Gas exchange

On this page
🫁 The Alveolar Exchange Engine: Precision Gas Trading
Every breath you take orchestrates a molecular trade worth your life-oxygen flooding in, carbon dioxide rushing out-across a surface area the size of a tennis court folded inside your chest. You'll master how alveoli and hemoglobin execute this precision exchange, why mismatches between ventilation and perfusion create hypoxemia, and how to recognize and correct gas exchange failures at the bedside. This journey moves from membrane physics to molecular cargo systems to the clinical reasoning that transforms struggling lungs back into efficient trading floors.
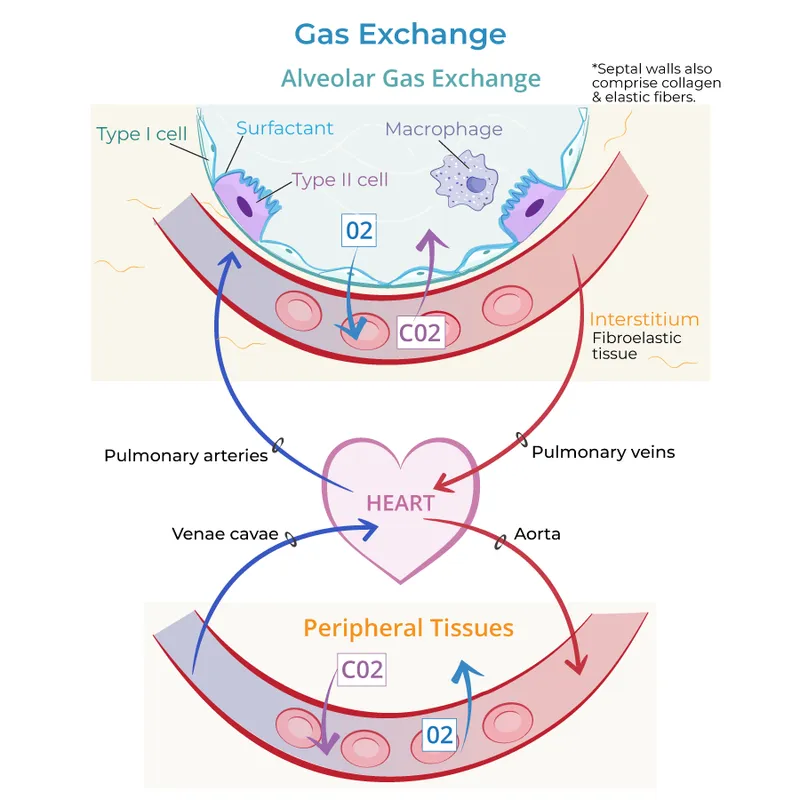
Gas exchange represents the fundamental currency of cellular life - the precise trading of oxygen for carbon dioxide across the alveolar-capillary barrier. This process involves four critical components: ventilation (air movement), diffusion (gas transfer), perfusion (blood flow), and transport (hemoglobin binding). The efficiency depends on maintaining optimal ventilation-perfusion ratios of 0.8, diffusion gradients of 60 mmHg for oxygen, and hemoglobin saturation above 95%.
📌 Remember: DIVE for gas exchange components - Diffusion across membrane, Inspiration/ventilation, Vascular perfusion, Erythrocyte transport. Each component must function at >90% efficiency for normal oxygenation.
The alveolar-arterial oxygen gradient (A-a gradient) serves as the master diagnostic tool, calculated as: A-a gradient = PAO₂ - PaO₂. Normal values remain <10 mmHg in young adults and <25 mmHg in elderly patients. Elevated gradients indicate diffusion impairment, V/Q mismatch, or shunting.
