Bohr effect and rightward shifts US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Bohr effect and rightward shifts. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Bohr effect and rightward shifts US Medical PG Question 1: A 33-year-old woman is brought to the emergency department 30 minutes after being rescued from a fire in her apartment. She reports nausea, headache, and dizziness. Physical examination shows black discoloration of her oral mucosa. Pulse oximetry shows an oxygen saturation of 99% on room air. The substance most likely causing symptoms in this patient primarily produces toxicity by which of the following mechanisms?
- A. Inhibition of mitochondrial complex V
- B. Degradation of 2,3-bisphosphoglycerate
- C. Oxidation of Fe2+
- D. Rise in serum pH
- E. Competitive binding to heme (Correct Answer)
Bohr effect and rightward shifts Explanation: ***Competitive binding to heme***
- The patient's symptoms (nausea, headache, dizziness, black oral mucosa) and history of being rescued from a fire strongly suggest **carbon monoxide (CO) poisoning** [1].
- **Carbon monoxide** primarily exerts its toxicity by competitively binding to the **heme iron** in hemoglobin with an affinity 200-250 times greater than oxygen, forming **carboxyhemoglobin (COHb)** and displacing oxygen [2].
*Inhibition of mitochondrial complex V*
- **Cyanide poisoning** inhibits **mitochondrial complex IV (cytochrome c oxidase)**, not complex V, leading to impaired cellular respiration.
- While both cyanide and CO poisoning can occur in fires, CO is more common due to incomplete combustion, and the specific presentation points toward CO.
*Degradation of 2,3-bisphosphoglycerate*
- **2,3-BPG** is an important regulator of oxygen affinity for hemoglobin, promoting oxygen release to tissues [2]. Its degradation would increase hemoglobin's affinity for oxygen, thus reducing oxygen unloading, but this is not the primary mechanism of toxicity for CO or common fire-related toxins.
- No common toxin directly causes widespread degradation of 2,3-BPG as its primary mechanism of acute toxicity or symptoms.
*Oxidation of Fe2+*
- The oxidation of **ferrous iron (Fe2+)** to **ferric iron (Fe3+)** in hemoglobin leads to the formation of **methemoglobin**, which cannot bind oxygen. This occurs in **methemoglobinemia** induced by certain drugs or toxins (e.g., nitrites, dapsone).
- While **methemoglobinemia** impairs oxygen transport, it does not explain the black oral mucosa or the strong association with fire smoke toxicity in the context of CO.
*Rise in serum pH*
- A rise in serum pH (alkalosis) is not a direct or primary mechanism of toxicity for common fire-related toxins like carbon monoxide or cyanide.
- Most severe forms of toxicity, including CO and cyanide poisoning, tend to cause **lactic acidosis** due to cellular hypoxia and anaerobic metabolism, leading to a
**decrease** in serum pH.
Bohr effect and rightward shifts US Medical PG Question 2: Which region of the nephron reabsorbs the highest percentage of filtered bicarbonate?
- A. Collecting duct
- B. Thick ascending limb
- C. Distal tubule
- D. Proximal tubule (Correct Answer)
Bohr effect and rightward shifts Explanation: ***Proximal tubule***
- The **proximal convoluted tubule (PCT)** reabsorbs approximately 80-90% of the **filtered bicarbonate** through a process involving **carbonic anhydrase** and the **Na+/H+ exchanger**.
- This vital function ensures that the majority of bicarbonate, a key buffer, is returned to the blood to maintain **acid-base balance**.
*Collecting duct*
- While the collecting duct does have the ability to reabsorb and secrete bicarbonate, its contribution is minor compared to the PCT, primarily for fine-tuning acid-base balance.
- Cells in the collecting duct, particularly **Type A intercalated cells**, are important for secreting acid (H+) in acidosis and therefore reabsorbing bicarbonate, but not the bulk of it.
*Thick ascending limb*
- The primary role of the **thick ascending limb** is the reabsorption of **sodium**, **potassium**, and **chloride** to create a concentrated interstitium, not significant bicarbonate reabsorption.
- It is largely impermeable to water and is relatively impermeable to bicarbonate.
*Distal tubule*
- The **distal convoluted tubule (DCT)** reabsorbs a small percentage of filtered bicarbonate, but its main role is regulated reabsorption of **sodium** and **calcium**, and secretion of **potassium** and **hydrogen ions**.
