Integration of metabolic regulation US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Integration of metabolic regulation. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Integration of metabolic regulation US Medical PG Question 1: A 35-year-old woman presents to the clinic for a several-month history of heat intolerance. She lives in a small apartment with her husband and reports that she always feels hot and sweaty, even when their air conditioning is on high. On further questioning, she's also had a 4.5 kg (10 lb) unintentional weight loss. The vital signs include: heart rate 102/min and blood pressure 150/80 mm Hg. The physical exam is notable for warm and slightly moist skin. She also exhibits a fine tremor in her hands when her arms are outstretched. Which of the following laboratory values is most likely low in this patient?
- A. Triiodothyronine (T3)
- B. Thyroxine (T4)
- C. Calcitonin
- D. Glucose
- E. Thyroid-stimulating hormone (Correct Answer)
Integration of metabolic regulation Explanation: ***Thyroid-stimulating hormone***
- The patient's symptoms (heat intolerance, weight loss, tachycardia, hypertension, warm/moist skin, fine tremor) are classic for **hyperthyroidism**.
- In primary hyperthyroidism, the thyroid gland overproduces T3 and T4, which **negatively feedbacks** on the pituitary, leading to a **low TSH** level.
*Triiodothyronine (T3)*
- In hyperthyroidism, **T3 levels are typically elevated**, not low, as the thyroid gland is overactive.
- T3 is one of the primary thyroid hormones responsible for the patient's metabolic symptoms.
*Thyroxine (T4)*
- In hyperthyroidism, **T4 levels are typically elevated**, not low, alongside T3.
- T4 is the other key thyroid hormone produced in excess, contributing to the hypermetabolic state.
*Calcitonin*
- Calcitonin is a hormone involved in **calcium regulation** and is produced by the parafollicular C cells of the thyroid gland.
- Its levels are not directly affected by hyperthyroidism and would not be consistently low in this scenario.
*Glucose*
- While hyperthyroidism can affect glucose metabolism, causing increased gluconeogenesis and glycogenolysis, it more commonly leads to **elevated or normal glucose levels**, not consistently low levels.
- Low glucose would typically suggest other conditions like insulinoma or adrenal insufficiency.
Integration of metabolic regulation US Medical PG Question 2: A 24-year-old man presents for an annual check-up. He is a bodybuilder and tells you he is on a protein-rich diet that only allows for minimal carbohydrate intake. His friend suggests he try exogenous glucagon to help him lose some excess weight before an upcoming competition. Which of the following effects of glucagon is he attempting to exploit?
- A. Increased glucose utilization by tissues
- B. Decreased blood cholesterol level
- C. Increased hepatic gluconeogenesis
- D. Increased lipolysis in adipose tissues (Correct Answer)
- E. Increased hepatic glycogenolysis
Integration of metabolic regulation Explanation: ***Increased lipolysis in adipose tissues***
- While **glucagon's primary target is the liver**, it can have **modest lipolytic effects** on adipose tissue by opposing insulin's anti-lipolytic actions.
- Glucagon stimulates cAMP production, which can activate **hormone-sensitive lipase** to break down triglycerides into **fatty acids** and **glycerol**.
- However, **catecholamines (epinephrine/norepinephrine)** are far more potent direct stimulators of adipose tissue lipolysis than glucagon.
- The friend is attempting to exploit this lipolytic effect for fat loss, though **exogenous glucagon is not an evidence-based or safe weight-loss strategy**.
*Increased glucose utilization by tissues*
- This is **opposite** to glucagon's actual effect. **Glucagon raises blood glucose** levels; it does not promote glucose uptake by peripheral tissues.
- **Insulin** is the hormone responsible for promoting glucose uptake and utilization by muscle, adipose, and other tissues.
*Decreased blood cholesterol level*
- Glucagon does not have a direct, clinically significant effect on reducing blood cholesterol levels.
- While glucagon affects overall lipid metabolism through its catabolic actions, it is not used therapeutically for hypercholesterolemia.
*Increased hepatic gluconeogenesis*
- **Glucagon strongly stimulates hepatic gluconeogenesis**, which is the synthesis of glucose from non-carbohydrate precursors (amino acids, lactate, glycerol) in the liver.