| Parameter | Normal Value | Pathological Threshold | Clinical Significance | Compensation Mechanism | Time to Correction |
|---|---|---|---|---|---|
| PaO₂ | 80-100 mmHg | <60 mmHg | Respiratory failure | Increased ventilation | 2-5 minutes |
| A-a Gradient | <15 mmHg | >25 mmHg | Gas exchange impairment | Increased FiO₂ | 15-30 minutes |
| V/Q Ratio | 0.8 | <0.5 or >2.0 | Mismatch pathology | Vascular redistribution | 30-60 minutes |
| Diffusion Capacity | 25-30 mL/min/mmHg | <20 mL/min/mmHg | Interstitial disease | Increased cardiac output | Hours to days |
| Shunt Fraction | <5% | >15% | Intrapulmonary shunt | Positive pressure ventilation | Variable |
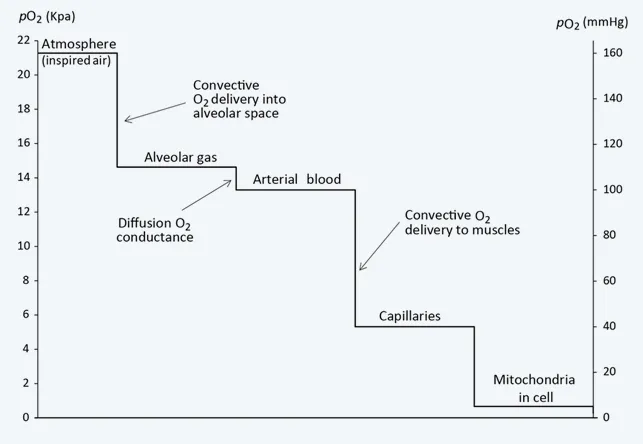
The oxygen cascade demonstrates the progressive decline in oxygen partial pressure from atmosphere to mitochondria. Atmospheric PO₂ of 159 mmHg drops to alveolar PO₂ of 100 mmHg, then arterial PO₂ of 95 mmHg, mixed venous PO₂ of 40 mmHg, and finally mitochondrial PO₂ of 1-3 mmHg. Each step represents a critical control point where pathology can disrupt oxygen delivery.
💡 Master This: The alveolar gas equation - PAO₂ = FiO₂(PB - PH₂O) - PaCO₂/RQ - predicts alveolar oxygen tension. At sea level with room air: PAO₂ = 0.21(760-47) - 40/0.8 = 100 mmHg. This calculation enables precise A-a gradient determination for any clinical scenario.
Understanding gas exchange mechanics unlocks the logic behind every respiratory pathophysiology pattern, from simple pneumonia to complex ARDS presentations.
🫁 The Alveolar Exchange Engine: Precision Gas Trading
⚡ Hemoglobin's Molecular Cargo System: The Oxygen Shuttle
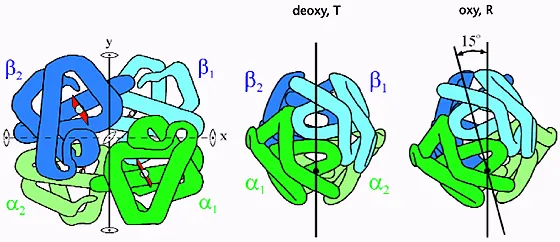
Hemoglobin's cooperative binding creates the characteristic S-shaped oxygen-hemoglobin dissociation curve. The P50 value of 27 mmHg represents the oxygen tension at 50% saturation, serving as the critical reference point. Cooperative binding means the first oxygen molecule binding increases affinity for subsequent molecules by 300%, while the fourth molecule binds with 10-fold higher affinity than the first.
📌 Remember: CADET face Right for rightward curve shifts (decreased oxygen affinity) - CO₂ increase, Acid increase (↓pH), DPG increase, Exercise/temperature increase, Tissue metabolism increase. Each factor shifts P50 from 27 to 35+ mmHg.
The mathematical relationship follows the Hill equation: Y = (PO₂ⁿ)/(P50ⁿ + PO₂ⁿ), where n = 2.8 represents the cooperativity coefficient. This sigmoidal curve ensures efficient oxygen loading in lungs (PO₂ = 100 mmHg, 97% saturation) and effective unloading in tissues (PO₂ = 40 mmHg, 75% saturation).