- Its contribution to bicarbonate reabsorption is much less significant than that of the proximal tubule.
Bohr effect and rightward shifts US Medical PG Question 3: A 24-year-old professional athlete is advised to train in the mountains to enhance his performance. After 5 months of training at an altitude of 1.5 km (5,000 feet), he is able to increase his running pace while competing at sea-level venues. Which of the following changes would produce the same effect on the oxygen-hemoglobin dissociation curve as this athlete's training did?
- A. Decreased 2,3-bisphosphoglycerate (Correct Answer)
- B. Increased carbon monoxide inhalation
- C. Decreased temperature
- D. Decreased pH
- E. Increased partial pressure of oxygen
Bohr effect and rightward shifts Explanation: ***Decreased 2,3-bisphosphoglycerate***
- This is **NOT** the correct physiological adaptation from altitude training, making this question conceptually flawed.
- Altitude training causes **increased erythropoietin → polycythemia → increased total hemoglobin**, which increases oxygen-carrying capacity.
- 2,3-BPG is **initially increased** at altitude (right shift) to facilitate O2 release, and remains elevated or returns to normal with acclimatization, **not decreased**.
- While decreased 2,3-BPG would cause a left shift (increased O2 affinity), this does NOT replicate altitude training adaptations.
*Increased carbon monoxide inhalation*
- Carbon monoxide binds hemoglobin with **200-250× higher affinity** than oxygen, forming carboxyhemoglobin.
- This **reduces oxygen-carrying capacity** and causes a left shift for remaining hemoglobin.
- This is harmful and does NOT replicate beneficial altitude adaptations.
*Decreased temperature*
- Decreases metabolic rate and causes a **left shift** (increased O2 affinity).
- Oxygen is held more tightly and released less readily to tissues.
- This does NOT replicate altitude training benefits.
*Decreased pH*
- Acidosis causes the **Bohr effect**: **right shift** (decreased O2 affinity).
- Facilitates O2 release to tissues during exercise.
- This is beneficial during exercise but does NOT replicate the chronic altitude adaptation of increased oxygen-carrying capacity.
*Increased partial pressure of oxygen*
- Higher PO2 increases hemoglobin saturation but does NOT shift the curve.
- This increases oxygen availability but does NOT replicate the physiological adaptation (polycythemia) from altitude training.
**Note:** This question is conceptually problematic as none of the options accurately replicate the primary altitude training adaptation (increased RBC mass/hemoglobin concentration).
Bohr effect and rightward shifts US Medical PG Question 4: An investigator is studying the changes that occur in the oxygen-hemoglobin dissociation curve of different types of hemoglobin under various conditions. The blood obtained from a male infant shows decreased affinity for 2,3-bisphosphoglyceric acid. Which of the following is the most likely composition of the hemoglobin molecule in this sample?
- A. α2βS2
- B. α2β2
- C. α2δ2
- D. α2γ2 (Correct Answer)
- E. β4
Bohr effect and rightward shifts Explanation: ***α2γ2***
- This formula represents **fetal hemoglobin (HbF)**, which is the predominant hemoglobin in infants.
- HbF has **decreased affinity for 2,3-bisphosphoglyceric acid (2,3-BPG)** compared to adult hemoglobin (HbA) because 2,3-BPG binds less avidly to the gamma chains.
- This decreased 2,3-BPG binding results in HbF having **higher oxygen affinity** than HbA (left-shifted oxygen-hemoglobin dissociation curve).
- The higher oxygen affinity allows fetal blood to efficiently extract oxygen from maternal blood across the placenta.
*α2βS2*
- This represents **hemoglobin S (HbS)**, found in **sickle cell disease**.
- HbS has similar 2,3-BPG binding to HbA, not decreased affinity.
- Its primary characteristic is polymerization and red blood cell sickling under deoxygenated conditions.
*α2β2*
- This represents **adult hemoglobin (HbA)**, the most common type of hemoglobin in adults.
- HbA has **higher affinity for 2,3-BPG** compared to HbF because 2,3-BPG binds strongly to the beta chains.
- The binding of 2,3-BPG to HbA decreases oxygen affinity, facilitating oxygen release to tissues.
*α2δ2*
- This represents **hemoglobin A2 (HbA2)**, a minor component of adult hemoglobin (typically <3.5%).
