Insulin secretion and action US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Insulin secretion and action. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Insulin secretion and action US Medical PG Question 1: A 57-year-old woman presents to the emergency department with acute onset confusion, sweating, weakness, and tremors. She says that the symptoms started when she went to dinner with friends and had several drinks of alcohol without eating much food. Her past medical history is significant for type 2 diabetes, and she was recently started on a new medication for this disease. She mentions that her doctor warned her about the risk of low blood sugar, especially if she drinks alcohol or skips meals. Which of the following describes the mechanism of action for the most likely diabetes drug that this patient started taking?
- A. Inhibiting dipeptidyl peptidase
- B. Closing potassium channels (Correct Answer)
- C. Inhibiting alpha-glucosidase
- D. Decreasing hepatic gluconeogenesis
- E. Binding to peroxisome proliferator-activating receptors
Insulin secretion and action Explanation: ***Closing potassium channels***
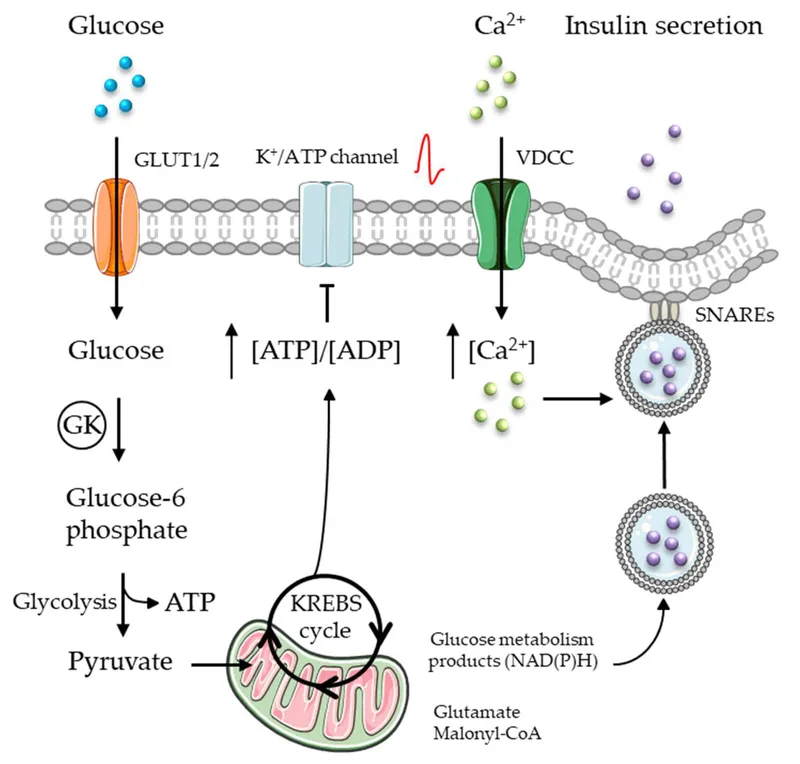
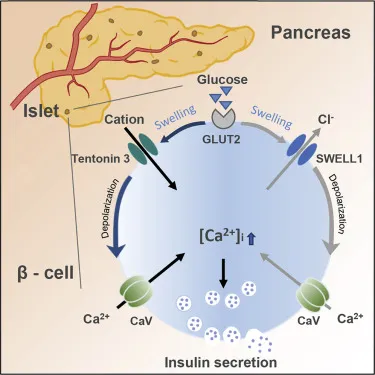
The patient's symptoms (confusion, sweating, weakness, tremors) along with recent alcohol consumption and a new diabetes medication strongly suggest **hypoglycemia**. **Sulfonylureas** are a class of diabetes drugs known to cause hypoglycemia, especially with alcohol, by binding to and **closing ATP-sensitive potassium channels** on pancreatic beta cells, leading to depolarization and insulin release. The doctor's warning about hypoglycemia risk with alcohol or skipped meals is classic for sulfonylureas.
*Inhibiting dipeptidyl peptidase*
This mechanism describes **DPP-4 inhibitors** (gliptins), which increase levels of endogenous incretins (GLP-1 and GIP) by preventing their breakdown. These drugs have a **low risk of hypoglycemia** when used alone because they work in a glucose-dependent manner. They do not typically cause severe hypoglycemia, especially not to the extent described in this case.