| Condition | P50 (mmHg) | Oxygen Affinity | Tissue Delivery | Clinical Impact | Compensation Time |
|---|---|---|---|---|---|
| Normal | 27 | Baseline | Optimal | Standard function | N/A |
| Acidosis (pH 7.2) | 35 | Decreased | Increased | Enhanced unloading | 2-4 hours |
| Alkalosis (pH 7.6) | 20 | Increased | Decreased | Impaired unloading | 4-8 hours |
| Hyperthermia (40°C) | 32 | Decreased | Increased | Fever compensation | 1-2 hours |
| High 2,3-DPG | 31 | Decreased | Increased | Chronic hypoxia adaptation | 24-48 hours |
| Carbon Monoxide | 15 | Markedly increased | Severely impaired | Life-threatening | 4-6 hours with O₂ |
2,3-diphosphoglycerate (2,3-DPG) serves as the primary physiological modulator of oxygen affinity. Chronic hypoxia increases 2,3-DPG levels by 50-100% within 24-48 hours, shifting the curve rightward to enhance tissue oxygen delivery. Stored blood loses 2,3-DPG within 7-14 days, reducing oxygen delivery capacity by 25%.
💡 Master This: Bohr effect quantification - pH decrease of 0.1 units shifts P50 rightward by 2-3 mmHg, while CO₂ increase of 10 mmHg produces similar shifts. This ensures automatic oxygen unloading in metabolically active tissues producing acid and CO₂.
The fetal hemoglobin advantage demonstrates evolutionary optimization: HbF P50 = 19 mmHg versus HbA P50 = 27 mmHg, creating a left-shifted curve that facilitates placental oxygen transfer from maternal to fetal circulation across oxygen gradients of 20-30 mmHg.
Mastering hemoglobin kinetics reveals the molecular precision behind every oxygen delivery calculation and therapeutic intervention strategy.
⚡ Hemoglobin's Molecular Cargo System: The Oxygen Shuttle
🎯 Clinical Pattern Recognition: The Hypoxemia Detective Framework
Systematic Hypoxemia Approach:
- Step 1: Calculate A-a gradient using PAO₂ - PaO₂
- Normal: <10 mmHg (age <40) or <25 mmHg (elderly)
- Elevated: >25 mmHg indicates pulmonary disease
- Step 2: Assess oxygen response to 100% FiO₂
- Shunt: <50 mmHg improvement suggests >20% shunt fraction
- V/Q mismatch: >100 mmHg improvement typical
- Step 3: Evaluate CO₂ retention patterns
- Hypoventilation: PaCO₂ >45 mmHg with normal A-a gradient
- Dead space: Increased minute ventilation with CO₂ retention
📌 Remember: SHAVED for hypoxemia mechanisms - Shunt (anatomic/physiologic), Hypoventilation (central/peripheral), Altitude (low FiO₂), V/Q mismatch (most common), Equilibration defect (diffusion limitation), Diffusion impairment (interstitial disease).
| Mechanism | A-a Gradient | O₂ Response | PaCO₂ Pattern | Common Causes | Diagnostic Test |
|---|---|---|---|---|---|
| Hypoventilation | Normal (<15) | Excellent | Elevated (>45) | Drug overdose, CNS disease | Ventilatory drive testing |
| V/Q Mismatch | Elevated (>25) | Good (>100 mmHg) | Variable | Pneumonia, asthma, COPD | Chest CT, V/Q scan |
| Shunt | Markedly elevated | Poor (<50 mmHg) | Normal/low | ARDS, pneumonia, AVM | Echocardiography, angiography |
| Diffusion Limitation | Elevated | Moderate | Normal/low | Pulmonary fibrosis, emphysema | DLCO measurement |
| Low FiO₂ | Normal | Excellent | Normal/low | Altitude, equipment failure | Environmental assessment |
Exercise-induced hypoxemia reveals diffusion limitation in interstitial lung disease. Resting DLCO <60% predicted with >4% oxygen desaturation during 6-minute walk test indicates significant diffusion impairment requiring supplemental oxygen during activity.
💡 Master This: Oxygen content equation - CaO₂ = (1.34 × Hgb × SaO₂) + (0.003 × PaO₂) - reveals that hemoglobin binding contributes >95% of oxygen transport. Anemia with Hgb <7 g/dL can cause tissue hypoxia despite normal PaO₂ and SaO₂.