- HbA2 has normal 2,3-BPG binding similar to HbA, not decreased affinity.
- This doesn't fit the clinical description of an infant with decreased 2,3-BPG affinity.
*β4*
- This represents **hemoglobin H (HbH)**, which occurs in **alpha-thalassemia** where there is an excess of beta chains that form tetramers.
- HbH has **extremely high oxygen affinity** and does not release oxygen well to tissues.
- While HbH also has decreased 2,3-BPG binding, it is not found in healthy infants and represents a pathological condition.
Bohr effect and rightward shifts US Medical PG Question 5: During a study on chronic obstructive pulmonary disease (COPD), researchers discovered an agent that markedly inhibits the carbon dioxide-carrying capacity of the venous blood. Which of the following is the most likely mechanism underlying this agent’s effects?
- A. Decreased amount of dissolved plasma carbon dioxide
- B. Decreased carbon dioxide binding to carbamino compounds
- C. Decreased capillary permeability to carbon dioxide
- D. Increased solubility of carbon dioxide in plasma
- E. Inhibition of erythrocyte carbonic anhydrase (Correct Answer)
Bohr effect and rightward shifts Explanation: ***Inhibition of erythrocyte carbonic anhydrase***
- **Carbonic anhydrase** in red blood cells is crucial for the rapid conversion of **carbon dioxide (CO2)** and water into **carbonic acid (H2CO3)**, which then dissociates into **bicarbonate (HCO3-)** and hydrogen ions. Bicarbonate is the primary form in which CO2 is transported in the blood.
- Inhibiting this enzyme would significantly slow down the formation of bicarbonate, thus reducing the **CO2-carrying capacity** of the venous blood, as most CO2 is transported as bicarbonate.
*Decreased amount of dissolved plasma carbon dioxide*
- While a small amount of CO2 is transported as dissolved plasma CO2, this represents only about **5-7%** of the total CO2 transport.
- An agent that primarily decreases dissolved CO2 would not "markedly" inhibit the overall CO2-carrying capacity, as the **bicarbonate system** is the dominant mechanism.
*Decreased carbon dioxide binding to carbamino compounds*
- **Carbaminohemoglobin** is formed when CO2 binds directly to amino groups on hemoglobin, accounting for about **20-30%** of CO2 transport.
- While a decrease in this binding would impact CO2 transport, it is less significant than the bicarbonate mechanism, and directly inhibiting carbonic anhydrase would have a more profound effect on overall CO2 carrying capacity.
*Decreased capillary permeability to carbon dioxide*
- The permeability of capillaries to CO2 is typically very high and not usually a limiting factor in CO2 transport.
- A decrease in permeability would hinder CO2 exchange at the tissue level, but it would not directly affect the **chemical capacity** of the blood to carry CO2 once it has entered the bloodstream.
*Increased solubility of carbon dioxide in plasma*
- An increase in plasma solubility would actually **enhance CO2 carrying capacity**, as more CO2 could be transported in dissolved form.
- This effect would contradict the observed inhibition of CO2-carrying capacity described in the question.
Bohr effect and rightward shifts US Medical PG Question 6: During normal respiration in the lungs, oxygen is absorbed into the bloodstream and carbon dioxide is released. The oxygen is used in cells as the final electron acceptor during oxidative phosphorylation, and carbon dioxide is generated during each turn of the tricarboxylic citric acid cycle (TCA). Which of the following steps in the TCA cycle represents the first decarboxylation reaction that generates carbon dioxide?
- A. Isocitrate to alpha ketoglutarate (Correct Answer)
- B. Fumarate to Malate
- C. Citrate to isocitrate
- D. Malate to oxaloacetate
- E. Alpha-ketoglutarate to Succinyl-CoA
Bohr effect and rightward shifts Explanation: ***Isocitrate to alpha ketoglutarate***
- This is the **first decarboxylation reaction** in the TCA cycle, catalyzed by **isocitrate dehydrogenase**.
- During this reaction, **isocitrate** is oxidized and a molecule of **carbon dioxide** is released, along with the reduction of NAD+ to NADH.
- This is one of the three irreversible steps in the TCA cycle and a key regulatory point.
*Fumarate to Malate*
- This step involves the **hydration** of **fumarate** to **malate** by the enzyme **fumarase**.