- This action **raises blood glucose** levels and would not directly contribute to fat loss or weight reduction.
- In the context of a low-carbohydrate diet, increased gluconeogenesis would maintain blood glucose but not promote the fat loss the bodybuilder seeks.
*Increased hepatic glycogenolysis*
- **Glucagon is a potent stimulator of hepatic glycogenolysis**, the breakdown of stored liver glycogen into glucose.
- This rapidly increases blood glucose levels during fasting or hypoglycemia.
- However, this does not directly target adipose tissue for fat loss; it mobilizes glucose stores rather than fat stores, so it's not the mechanism relevant to weight loss goals.
Integration of metabolic regulation US Medical PG Question 3: A 65-year-old male prisoner goes on a hunger strike to protest the conditions of his detainment. After 5 days without food, he suffers a seizure for which he is taken into a medical facility. On physical examination, he looks pale and diaphoretic. His blood glucose level is 50 mg/dL. In order to keep a constant supply of energy to his brain, which of the following molecules is his liver releasing into the bloodstream?
- A. Glycogen
- B. Glucose-6-phosphate
- C. ß-hydroxybutyric acid (Correct Answer)
- D. Fatty acids
- E. Glucose-1-phosphate
Integration of metabolic regulation Explanation: ***ß-hydroxybutyric acid***
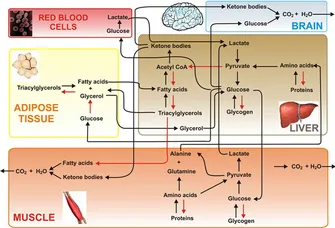
- After 5 days of a hunger strike, **glycogen stores** are depleted, forcing the body to rely on **fatty acid oxidation** and **ketone body production** in the liver as an alternative fuel source for the brain.
- **ß-hydroxybutyrate** is one of the primary ketone bodies released by the liver into the bloodstream to provide energy, especially for the brain, during prolonged fasting.
*Glycogen*
- **Glycogenolysis** (breakdown of glycogen) is a short-term response to low blood glucose and supplies glucose for only about 24-36 hours of fasting. After 5 days, **hepatic glycogen stores** would be largely depleted.
- The liver releases **free glucose** into the bloodstream, not intact glycogen, from glycogen breakdown.
*Glucose-6-phosphate*
- **Glucose-6-phosphate** is an intermediate in glycolysis and gluconeogenesis, but it is not directly released into the bloodstream by the liver.
- It must be converted to **free glucose** by glucose-6-phosphatase before it can exit the hepatocyte and enter circulation.
*Fatty acids*
- The liver takes up **fatty acids** from adipose tissue breakdown during prolonged fasting to convert them into **ketone bodies**.
- While fatty acids are a major energy source for other tissues, the **brain cannot directly utilize fatty acids** for energy due to the inability of long-chain fatty acids to cross the blood-brain barrier.
*Glucose-1-phosphate*
- **Glucose-1-phosphate** is an intermediate formed during the breakdown of glycogen (glycogenolysis).
- Like glucose-6-phosphate, it is not directly released into the bloodstream but is further metabolized within the hepatocyte, eventually leading to the release of **free glucose**.
Integration of metabolic regulation US Medical PG Question 4: A researcher is tracing the fate of C-peptide, a product of preproinsulin cleavage. Which of the following is a true statement regarding the fate of C-peptide?
- A. C-peptide exits the cells via a protein channel
- B. C-peptide is further cleaved into insulin
- C. C-peptide is packaged with insulin in secretory vesicles (Correct Answer)
- D. C-peptide is immediately degraded by the proteasome
- E. C-peptide activates an intracellular signaling cascade
Integration of metabolic regulation Explanation: ***C-peptide is packaged with insulin in secretory vesicles***
- Preproinsulin is cleaved in the **endoplasmic reticulum** to proinsulin (signal peptide removal), which is then transported to the **Golgi apparatus**.
- In the Golgi, proinsulin is cleaved by **prohormone convertases** into **insulin** and **C-peptide**, and both are stored together in **secretory vesicles** within the pancreatic beta cells.
- Upon stimulation, both insulin and C-peptide are **co-secreted** via exocytosis in equimolar amounts, making C-peptide a useful marker of endogenous insulin secretion.