Methemoglobinemia presents the classic "chocolate blood" appearance with SpO₂ plateau at 85% despite normal PaO₂. Co-oximetry reveals methemoglobin levels >15%, requiring methylene blue 1-2 mg/kg IV for symptomatic patients.
Understanding these recognition patterns transforms complex presentations into systematic diagnostic pathways with predictable therapeutic responses.
🎯 Clinical Pattern Recognition: The Hypoxemia Detective Framework
🔬 Ventilation-Perfusion Dynamics: The Lung's Matching Game
Gravitational effects create three distinct lung zones with different V/Q characteristics. Zone 1 (apex): V/Q >1.0 due to minimal perfusion and maximal ventilation. Zone 2 (middle): V/Q ≈ 0.8 representing optimal matching. Zone 3 (base): V/Q <0.8 due to increased perfusion and relatively decreased ventilation.
V/Q Ratio Physiology:
- Normal V/Q = 0.8 (4 L/min ventilation ÷ 5 L/min perfusion)
- Dead space: V/Q >1.0 - ventilation without perfusion
- Shunt: V/Q <0.1 - perfusion without ventilation
- Silent unit: V/Q = 0 - neither ventilation nor perfusion
📌 Remember: APEX-BASE gradient effects - Apex has Poor perfusion, Excellent ventilation, Xtra dead space; Base has Abundant perfusion, Suboptimal ventilation, Excess shunt tendency.
| Lung Zone | V/Q Ratio | Ventilation | Perfusion | PO₂ (mmHg) | PCO₂ (mmHg) | Clinical Relevance |
|---|---|---|---|---|---|---|
| Zone 1 (Apex) | 3.0-∞ | High | Minimal | 130-150 | 28-32 | Dead space, PE risk |
| Zone 2 (Middle) | 0.8-1.2 | Moderate | Moderate | 100-110 | 35-40 | Optimal gas exchange |
| Zone 3 (Base) | 0.3-0.8 | Lower | High | 85-95 | 42-46 | Shunt tendency |
| Pathological Shunt | 0-0.1 | Variable | High | 40-60 | 45-50 | Requires PEEP |
| Pathological Dead Space | >3.0 | High | Minimal | >130 | <30 | Requires volume |
Pulmonary embolism creates acute dead space with V/Q ratios >3.0 in affected segments. D-dimer >500 ng/mL with sudden dyspnea and normal chest X-ray suggests PE probability >85%. CTPA remains gold standard with sensitivity >95% and specificity >90%.
ARDS pathophysiology demonstrates heterogeneous V/Q distribution with baby lung concept - only 20-30% of lung tissue remains recruitable with normal V/Q ratios. Prone positioning improves V/Q matching by redistributing perfusion to previously dependent regions.
💡 Master This: Shunt equation - Qs/Qt = (PAO₂ - PaO₂) × 0.003 / [(PAO₂ - PaO₂) × 0.003 + 1.34 × Hgb × (SaO₂ - SvO₂)] - calculates true shunt fraction. Values >20% indicate severe V/Q mismatch requiring mechanical ventilation.
Hypoxic pulmonary vasoconstriction serves as the lung's automatic V/Q matching system. Alveolar PO₂ <60 mmHg triggers local vasoconstriction within 2-5 minutes, redirecting blood flow to better-ventilated regions. This mechanism becomes maladaptive in global hypoxia, causing pulmonary hypertension.
Exercise physiology reveals V/Q optimization - cardiac output increases 4-6 fold while minute ventilation increases 10-15 fold, improving overall V/Q matching from 0.8 to 1.2 during maximal exercise.
These V/Q principles guide ventilator management, positioning therapy, and PEEP optimization in critically ill patients.
🔬 Ventilation-Perfusion Dynamics: The Lung's Matching Game
⚕️ Therapeutic Gas Exchange Optimization: The Clinical Command Center
ARDS management follows the ARDSNet protocol with low tidal volume ventilation (6 mL/kg predicted body weight), PEEP titration to maintain plateau pressure <30 cmH₂O, and FiO₂ optimization targeting SpO₂ 88-95%. This approach reduces mortality by 22% compared to traditional ventilation.