- There is no release of carbon dioxide in this reaction; it's a simple addition of water.
*Citrate to isocitrate*
- This is an **isomerization** reaction, catalyzed by **aconitase**, where **citrate** is rearranged into its isomer, **isocitrate**.
- This step does not involve the removal of carbon atoms or the production of carbon dioxide.
*Malate to oxaloacetate*
- In this step, **malate** is oxidized to **oxaloacetate** by **malate dehydrogenase**, which produces NADH.
- This is an **oxidation** reaction, not a decarboxylation reaction, and no carbon dioxide is released.
*Alpha-ketoglutarate to Succinyl-CoA*
- This is the **second decarboxylation** step in the TCA cycle, catalyzed by the **alpha-ketoglutarate dehydrogenase complex**.
- While this step also produces carbon dioxide and reduces NAD+ to NADH, it occurs after the isocitrate to alpha-ketoglutarate step, making it the second rather than the first decarboxylation reaction.
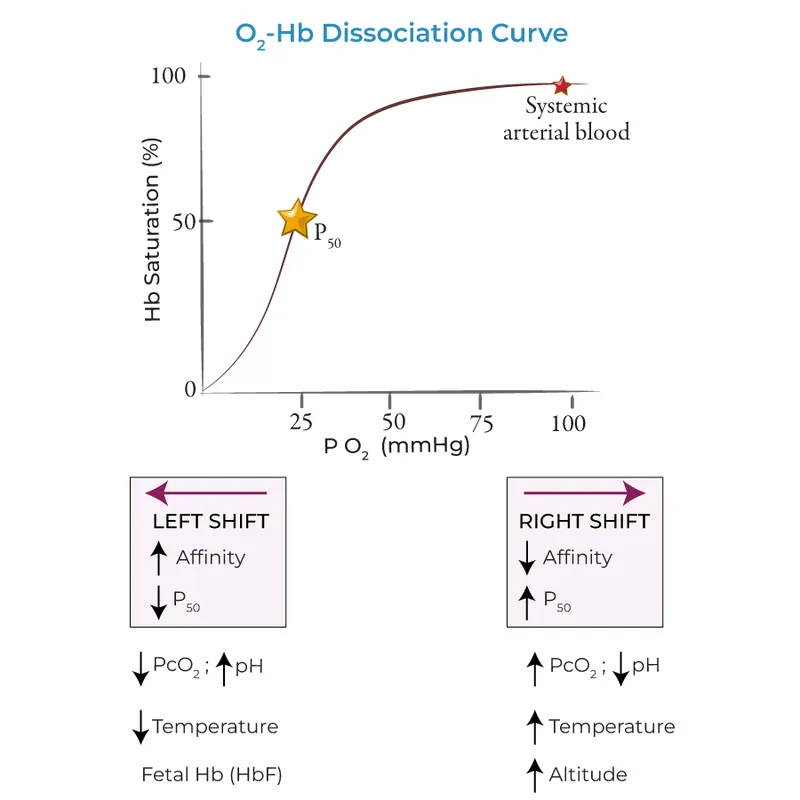
Bohr effect and rightward shifts US Medical PG Question 7: An investigator is conducting a study on hematological factors that affect the affinity of hemoglobin for oxygen. An illustration of two graphs (A and B) that represent the affinity of hemoglobin for oxygen is shown. Which of the following best explains a shift from A to B?
- A. Decreased serum pCO2
- B. Increased serum pH
- C. Decreased serum 2,3-bisphosphoglycerate concentration
- D. Increased body temperature (Correct Answer)
- E. Increased hemoglobin γ-chain synthesis
Bohr effect and rightward shifts Explanation: ***Increased body temperature***
- A shift from A to B represents a **rightward shift** of the oxygen-hemoglobin dissociation curve, indicating **decreased hemoglobin affinity for oxygen**.
- **Increased body temperature** (e.g., during exercise, fever) reduces hemoglobin's affinity for oxygen, facilitating **oxygen release to tissues**.
*Decreased serum pCO2*
- A **decrease in serum pCO2** leads to an **increase in pH** (alkalosis) and a **leftward shift** of the curve, meaning an increased affinity of hemoglobin for oxygen.
- This is part of the **Bohr effect**, where lower CO2 levels signal decreased tissue metabolic activity, thus reducing oxygen unloading.