*C-peptide exits the cells via a protein channel*
- C-peptide exits the beta cells via **exocytosis** of secretory granules, not through specific protein channels.
- It is **co-secreted with insulin** when secretory vesicles fuse with the plasma membrane.
- Its presence in the bloodstream in equimolar amounts with insulin makes it an indirect measure of **insulin secretion**.
*C-peptide is further cleaved into insulin*
- **C-peptide** is a product of proinsulin cleavage, alongside insulin; it is not further processed into insulin.
- Insulin itself is composed of two **peptide chains (A and B)** linked by disulfide bonds, formed after C-peptide is removed from proinsulin.
*C-peptide is immediately degraded by the proteasome*
- C-peptide is not immediately degraded by the **proteasome** upon synthesis.
- After secretion, it circulates in the blood with a **longer half-life** than insulin (approximately 30 minutes versus 4-6 minutes), allowing it to be a useful marker of endogenous insulin production.
- Its degradation occurs primarily in the **kidney**.
*C-peptide activates an intracellular signaling cascade*
- While there is some research suggesting C-peptide may have independent **biological activity** and activate certain signaling pathways extracellularly, its primary role in the context of the insulin synthesis pathway is as a **byproduct** of proinsulin processing.
- Its clinical utility is primarily as a **biomarker** of endogenous insulin secretion, particularly useful in distinguishing between endogenous and exogenous insulin in diabetic patients.
Integration of metabolic regulation US Medical PG Question 5: A 46-year-old man comes to the physician for a follow-up examination. He has type 2 diabetes mellitus and hypertension. Current medications include metformin and lisinopril. He reports that he has adhered to his diet and medication regimen. His hemoglobin A1c is 8.6%. Insulin glargine is added to his medication regimen. Which of the following sets of changes is most likely to occur in response to this new medication?
$$$ Glycolysis %%% Glycogenesis %%% Lipolysis %%% Gluconeogenesis $$$
- A. ↓ ↓ ↑ ↑
- B. ↑ ↓ ↑ ↓
- C. ↓ ↑ ↓ ↑
- D. ↑ ↑ ↓ ↓ (Correct Answer)
- E. ↑ ↓ ↑ ↑
Integration of metabolic regulation Explanation: ***↑ ↑ ↓ ↓***
- Insulin **increases glucose utilization** by promoting glycolysis and increases glucose storage by promoting glycogenesis.
- Insulin **inhibits glucose production** by decreasing lipolysis and gluconeogenesis.
*↓ ↓ ↑ ↑*
- This option incorrectly suggests that insulin would decrease glycolysis and glycogenesis, which are pathways for glucose utilization and storage.
- It also incorrectly suggests that insulin would increase lipolysis and gluconeogenesis, which are pathways for glucose production.
*↑ ↓ ↑ ↓*
- This option correctly indicates an increase in glycolysis and a decrease in gluconeogenesis, but incorrectly suggests a decrease in glycogenesis and an increase in lipolysis.
- Insulin's primary role is to lower blood glucose, which involves promoting both glucose utilization (glycolysis) and storage (glycogenesis).
*↓ ↑ ↓ ↑*
- This option incorrectly suggests a decrease in glycolysis and an increase in gluconeogenesis, which would lead to higher blood glucose.
- While it correctly shows an increase in glycogenesis and a decrease in lipolysis, the overall pattern does not match insulin's coordinated metabolic effects.
*↑ ↓ ↑ ↑*
- This option incorrectly suggests a decrease in glycogenesis and an increase in lipolysis and gluconeogenesis, which would lead to higher blood glucose.
- Insulin's main actions are to promote glucose uptake and storage and to inhibit glucose production.
Integration of metabolic regulation US Medical PG Question 6: Researchers are experimenting with hormone levels in mice in fasting and fed states. To test hormone levels in the fed state, the mice are given an oral glucose load and various hormones are measured in a blood sample. Researchers are most interested in the hormone whose blood levels track evenly with C-peptide levels. The hormone the researchers are most interested in is responsible for which of the following actions in the body?