PEEP optimization uses the decremental PEEP trial - start at 20 cmH₂O, decrease by 2 cmH₂O every 5 minutes while monitoring compliance and oxygenation. Optimal PEEP occurs at maximum compliance with adequate oxygenation (PaO₂ >60 mmHg on FiO₂ <0.6).
| Intervention | Mechanism | Oxygenation Improvement | Time to Effect | Monitoring Parameter | Success Rate |
|---|---|---|---|---|---|
| PEEP 5-15 cmH₂O | Alveolar recruitment | 20-50 mmHg | 5-15 minutes | Compliance, PaO₂ | 85-90% |
| Prone positioning | V/Q redistribution | 30-80 mmHg | 2-6 hours | PaO₂/FiO₂ ratio | 70-80% |
| Recruitment maneuvers | Alveolar opening | 40-100 mmHg | 15-30 minutes | Static compliance | 60-75% |
| Inhaled NO 20-40 ppm | Selective vasodilation | 10-30 mmHg | 5-10 minutes | PVR, oxygenation | 50-60% |
| ECMO | Extracorporeal support | Variable | 2-4 hours | Sweep gas flow | 60-70% survival |
Oxygen toxicity becomes significant at FiO₂ >0.6 for >48 hours, causing absorption atelectasis and inflammatory lung injury. Target SpO₂ 88-95% in ARDS patients to minimize FiO₂ exposure while maintaining adequate oxygen delivery.
📌 Remember: PROVE for ARDS ventilation - Plateau pressure <30 cmH₂O, Respiratory rate 6-35/min, Oxygenation SpO₂ 88-95%, Volume 6 mL/kg PBW, Expiratory time adequate (I:E 1:1 to 1:3).
Extracorporeal membrane oxygenation (ECMO) serves as rescue therapy for refractory hypoxemia with PaO₂/FiO₂ <80 despite optimal ventilation. VV-ECMO provides gas exchange support with blood flow 3-5 L/min and sweep gas 1-10 L/min, achieving CO₂ removal and oxygenation while allowing lung-protective ventilation.
Inhaled nitric oxide causes selective pulmonary vasodilation in ventilated lung regions, improving V/Q matching without systemic hypotension. Doses of 20-40 ppm improve oxygenation by 15-25% in 50-60% of patients, but mortality benefit remains unproven.
💡 Master This: Oxygen delivery equation - DO₂ = CO × CaO₂ × 10 = CO × (1.34 × Hgb × SaO₂ + 0.003 × PaO₂) × 10. Normal DO₂ = 1000 mL/min. Critical threshold <400 mL/min triggers anaerobic metabolism and lactic acidosis.
High-frequency oscillatory ventilation (HFOV) delivers tidal volumes <dead space at frequencies 3-15 Hz, maintaining continuous alveolar recruitment while minimizing barotrauma. Mean airway pressure 25-35 cmH₂O provides oxygenation while oscillatory amplitude controls CO₂ elimination.
These advanced interventions require continuous monitoring and systematic titration to optimize gas exchange while minimizing complications in critically ill patients.
⚕️ Therapeutic Gas Exchange Optimization: The Clinical Command Center
🧬 Molecular Gas Transport Integration: The Cellular Delivery Network
Oxygen transport integration involves four coordinated systems: pulmonary gas exchange, hemoglobin binding, cardiovascular delivery, and cellular extraction. Fick equation quantifies this integration: VO₂ = CO × (CaO₂ - CvO₂), where normal oxygen consumption = 250 mL/min with cardiac output = 5 L/min and arteriovenous difference = 5 mL/dL.
Tissue oxygen extraction varies dramatically by organ: brain extracts 35% (high metabolic demand), kidney extracts 10% (high blood flow), heart extracts 75% (maximal extraction), and skeletal muscle extracts 25-85% (exercise-dependent). Mixed venous saturation (SvO₂) = 75% reflects global oxygen balance.
📌 Remember: HEART organs for oxygen extraction - Heart (75%), Exercising muscle (85%), Arterial system (25% average), Renal (10%), Tissue average (25%). SvO₂ <65% indicates inadequate oxygen delivery or excessive consumption.