*Increased serum pH*
- An **increase in serum pH** (alkalosis) causes a **leftward shift** of the oxygen-hemoglobin dissociation curve, signifying **increased hemoglobin affinity for oxygen**.
- This response is beneficial in the lungs, where higher pH promotes oxygen binding to hemoglobin.
*Decreased serum 2,3-bisphosphoglycerate concentration*
- A **decrease in 2,3-BPG** concentration leads to a **leftward shift** of the curve, representing **increased hemoglobin affinity for oxygen**.
- 2,3-BPG typically binds to deoxyhemoglobin, stabilizing its T-state and promoting oxygen release; thus, less 2,3-BPG means less release.
*Increased hemoglobin γ-chain synthesis*
- Increased **hemoglobin γ-chain synthesis** is characteristic of **fetal hemoglobin (HbF)**, which has a **higher affinity for oxygen** than adult hemoglobin (HbA).
- This would result in a **leftward shift** of the oxygen-hemoglobin dissociation curve, enhancing oxygen uptake by the fetus.
Bohr effect and rightward shifts US Medical PG Question 8: A 29-year-old man presents for the evaluation of infertility. He has a history of recurrent lower respiratory tract infections, productive cough, abdominal pain, and diarrhea. Physical examination reveals clubbing and bilateral crackles on chest auscultation. Chest X-ray reveals increased pulmonary markings and peripheral bronchi with a ‘tram track’ appearance. Which of the following pathophysiologies is responsible for the patient’s condition?
- A. Fibrosis of the lung parenchyma
- B. Bronchial hypersensitivity
- C. Abnormal ciliary motion
- D. Gluten hypersensitivity
- E. Defective chloride transport (Correct Answer)
Bohr effect and rightward shifts Explanation: ***Defective chloride transport***
- The patient's presentation with **recurrent respiratory infections**, **bronchiectasis** (tram track appearance on CXR), **clubbing**, and **infertility** is highly suggestive of **cystic fibrosis**.
- **Cystic fibrosis** is caused by mutations in the **CFTR gene**, leading to **defective chloride transport** across epithelial cells, resulting in thick, viscous secretions.
*Fibrosis of the lung parenchyma*
- While chronic lung disease can lead to some **pulmonary fibrosis**, it is not the primary underlying pathophysiology described here.
- Pulmonary fibrosis typically presents with **restrictive lung disease** and interstitial patterns on imaging, rather than the prominent **bronchiectasis** seen in this patient.
*Bronchial hypersensitivity*
- This is characteristic of **asthma**, which involves airway inflammation and bronchoconstriction, but typically does not cause the extensive **recurrent infections**, **bronchiectasis**, or **infertility** seen in this case.
- Asthma is less likely to result in **clubbing** or the progressive lung damage implied by a "tram track" appearance.
*Abnormal ciliary motion*
- This describes **primary ciliary dyskinesia (PCD)**, which can also cause recurrent respiratory infections and male infertility due to **immotile sperm**.
- However, PCD typically presents with **situs inversus** in a significant proportion of cases and does not involve the characteristic **exocrine gland dysfunction** (e.g., severe abdominal symptoms, pancreatic insufficiency leading to diarrhea) often seen in cystic fibrosis implied by the broad clinical picture.
*Gluten hypersensitivity*
- Also known as **celiac disease**, this is primarily a **gastrointestinal condition** characterized by malabsorption due to immune reactions to gluten.
- While celiac disease can cause **abdominal pain** and **diarrhea**, it does not explain the **recurrent respiratory infections**, **bronchiectasis**, **clubbing**, or **male infertility**.
Bohr effect and rightward shifts US Medical PG Question 9: A 67-year-old man presents to the surgical clinic with swelling of his right leg, fever, and chills for 2 days. The maximum recorded temperature was 38.3°C (101.0°F) at home. His right leg is red and swollen from the dorsum of the foot to the thigh with an ill-defined edge. Venous stasis ulcers are present in both of his limbs, but those on the right have a yellow discharge. His vitals include the following: blood pressure is 120/78 mm Hg, heart rate is 94/min, temperature is 38.3°C (101.0°F), and respiratory rate is 16/min. On physical examination, there is tenderness and warmth compared with his normal leg. Dorsalis pedis pulses are present on both of the ankles. What is the most likely cause of the right shift of the hemoglobin dissociation curve for his condition?
