct Answer)
- E. Non-anion gap metabolic acidosis
Chloride shift mechanism Explanation: ***Non-anion gap metabolic acidosis with respiratory compensation***
- This patient has significant **diarrhea**, which causes a loss of **bicarbonate** from the gastrointestinal tract, leading to a **non-anion gap metabolic acidosis**.
- The **serum pH of 7.3** confirms acidosis, and the **Pco2 of 38 mm Hg** (which is slightly below the normal range, considering the acidosis) indicates effective **respiratory compensation** for the metabolic disturbance. Calculating the **anion gap** = Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L** (normal range 8-12 mEq/L), which is within normal limits.
*Chronic respiratory acidosis*
- This would involve an elevated **Pco2** and a compensatory increase in **bicarbonate**, neither of which are observed in this patient.
- The patient's primary problem is loss of bicarbonate due to diarrhea, not impaired CO2 excretion.
*Anion gap metabolic acidosis with respiratory compensation*
- An **anion gap metabolic acidosis** would show an elevated anion gap (>12 mEq/L), which is not present here (anion gap is 10 mEq/L).
- While respiratory compensation is occurring, the underlying acidosis is **non-anion gap**.
*Anion gap metabolic acidosis*
- This diagnosis requires an **elevated anion gap**, which is calculated as Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L**.
- Since the anion gap is within the normal range, an anion gap metabolic acidosis is excluded.
*Non-anion gap metabolic acidosis*
- While the patient does have a **non-anion gap metabolic acidosis** due to bicarbonate loss from diarrhea, this option doesn't account for the **respiratory compensation** indicated by the Pco2.
- The slightly reduced Pco2 demonstrates the body's attempt to normalize pH, making "with respiratory compensation" a more complete description.
Chloride shift mechanism US Medical PG Question 7: During normal respiration in the lungs, oxygen is absorbed into the bloodstream and carbon dioxide is released. The oxygen is used in cells as the final electron acceptor during oxidative phosphorylation, and carbon dioxide is generated during each turn of the tricarboxylic citric acid cycle (TCA). Which of the following steps in the TCA cycle represents the first decarboxylation reaction that generates carbon dioxide?
- A. Isocitrate to alpha ketoglutarate (Correct Answer)
- B. Fumarate to Malate
- C. Citrate to isocitrate
- D. Malate to oxaloacetate
- E. Alpha-ketoglutarate to Succinyl-CoA
Chloride shift mechanism Explanation: ***Isocitrate to alpha ketoglutarate***
- This is the **first decarboxylation reaction** in the TCA cycle, catalyzed by **isocitrate dehydrogenase**.
- During this reaction, **isocitrate** is oxidized and a molecule of **carbon dioxide** is released, along with the reduction of NAD+ to NADH.
- This is one of the three irreversible steps in the TCA cycle and a key regulatory point.
*Fumarate to Malate*
- This step involves the **hydration** of **fumarate** to **malate** by the enzyme **fumarase**.
- There is no release of carbon dioxide in this reaction; it's a simple addition of water.
*Citrate to isocitrate*
- This is an **isomerization** reaction, catalyzed by **aconitase**, where **citrate** is rearranged into its isomer, **isocitrate**.
- This step does not involve the removal of carbon atoms or the production of carbon dioxide.
*Malate to oxaloacetate*
- In this step, **malate** is oxidized to **oxaloacetate** by **malate dehydrogenase**, which produces NADH.
- This is an **oxidation** reaction, not a decarboxylation reaction, and no carbon dioxide is released.
*Alpha-ketoglutarate to Succinyl-CoA*
- This is the **second decarboxylation** step in the TCA cycle, catalyzed by the **alpha-ketoglutarate dehydrogenase complex**.
- While this step also produces carbon dioxide and reduces NAD+ to NADH, it occurs after the isocitrate to alpha-ketoglutarate step, making it the second rather than the first decarboxylation reaction.
Chloride shift mechanism US Medical PG Question 8: A 55-year-old man with a history of congestive heart failure, hypertension, and hyperlipidemia presents to his primary care clinic. He admits he did not adhere to a low salt diet on a recent vacation. He now has progressive leg swelling and needs two pillows to sleep because he gets short of breath when lying flat. Current medications include aspirin, metoprolol, lisinopril, atorvastatin, and furosemide. His physician decides to increase the dosage and frequency of the patient’s furosemide. Which of the following electrolyte abnormalities is associated with loop diuretics?
- A. Hyperchloremia
- B. Hypocalcemia
- C. Hypermagnesemia
- D. Hypouricemia
- E. Hypokalemia (Correct Answer)
Chloride shift mechanism Explanation: ***Hypokalemia***
- **Loop diuretics** are most commonly associated with **hypokalemia**, which is one of their most clinically significant electrolyte disturbances.
- Loop diuretics inhibit the **Na-K-2Cl cotransporter** in the thick ascending limb, increasing sodium delivery to the collecting duct.
- This stimulates **aldosterone-mediated potassium secretion** via principal cells, leading to increased urinary potassium loss.
- **Clinical significance**: Hypokalemia can cause muscle weakness, cardiac arrhythmias, and potentiates digoxin toxicity—particularly important in heart failure patients.