Carbon dioxide transport demonstrates superior efficiency compared to oxygen: CO₂ content = 48 mL/dL versus O₂ content = 20 mL/dL in venous blood. Three transport mechanisms: dissolved CO₂ (10%), carbamino compounds (30%), and bicarbonate (60%) via carbonic anhydrase.
| Transport System | O₂ Capacity | CO₂ Capacity | Binding Affinity | Response Time | Efficiency Factor |
|---|---|---|---|---|---|
| Dissolved gas | 0.3 mL/dL | 2.4 mL/dL | Linear | Instantaneous | 1x baseline |
| Hemoglobin binding | 20 mL/dL | 14 mL/dL | Cooperative | 0.25 seconds | 67x dissolved |
| Bicarbonate system | N/A | 32 mL/dL | Enzymatic | 0.1 seconds | 13x dissolved |
| Carbamino binding | N/A | 2 mL/dL | Direct | 0.05 seconds | 5x dissolved |
| Total capacity | 20.3 mL/dL | 48.4 mL/dL | Combined | Variable | Integrated |
Chloride shift (Hamburger phenomenon) maintains electroneutrality during CO₂ transport. Bicarbonate efflux from red blood cells triggers chloride influx, causing venous hematocrit to be 3-5% higher than arterial hematocrit due to osmotic cell swelling.
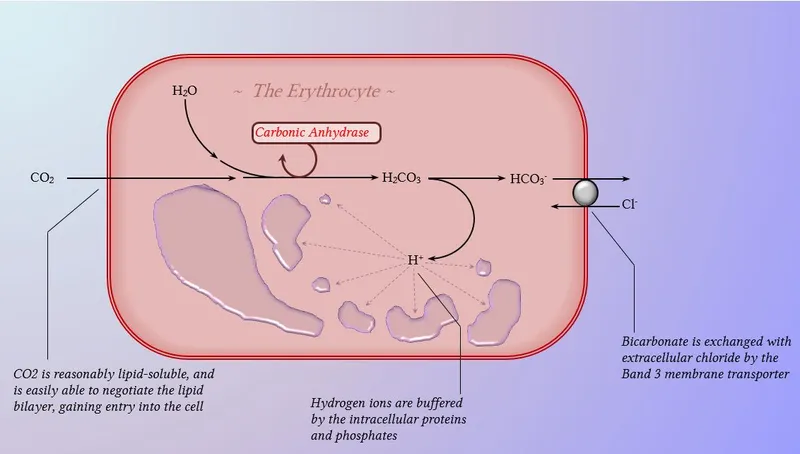
Carbonic anhydrase catalyzes CO₂ + H₂O ↔ H₂CO₃ ↔ H⁺ + HCO₃⁻ with turnover rate = 10⁶ reactions/second. Acetazolamide inhibition reduces CO₂ transport efficiency by 60%, causing metabolic acidosis and compensatory hyperventilation.
💡 Master This: Bohr and Haldane effects work synergistically - tissue acidosis promotes O₂ unloading (Bohr) while O₂ unloading promotes CO₂ loading (Haldane). This dual effect increases gas exchange efficiency by 85% compared to non-cooperative binding.
Exercise integration demonstrates maximal system coordination: cardiac output increases 5-fold, oxygen extraction increases 3-fold, and ventilation increases 15-fold, achieving VO₂max = 3.5 L/min in trained athletes. Lactate threshold occurs when oxygen delivery becomes insufficient for aerobic metabolism.
Pathological integration in sepsis shows dysregulated oxygen utilization: elevated cardiac output with decreased oxygen extraction due to mitochondrial dysfunction. SvO₂ >80% with elevated lactate indicates cytopathic hypoxia despite adequate oxygen delivery.
Fetal circulation optimizes oxygen extraction across placental gradients: fetal hemoglobin P50 = 19 mmHg versus maternal P50 = 27 mmHg creates oxygen affinity gradient enabling transfer despite low placental PO₂ = 30-35 mmHg.