- A. Protein catabolism
- B. Fatty acid breakdown
- C. Fatty acid synthesis (Correct Answer)
- D. Ketogenesis
- E. Lipolysis
Integration of metabolic regulation Explanation: ***Fatty acid synthesis***
- The hormone whose blood levels track evenly with **C-peptide** levels after a glucose load is **insulin**.
- Insulin is a key anabolic hormone that promotes **fatty acid synthesis** from excess glucose in the fed state, particularly in the liver and adipose tissue.
*Protein catabolism*
- **Insulin** is an anabolic hormone that generally **inhibits protein catabolism** and promotes protein synthesis.
- Conditions like **glucagon excess** or **cortisol excess** promote protein catabolism, not insulin.
*Fatty acid breakdown*
- **Insulin inhibits fatty acid breakdown** (beta-oxidation) by suppressing hormone-sensitive lipase.
- **Glucagon** and **epinephrine** promote fatty acid breakdown, especially during fasting.
*Ketogenesis*
- **Insulin inhibits ketogenesis** by reducing the supply of fatty acids to the liver and inhibiting the enzymes involved in ketone body formation.
- **Glucagon** and **low insulin levels** (as in uncontrolled diabetes or prolonged fasting) promote ketogenesis.
*Lipolysis*
- **Insulin is a potent inhibitor of lipolysis** (breakdown of triglycerides into fatty acids and glycerol) in adipose tissue.
- **Glucagon**, **catecholamines**, and **growth hormone** stimulate lipolysis.
Integration of metabolic regulation US Medical PG Question 7: Certain glucose transporters that are expressed predominantly on skeletal muscle cells and adipocytes are unique compared to those transporters found on other cell types within the body. Without directly affecting glucose transport in other cell types, which of the following would be most likely to selectively increase glucose uptake in skeletal muscle cells and adipocytes?
- A. Increased plasma glucose concentration
- B. It is physiologically impossible to selectively increase glucose uptake in specific cells
- C. Increased levels of circulating insulin (Correct Answer)
- D. Decreased plasma glucose concentration
- E. Decreased levels of circulating insulin
Integration of metabolic regulation Explanation: ***Increased levels of circulating insulin***
- Insulin stimulates the translocation of **GLUT4 transporters** from intracellular vesicles to the cell membrane in **skeletal muscle** and **adipocytes**, thereby increasing glucose uptake.
- This mechanism is **selective** because other cell types (e.g., brain, liver) primarily use insulin-independent glucose transporters (e.g., GLUT1, GLUT2, GLUT3) that are constitutively active or respond to different signals.
*Increased plasma glucose concentration*
- While increased glucose concentration would drive glucose uptake in many cells, it is not **selective** for skeletal muscle and adipocytes since other cells also take up glucose.
- Insulin-independent tissues would also increase glucose uptake, making this a non-specific effect.
*It is physiologically impossible to selectively increase glucose uptake in specific cells*
- This statement is incorrect because the body has mechanisms, such as **insulin-mediated GLUT4 translocation**, that specifically regulate glucose uptake in certain cell types like skeletal muscle and adipocytes.
- This regulatory specificity is crucial for maintaining **glucose homeostasis**.
*Decreased plasma glucose concentration*
- A decrease in plasma glucose would generally **reduce** glucose uptake across all cell types, including skeletal muscle and adipocytes.
- It would not selectively increase uptake in any specific cell population.
*Decreased levels of circulating insulin*
- Decreased insulin levels would lead to **reduced** glucose uptake in insulin-sensitive tissues like skeletal muscle and adipocytes, as GLUT4 transporters would remain sequestered intracellularly.
- This would result in higher circulating glucose levels rather than increased uptake.
Integration of metabolic regulation US Medical PG Question 8: A 60-year-old man with type 2 diabetes on metformin and insulin presents with 3 days of nausea, vomiting, and diffuse abdominal pain. He appears ill and confused. Vital signs: BP 95/60 mmHg, HR 115/min, RR 28/min, T 37.2°C. Labs show glucose 380 mg/dL, pH 7.28, HCO3 18 mEq/L, anion gap 24, serum osmolality 310 mOsm/kg, negative urine ketones, creatinine 2.8 mg/dL (baseline 1.1), lactate 8.2 mmol/L. Apply physiological principles to determine the primary acid-base and metabolic disturbance.