*Hyperchloremia*
- Loop diuretics cause **hypochloremia**, not hyperchloremia.
- Chloride reabsorption is blocked in the thick ascending limb, leading to increased chloride excretion.
*Hypocalcemia*
- Loop diuretics increase **urinary calcium excretion** (hypercalciuria) by reducing the positive luminal charge needed for paracellular calcium reabsorption.
- However, this typically does **not cause clinically significant hypocalcemia** in most patients.
- In contrast, thiazide diuretics decrease calcium excretion.
*Hypermagnesemia*
- Loop diuretics cause **hypomagnesemia**, not hypermagnesemia.
- They disrupt the positive lumen potential necessary for magnesium reabsorption in the thick ascending limb.
*Hypouricemia*
- Loop diuretics cause **hyperuricemia**, not hypouricemia.
- They compete with uric acid for secretion in the proximal tubule, promoting uric acid reabsorption and decreasing its excretion.
Chloride shift mechanism US Medical PG Question 9: A 19-year-old male soccer player undergoes an exercise tolerance test to measure his maximal oxygen uptake during exercise. Which of the following changes are most likely to occur during exercise?
- A. Increased apical ventilation-perfusion ratio
- B. Decreased physiologic dead space (Correct Answer)
- C. Decreased alveolar-arterial oxygen gradient
- D. Increased arterial partial pressure of oxygen
- E. Increased pulmonary vascular resistance
Chloride shift mechanism Explanation: **Decreased physiologic dead space**
- During exercise, there is improved perfusion to previously underperfused areas of the lung, leading to a **more uniform ventilation-perfusion (V/Q) matching** and thus a decrease in physiologic dead space.
- The increased cardiac output helps to perfuse more capillaries, reducing the amount of ventilated air that does not participate in gas exchange.
*Increased apical ventilation-perfusion ratio*
- At rest, the **apical V/Q ratio is already high** due to gravity-dependent differences in blood flow; exercise partially normalizes these differences.
- While overall V/Q matching improves, the relative V/Q differences between apical and basal regions may become less pronounced, not necessarily a further increase in the apical ratio.
*Decreased alveolar-arterial oxygen gradient*
- During severe exercise, the **A-a gradient often increases slightly** due to increased oxygen diffusion limitations and V/Q mismatch.
- Although overall gas exchange efficiency improves, the sheer volume of oxygen demand can reveal small imbalances, rather than fully eliminating the gradient.
*Increased arterial partial pressure of oxygen*
- Exercise typically leads to **stable or slightly decreased arterial PO2** in healthy individuals due to the increased metabolic demand and potential small V/Q mismatches.
- The body maintains arterial PO2 remarkably well even at high exertion, but it does not usually significantly increase.
*Increased pulmonary vascular resistance*
- During exercise, **pulmonary vascular resistance (PVR) generally decreases** due to recruitment and distension of pulmonary capillaries.
- This decrease in PVR helps to accommodate the increased cardiac output without a significant rise in pulmonary arterial pressure.
Chloride shift mechanism US Medical PG Question 10: An investigator is conducting a study on hematological factors that affect the affinity of hemoglobin for oxygen. An illustration of two graphs (A and B) that represent the affinity of hemoglobin for oxygen is shown. Which of the following best explains a shift from A to B?
- A. Decreased serum pCO2
- B. Increased serum pH
- C. Decreased serum 2,3-bisphosphoglycerate concentration
- D. Increased body temperature (Correct Answer)
- E. Increased hemoglobin γ-chain synthesis
Chloride shift mechanism Explanation: ***Increased body temperature***
- A shift from A to B represents a **rightward shift** of the oxygen-hemoglobin dissociation curve, indicating **decreased hemoglobin affinity for oxygen**.
- **Increased body temperature** (e.g., during exercise, fever) reduces hemoglobin's affinity for oxygen, facilitating **oxygen release to tissues**.
*Decreased serum pCO2*
- A **decrease in serum pCO2** leads to an **increase in pH** (alkalosis) and a **leftward shift** of the curve, meaning an increased affinity of hemoglobin for oxygen.
- This is part of the **Bohr effect**, where lower CO2 levels signal decreased tissue metabolic activity, thus reducing oxygen unloading.
*Increased serum pH*
- An **increase in serum pH** (alkalosis) causes a **leftward shift** of the oxygen-hemoglobin dissociation curve, signifying **increased hemoglobin affinity for oxygen**.
- This response is beneficial in the lungs, where higher pH promotes oxygen binding to hemoglobin.
*Decreased serum 2,3-bisphosphoglycerate concentration*
- A **decrease in 2,3-BPG** concentration leads to a **leftward shift** of the curve, representing **increased hemoglobin affinity for oxygen**.
- 2,3-BPG typically binds to deoxyhemoglobin, stabilizing its T-state and promoting oxygen release; thus, less 2,3-BPG means less release.
*Increased hemoglobin γ-chain synthesis*
- Increased **hemoglobin γ-chain synthesis** is characteristic of **fetal hemoglobin (HbF)**, which has a **higher affinity for oxygen** than adult hemoglobin (HbA).
- This would result in a **leftward shift** of the oxygen-hemoglobin dissociation curve, enhancing oxygen uptake by the fetus.
More Chloride shift mechanism US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.