Understanding molecular integration reveals how genetic variations, environmental factors, and disease states affect oxygen transport efficiency and therapeutic responses.
🧬 Molecular Gas Transport Integration: The Cellular Delivery Network
🎯 Gas Exchange Mastery Arsenal: Clinical Command Tools
Essential Clinical Calculations:
- A-a Gradient = PAO₂ - PaO₂ = [FiO₂(760-47) - PaCO₂/0.8] - PaO₂
- Shunt Fraction = (CcO₂ - CaO₂)/(CcO₂ - CvO₂) × 100%
- Oxygen Delivery = CO × 1.34 × Hgb × SaO₂ × 10 mL/min
- P/F Ratio = PaO₂/FiO₂ (normal >400, ARDS <300)
- Dead Space = (PaCO₂ - PECO₂)/PaCO₂ × 100%
📌 Remember: RAPID assessment protocol - Respiratory rate and effort, A-a gradient calculation, Pulse oximetry and ABG, Imaging interpretation, Differential diagnosis formation. Complete assessment within 3-5 minutes for optimal outcomes.
Critical Threshold Values:
- PaO₂ <60 mmHg: Respiratory failure requiring intervention
- A-a gradient >25 mmHg: Pulmonary pathology present
- Shunt fraction >15%: Consider PEEP or prone positioning
- P/F ratio <200: Severe ARDS, consider ECMO evaluation
- SvO₂ <65%: Inadequate oxygen delivery or excessive consumption
| Clinical Scenario | Key Parameters | Immediate Action | Target Goals | Monitoring Frequency | Success Metrics |
|---|---|---|---|---|---|
| ARDS (P/F <200) | PEEP, Compliance | Low TV, High PEEP | SpO₂ 88-95% | Every 4 hours | Mortality reduction |
| Massive PE | RV strain, D-dimer | Anticoagulation/lysis | Hemodynamic stability | Continuous | Clot resolution |
| Severe Asthma | Peak flow, CO₂ | Bronchodilators, steroids | Normal ventilation | Every 15 minutes | Symptom resolution |
| Pneumonia | Infiltrates, WBC | Antibiotics, O₂ | Infection clearance | Daily | Clinical improvement |
| Pulmonary Edema | BNP, Echo | Diuretics, afterload reduction | Euvolemia | Every 2 hours | Symptom relief |
Ventilator Liberation Protocol:
- Daily sedation interruption with spontaneous breathing trial
- RSBI <105 (respiratory rate/tidal volume in L)
- PEEP ≤8 cmH₂O and FiO₂ ≤0.4
- Hemodynamic stability without vasopressors
- Adequate cough and airway protection
💡 Master This: Oxygen cascade optimization - atmospheric (159 mmHg) → alveolar (100 mmHg) → arterial (95 mmHg) → tissue (40 mmHg) → mitochondrial (1-3 mmHg). Each step represents intervention opportunity: FiO₂, ventilation, perfusion, hemoglobin, extraction.
Advanced Monitoring Tools:
- Volumetric capnography: Dead space calculation and CO₂ elimination efficiency
- Electrical impedance tomography: Real-time ventilation distribution and PEEP optimization
- Transpulmonary pressure: Lung stress assessment and recruitment potential
- Extravascular lung water: Pulmonary edema quantification and fluid management
Emergency Response Algorithms for life-threatening hypoxemia: 100% oxygen, bag-mask ventilation, intubation preparation, PEEP application, hemodynamic support. Target SpO₂ >90% within 5 minutes to prevent cardiac arrest.
These mastery tools enable rapid assessment, evidence-based intervention, and continuous optimization of gas exchange in any clinical setting.
🎯 Gas Exchange Mastery Arsenal: Clinical Command Tools
Practice Questions: Gas exchange
Test your understanding with these related questions
A 33-year-old woman is brought to the emergency department 30 minutes after being rescued from a fire in her apartment. She reports nausea, headache, and dizziness. Physical examination shows black discoloration of her oral mucosa. Pulse oximetry shows an oxygen saturation of 99% on room air. The substance most likely causing symptoms in this patient primarily produces toxicity by which of the following mechanisms?