*Inhibiting alpha-glucosidase*
Alpha-glucosidase inhibitors (e.g., **acarbose**, miglitol) delay carbohydrate absorption in the small intestine by inhibiting brush border enzymes. While they can cause gastrointestinal side effects (flatulence, diarrhea), they have a **very low risk of hypoglycemia** because they do not affect insulin secretion directly.
*Decreasing hepatic gluconeogenesis*
This is the primary mechanism of action for **metformin**, which is a biguanide and typically the first-line agent for type 2 diabetes. Metformin reduces glucose production by the liver and has a **very low risk of hypoglycemia** when used as monotherapy, making it an unlikely cause of the patient's acute symptoms.
*Binding to peroxisome proliferator-activating receptors*
This mechanism describes **thiazolidinediones** (TZDs) like pioglitazone and rosiglitazone, which improve insulin sensitivity by activating PPAR-gamma receptors. They typically have a **low risk of hypoglycemia** when used as monotherapy. Their common side effects include weight gain, fluid retention, and increased fracture risk—not acute hypoglycemia.
Insulin secretion and action US Medical PG Question 2: A 46-year-old woman presents with palpitations, tremors, and anxiety. She says these symptoms have been present ever since a recent change in her diabetic medication. The most recent time she felt these symptoms, her blood glucose level was 65 mg/dL, and she felt better after eating a cookie. Which of the following is the mechanism of action of the drug most likely to have caused this patient's symptoms?
- A. Inhibition of α-glucosidase
- B. Blocking of the ATP-sensitive K+ channels (Correct Answer)
- C. Block reabsorption of glucose in proximal convoluted tubule (PCT)
- D. Inhibitor of dipeptidyl peptidase (DPP-IV)
- E. Decreased hepatic gluconeogenesis
Insulin secretion and action Explanation: ***Blocking of the ATP-sensitive K+ channels***
- The patient's symptoms of palpitations, tremors, anxiety, and a blood glucose level of 65 mg/dL, which improved after eating, are characteristic of **hypoglycemia**.
- **Sulfonylureas**, such as glyburide or glipizide, cause hypoglycemia by **blocking ATP-sensitive K+ channels** on pancreatic beta cells, leading to insulin release independent of blood glucose levels.
*Inhibition of α-glucosidase*
- This mechanism, characteristic of drugs like **acarbose** and **miglitol**, delays carbohydrate absorption in the gut.
- These drugs typically cause **gastrointestinal side effects** such as flatulence and diarrhea, not hypoglycemia or the associated adrenergic symptoms.
*Block reabsorption of glucose in proximal convoluted tubule (PCT)*
- This action describes **SGLT2 inhibitors** (e.g., canagliflozin, empagliflozin), which increase urinary glucose excretion.
- While they can cause **genitourinary infections** and **polyuria**, they have a very low risk of hypoglycemia unless combined with insulin or sulfonylureas.
*Inhibitor of dipeptidyl peptidase (DPP-IV)*
- **DPP-IV inhibitors** (e.g., sitagliptin, saxagliptin) prevent the breakdown of incretins, thus enhancing glucose-dependent insulin secretion and suppressing glucagon.
- These drugs typically have a **low risk of hypoglycemia** because their effects on insulin secretion are glucose-dependent.
*Decreased hepatic gluconeogenesis*
- This is the primary mechanism of **metformin**, which also increases insulin sensitivity in peripheral tissues.
- Metformin is associated with **lactic acidosis** and **gastrointestinal upset**, but it does not typically cause hypoglycemia as a monotherapy because it does not stimulate insulin secretion.
Insulin secretion and action US Medical PG Question 3: A 45-year-old woman with type 1 diabetes mellitus is brought to the emergency department by her husband because of polyuria, nausea, vomiting, and altered mental status for 4 hours. On arrival, she is unconscious. Treatment with a drug is begun that increases glucose transport to skeletal muscle and adipose tissue. Which of the following cellular events is most likely to also occur in response to this drug?