- A. Sepsis-induced lactic acidosis with stress hyperglycemia
- B. Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin (Correct Answer)
- C. Alcoholic ketoacidosis with concurrent diabetic emergency
- D. Diabetic ketoacidosis with renal failure from volume depletion
- E. Mixed metabolic acidosis from uremia and starvation ketosis
Integration of metabolic regulation Explanation: ***Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin***
- The patient exhibits features of **Hyperosmolar Hyperglycemic State (HHS)**, including significant hyperglycemia and confusion, but the **negative urine ketones** effectively rule out DKA.
- The severe **high anion gap metabolic acidosis** is driven by a **lactate of 8.2 mmol/L**, likely due to **Metformin-Associated Lactic Acidosis (MALA)** triggered by **acute kidney injury** (creatinine 2.8).
*Sepsis-induced lactic acidosis with stress hyperglycemia*
- While the patient is hypotensive and tachycardic, the **serum glucose of 380 mg/dL** and history of insulin use point primarily to a diabetic emergency rather than simple stress hyperglycemia.
- **Lactic acidosis** in sepsis usually occurs alongside clinical signs of infection, which are not the focus of this metformin-using patient's profile.
*Alcoholic ketoacidosis with concurrent diabetic emergency*
- **Alcoholic ketoacidosis** would typically present with a history of alcohol abuse and **positive ketones** (specifically beta-hydroxybutyrate), which contradicts the **negative urine ketones** found here.
- The primary source of acidosis in this patient is clearly identified as **lactate (8.2 mmol/L)**, not ketoacids.
*Diabetic ketoacidosis with renal failure from volume depletion*
- **Diabetic Ketoacidosis (DKA)** is unlikely given the **negative urine ketones** and a pH/bicarbonate profile that is less severe than typically seen in profound ketoacidosis.
- DKA usually presents with a lower glucose level (often <250-300 mg/dL) compared to the hyperosmolar states seen in **Type 2 Diabetes** patients.
*Mixed metabolic acidosis from uremia and starvation ketosis*
- While **uremia** contributes to the anion gap when creatinine is elevated (2.8 mg/dL), it is rarely the primary cause of an **anion gap of 24** without more advanced renal failure.
- **Starvation ketosis** would result in positive ketones and a much milder acidosis than the profound **lactic acidosis** (8.2 mmol/L) observed in this case.
Integration of metabolic regulation US Medical PG Question 9: A 38-year-old woman presents with hypertension (170/105 mmHg), hypokalemia (2.9 mEq/L), and metabolic alkalosis. Plasma aldosterone is elevated at 35 ng/dL (normal 4-31) and plasma renin activity is suppressed at 0.2 ng/mL/hr (normal 0.5-3.5). CT scan shows a 2.5 cm left adrenal mass. She also reports recent diagnosis of hyperthyroidism and is being evaluated for a neck mass. Synthesize these findings to evaluate for an underlying unifying diagnosis requiring modified treatment approach.
- A. Ectopic ACTH syndrome from thyroid carcinoma causing bilateral adrenal hyperplasia
- B. Multiple endocrine neoplasia type 2 requiring RET proto-oncogene testing and comprehensive screening (Correct Answer)
- C. Carney complex requiring cardiac myxoma screening before adrenal surgery
- D. Isolated aldosterone-producing adenoma requiring unilateral adrenalectomy only
- E. Coincidental adrenal adenoma and Graves' disease requiring separate standard treatments
Integration of metabolic regulation Explanation: ***Multiple endocrine neoplasia type 2 requiring RET proto-oncogene testing and comprehensive screening***
- The presence of an **adrenal mass** and a **neck mass** in a relatively young patient with hypertension points toward **Multiple Endocrine Neoplasia type 2 (MEN 2)**, specifically medullary thyroid cancer and potential pheochromocytoma.
- While the labs mimic **primary hyperaldosteronism**, the high-risk combination requires **RET proto-oncogene** testing to identify a syndromic association and prevent surgical catastrophes.
*Ectopic ACTH syndrome from thyroid carcinoma causing bilateral adrenal hyperplasia*
- Ectopic ACTH typically results from **small cell lung cancer** or bronchial carcinoids and presents with **hypercortisolism** (Cushing syndrome), not primary hyperaldosteronism.