- A. Decrease in temperature
- B. Increase in CO2 production
- C. Increase in pH
- D. Increase in temperature (Correct Answer)
- E. Decrease in 2,3-DPG
Bohr effect and rightward shifts Explanation: ***Increase in temperature***
- The patient presents with **fever (38.3°C)**, which is explicitly mentioned multiple times in the clinical scenario and represents a **systemic response** to infection.
- **Increased temperature** directly causes a **right shift** in the oxygen-hemoglobin dissociation curve by **decreasing hemoglobin's affinity for oxygen**.
- This facilitates oxygen release to metabolically active tissues, particularly important in areas of infection and inflammation.
- While multiple factors can cause right shifts during infection, the **fever is the most prominently featured clinical finding** in this case and represents a measurable systemic change.
*Decrease in temperature*
- A **decrease in temperature** causes a **left shift** in the oxygen-hemoglobin dissociation curve, **increasing hemoglobin's affinity for oxygen**.
- This would impair oxygen release to tissues, which is counterproductive during infection when tissues require increased oxygen delivery.
*Increase in CO2 production*
- While **increased CO2 production** does occur during infection due to increased tissue metabolism and does cause a **right shift** via the **Bohr effect** (CO2 + H2O → H2CO3 → H+ + HCO3-, leading to decreased pH), this is not the primary factor being highlighted in this clinical presentation.
- The Bohr effect (acidosis from increased CO2 and metabolic acids) is an important physiological response, but the question emphasizes the **fever** as the key feature of this patient's condition.
- In the context of this question asking about "his condition," the **temperature elevation is the most direct and measurable systemic change** presented.
*Increase in pH*
- An **increase in pH** (alkalosis) causes a **left shift** in the oxygen-hemoglobin dissociation curve, **increasing hemoglobin's oxygen affinity**.
- This would hinder oxygen delivery to tissues, which is not beneficial during infection when tissue oxygen demand is elevated.
*Decrease in 2,3-DPG*
- A **decrease in 2,3-bisphosphoglycerate (2,3-DPG)** causes a **left shift** in the oxygen-hemoglobin dissociation curve.
- This increases hemoglobin's affinity for oxygen, making oxygen release to tissues more difficult.
- During infection, 2,3-DPG levels typically remain stable or may increase slightly, not decrease.
Bohr effect and rightward shifts US Medical PG Question 10: A male infant is born at 27 weeks following premature rupture of membranes and a precipitous labor to a G4P3 female. Given the speed of delivery steroids are not given. Shortly after delivery he develops respiratory distress and the decision is made to administer surfactant replacement therapy. While the components of the surfactant used in surfactant therapy may vary based on institution, what is the main component of pulmonary surfactant produced by type II pneumocytes?
- A. Cholesterol
- B. Protein S
- C. Surfactant-associated proteins
- D. Phospholipids (Correct Answer)
- E. Zinc finger protein
Bohr effect and rightward shifts Explanation: ***Phospholipids***
- The main component of **pulmonary surfactant** produced by **type II pneumocytes** is **dipalmitoylphosphatidylcholine (DPPC)**, a type of **phospholipid**.
- These **phospholipids** reduce **alveolar surface tension**, preventing alveolar collapse at the end of expiration.
*Cholesterol*
- While **cholesterol** is present in biological membranes, it is a minor component of pulmonary surfactant and does not primarily determine its function.
- Its role is mainly in regulating the fluidity of the **surfactant film**, rather than reducing surface tension.
*Protein S*
- **Protein S** is a **vitamin K-dependent plasma protein** that functions as a **natural anticoagulant**; it is not a component of pulmonary surfactant.
- Its deficiency is associated with **thrombotic disorders**.
*Surfactant-associated proteins*
- **Surfactant-associated proteins (SPs)**, such as SP-A, SP-B, SP-C, and SP-D, are crucial for the **function and regulation** of pulmonary surfactant.
- However, they constitute a much smaller proportion by mass compared to **phospholipids**, which are the main structural and functional components.
*Zinc finger protein*
- **Zinc finger proteins** are a diverse class of proteins that bind to DNA, RNA, or other proteins and are involved in various cellular processes, including **gene regulation**.
- They are not a structural or functional component of **pulmonary surfactant**.
More Bohr effect and rightward shifts US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.