- A. Dephosphorylation of fructose-1,6-bisphosphatase (Correct Answer)
- B. Increased activity of acyl-CoA dehydrogenases
- C. Cleavage of UDP from UDP-glucose
- D. Upregulation of glucose transporter type 3 expression
- E. Phosphorylation of glycogen phosphorylase kinase
Insulin secretion and action Explanation: ***Dephosphorylation of fructose-1,6-bisphosphatase***
- The patient is in diabetic ketoacidosis (DKA), and the drug administered is insulin
- Insulin promotes glucose utilization and storage, which involves inhibiting gluconeogenesis through the dephosphorylation and inactivation of fructose-1,6-bisphosphatase
- This is a key regulatory mechanism by which insulin suppresses hepatic glucose production
*Increased activity of acyl-CoA dehydrogenases*
- This enzyme is crucial for fatty acid oxidation, a process that is inhibited by insulin
- In DKA, fatty acid oxidation is elevated, leading to ketone body production, but insulin treatment reduces this activity
*Cleavage of UDP from UDP-glucose*
- This reaction occurs in the synthesis of glycogen from UDP-glucose by glycogen synthase, which is activated by insulin
- While insulin stimulates glycogen synthesis, the direct cleavage of UDP from UDP-glucose is part of the synthetic process, not a primary regulatory cellular event caused by insulin in the context of DKA treatment
*Upregulation of glucose transporter type 3 expression*
- Glucose transporter type 3 (GLUT3) is primarily found in neurons and has a high affinity for glucose, with its expression generally not significantly regulated by insulin
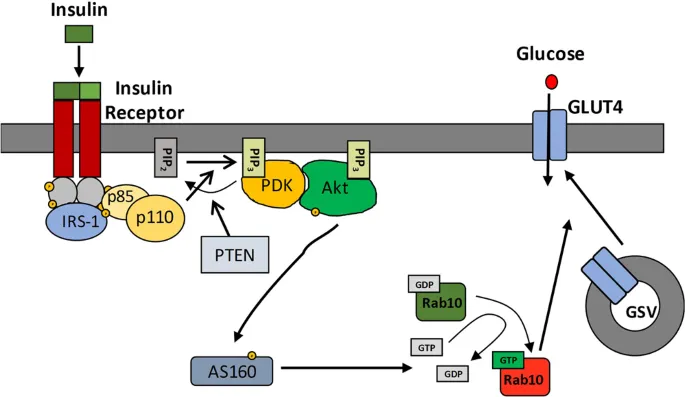
- Insulin primarily promotes GLUT4 translocation to the cell membrane in muscle and adipose tissue to increase glucose uptake
*Phosphorylation of glycogen phosphorylase kinase*
- Phosphorylation of glycogen phosphorylase kinase activates it, subsequently activating glycogen phosphorylase and promoting glycogen breakdown (glycogenolysis)
- Insulin inhibits glycogenolysis and promotes glycogen synthesis, meaning insulin would deactivate glycogen phosphorylase kinase through dephosphorylation
Insulin secretion and action US Medical PG Question 4: Certain glucose transporters that are expressed predominantly on skeletal muscle cells and adipocytes are unique compared to those transporters found on other cell types within the body. Without directly affecting glucose transport in other cell types, which of the following would be most likely to selectively increase glucose uptake in skeletal muscle cells and adipocytes?
- A. Increased plasma glucose concentration
- B. It is physiologically impossible to selectively increase glucose uptake in specific cells
- C. Increased levels of circulating insulin (Correct Answer)
- D. Decreased plasma glucose concentration
- E. Decreased levels of circulating insulin
Insulin secretion and action Explanation: ***Increased levels of circulating insulin***
- Insulin stimulates the translocation of **GLUT4 transporters** from intracellular vesicles to the cell membrane in **skeletal muscle** and **adipocytes**, thereby increasing glucose uptake.
- This mechanism is **selective** because other cell types (e.g., brain, liver) primarily use insulin-independent glucose transporters (e.g., GLUT1, GLUT2, GLUT3) that are constitutively active or respond to different signals.
*Increased plasma glucose concentration*
- While increased glucose concentration would drive glucose uptake in many cells, it is not **selective** for skeletal muscle and adipocytes since other cells also take up glucose.
- Insulin-independent tissues would also increase glucose uptake, making this a non-specific effect.
*It is physiologically impossible to selectively increase glucose uptake in specific cells*
- This statement is incorrect because the body has mechanisms, such as **insulin-mediated GLUT4 translocation**, that specifically regulate glucose uptake in certain cell types like skeletal muscle and adipocytes.