- The CT scan specifically identified a **unilateral 2.5 cm mass**, which is inconsistent with the **bilateral adrenal hyperplasia** seen in ACTH-secreting tumors.
*Carney complex requiring cardiac myxoma screening before adrenal surgery*
- Carney complex usually involves **Primary Pigmented Nodular Adrenocortical Disease (PPNAD)**, which presents with Cushing syndrome, not the mineralocorticoid excess seen here.
- This syndrome is characterized by **skin lentigines**, **blue nevi**, and **atrial myxomas**, which are not reported in this patient's clinical presentation.
*Isolated aldosterone-producing adenoma requiring unilateral adrenalectomy only*
- While the **high aldosterone** and **low renin** (ARR > 30) suggest an **aldosteronoma (Conn syndrome)**, this diagnosis does not account for the concurrent thyroid and neck masses.
- Proceeding with surgery based on an isolated diagnosis would be dangerous if the mass is actually a **pheochromocytoma** (common in MEN 2) disguised by confounding labs.
*Coincidental adrenal adenoma and Graves' disease requiring separate standard treatments*
- **Graves' disease** typically presents with a diffuse goiter and ophthalmopathy rather than a discrete **neck mass**, which is more indicative of a thyroid nodule or carcinoma.
- In medical examinations, clusters of endocrine findings are rarely coincidental; assuming they are unrelated misses the opportunity to screen for **hereditary syndromes**.
Integration of metabolic regulation US Medical PG Question 10: A 32-year-old pregnant woman at 28 weeks gestation with type 1 diabetes presents with recurrent severe hypoglycemia despite reducing her insulin dose. Her insulin requirements have decreased by 40% over the past week. She reports decreased fetal movement. Fetal ultrasound shows intrauterine fetal demise. Evaluate the physiological mechanism explaining her changing insulin requirements in the context of pregnancy loss.
- A. Increased maternal growth hormone from pituitary compensation for fetal loss
- B. Maternal thyroid hormone surge causing enhanced glucose utilization
- C. Loss of placental lactogen and other diabetogenic hormones that normally increase insulin resistance (Correct Answer)
- D. Increased maternal cortisol from stress of fetal loss improving insulin sensitivity
- E. Placental glucose consumption cessation leading to maternal hyperglycemia compensation
Integration of metabolic regulation Explanation: ***Loss of placental lactogen and other diabetogenic hormones that normally increase insulin resistance***
- Sudden **intrauterine fetal demise** leads to the cessation of placental function and a rapid drop in **human placental lactogen (hPL)**, which normally promotes maternal **insulin resistance**.
- Without these antagonizing hormones, the patient's **insulin sensitivity** returns to pre-pregnancy levels, causing a dramatic decrease in insulin requirements and severe **hypoglycemia**.
*Increased maternal growth hormone from pituitary compensation for fetal loss*
- Maternal **pituitary growth hormone** is actually suppressed during pregnancy as placental growth hormone takes over; there is no compensatory surge upon fetal loss.
- Even if growth hormone were to increase, it is a **diabetogenic hormone** that would increase insulin resistance rather than cause hypoglycemia.
*Maternal thyroid hormone surge causing enhanced glucose utilization*
- Pregnancy loss does not trigger a maternal **thyroid hormone surge**; thyroid levels typically stabilize or decrease following placental dysfunction.
- While hyperthyroidism can affect metabolism, it more commonly causes **glucose intolerance** rather than a 40% reduction in insulin needs.
*Increased maternal cortisol from stress of fetal loss improving insulin sensitivity*
- **Cortisol** is a stress hormone that increases gluconeogenesis and **decreases insulin sensitivity**, which would lead to hyperglycemia.
- Normal pregnancy is already a state of **physiologic hypercortisolism**; the loss of placental function actually reduces the contributions of placental CRH and cortisol.
*Placental glucose consumption cessation leading to maternal hyperglycemia compensation*
- While the fetus and placenta stop consuming glucose after demise, the loss of **anti-insulin hormones** has a much larger impact on the maternal metabolic state.
- The clinical presentation clearly shows **hypoglycemia**, which contradicts a compensatory mechanism for maternal hyperglycemia.
More Integration of metabolic regulation US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.