- This regulatory specificity is crucial for maintaining **glucose homeostasis**.
*Decreased plasma glucose concentration*
- A decrease in plasma glucose would generally **reduce** glucose uptake across all cell types, including skeletal muscle and adipocytes.
- It would not selectively increase uptake in any specific cell population.
*Decreased levels of circulating insulin*
- Decreased insulin levels would lead to **reduced** glucose uptake in insulin-sensitive tissues like skeletal muscle and adipocytes, as GLUT4 transporters would remain sequestered intracellularly.
- This would result in higher circulating glucose levels rather than increased uptake.
Insulin secretion and action US Medical PG Question 5: A 60-year-old African-American female presents to your office complaining of dysuria, paresthesias, and blurry vision. Her body mass index is 37.2 kg/m2. Which of the following drugs would most significantly increase the levels of C-peptide in the blood when administered to this patient?
- A. Acarbose
- B. Glipizide (Correct Answer)
- C. Insulin
- D. Metformin
- E. NPH
Insulin secretion and action Explanation: ***Glipizide***
- **Glipizide** is a **sulfonylurea** that stimulates insulin secretion from pancreatic beta cells, leading to increased C-peptide levels.
- Increased insulin secretion by glipizide is independent of meals and can cause **hypoglycemia**.
*Acarbose*
- **Acarbose** is an **alpha-glucosidase inhibitor** that delays glucose absorption from the gut, reducing post-prandial glucose spikes.
- It does not directly affect insulin secretion or C-peptide levels.
*Insulin*
- Administering exogenous **insulin** directly lowers blood glucose but does not stimulate endogenous insulin production, and therefore does not increase C-peptide.
- C-peptide is a marker of endogenous insulin secretion; an increase would only be seen if the body produced more insulin.
*Metformin*
- **Metformin** primarily reduces **hepatic glucose production** and improves insulin sensitivity in peripheral tissues.
- It does not directly stimulate insulin secretion from the pancreas and therefore does not increase C-peptide levels.
*NPH*
- **NPH (Neutral Protamine Hagedorn)** is an intermediate-acting exogenous insulin formulation.
- As an exogenous insulin, it does not stimulate the pancreas to produce more insulin or C-peptide.
Insulin secretion and action US Medical PG Question 6: A 46-year-old man comes to the physician for a follow-up examination. He has type 2 diabetes mellitus and hypertension. Current medications include metformin and lisinopril. He reports that he has adhered to his diet and medication regimen. His hemoglobin A1c is 8.6%. Insulin glargine is added to his medication regimen. Which of the following sets of changes is most likely to occur in response to this new medication?
$$$ Glycolysis %%% Glycogenesis %%% Lipolysis %%% Gluconeogenesis $$$
- A. ↓ ↓ ↑ ↑
- B. ↑ ↓ ↑ ↓
- C. ↓ ↑ ↓ ↑
- D. ↑ ↑ ↓ ↓ (Correct Answer)
- E. ↑ ↓ ↑ ↑
Insulin secretion and action Explanation: ***↑ ↑ ↓ ↓***
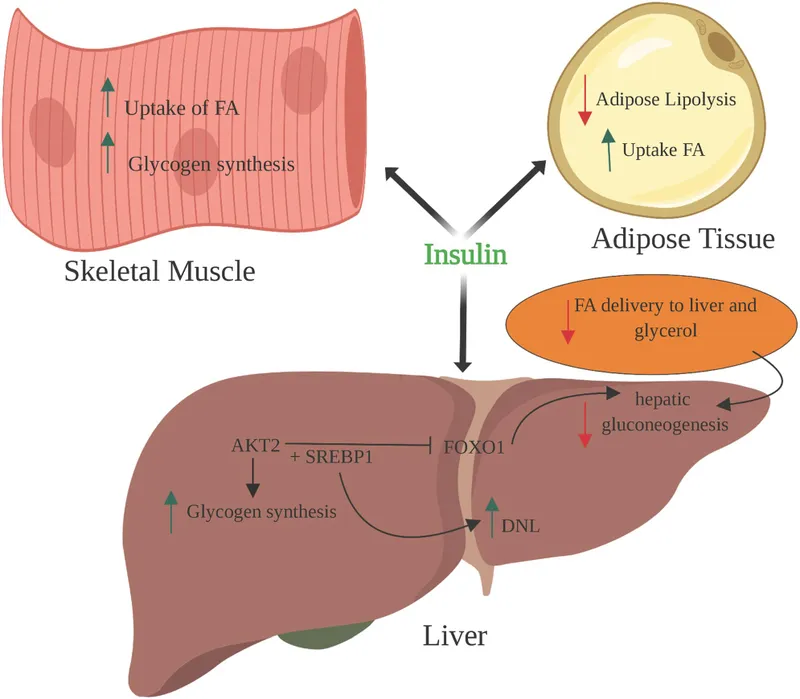
- Insulin **increases glucose utilization** by promoting glycolysis and increases glucose storage by promoting glycogenesis.
- Insulin **inhibits glucose production** by decreasing lipolysis and gluconeogenesis.
*↓ ↓ ↑ ↑*
- This option incorrectly suggests that insulin would decrease glycolysis and glycogenesis, which are pathways for glucose utilization and storage.
- It also incorrectly suggests that insulin would increase lipolysis and gluconeogenesis, which are pathways for glucose production.
*↑ ↓ ↑ ↓*
- This option correctly indicates an increase in glycolysis and a decrease in gluconeogenesis, but incorrectly suggests a decrease in glycogenesis and an increase in lipolysis.
- Insulin's primary role is to lower blood glucose, which involves promoting both glucose utilization (glycolysis) and storage (glycogenesis).
*↓ ↑ ↓ ↑*
- This option incorrectly suggests a decrease in glycolysis and an increase in gluconeogenesis, which would lead to higher blood glucose.
- While it correctly shows an increase in glycogenesis and a decrease in lipolysis, the overall pattern does not match insulin's coordinated metabolic effects.
*↑ ↓ ↑ ↑*
- This option incorrectly suggests a decrease in glycogenesis and an increase in lipolysis and gluconeogenesis, which would lead to higher blood glucose.
- Insulin's main actions are to promote glucose uptake and storage and to inhibit glucose production.
Insulin secretion and action US Medical PG Question 7: A group of scientists is studying the mechanism of action of various pancreatic hormones in rats. The scientists studied hormone A, which is secreted by the β-cells of the pancreas, and found that hormone A binds to a complex dimeric receptor on the cell membrane and exerts its effects via phosphorylation and subsequent downstream signaling that includes dephosphorylation of different intracellular proteins. Now they are studying hormone B, which is secreted by the α-cells and antagonizes the actions of hormone A. Which 2nd messenger system would hormone B utilize to exert its cellular effects?
- A. Direct cytoplasmic receptor binding
- B. Phospholipase C
- C. Tyrosine kinase
- D. Direct nuclear receptor binding
- E. Adenylyl cyclase-cyclic AMP (Correct Answer)
Insulin secretion and action Explanation: ***Adenylyl cyclase-cyclic AMP***
- Hormone B is **glucagon**, secreted by pancreatic α-cells, which antagonizes the effects of insulin (hormone A). Glucagon primarily acts through a **G protein-coupled receptor** that activates **adenylyl cyclase**, leading to an increase in intracellular **cyclic AMP (cAMP)**.
- Increased cAMP then activates **protein kinase A (PKA)**, which phosphorylates various intracellular proteins to promote **glycogenolysis** and **gluconeogenesis**, thereby raising blood glucose levels.
*Direct cytoplasmic receptor binding*
- This mechanism is characteristic of **steroid hormones**, which are lipid-soluble and can diffuse across the cell membrane to bind to receptors in the cytoplasm.
- Pancreatic hormones like glucagon are **peptide hormones**, which are water-soluble and typically bind to cell surface receptors.
*Phospholipase C*
- Activation of **phospholipase C (PLC)** leads to the production of **inositol triphosphate (IP3)** and **diacylglycerol (DAG)**, which mobilize intracellular calcium and activate protein kinase C, respectively.
- While some G protein-coupled receptors activate PLC, **glucagon's primary signaling pathway** involves adenylyl cyclase.
*Tyrosine kinase*
- **Tyrosine kinase receptors** are often associated with growth factors and insulin (hormone A) signaling, leading to phosphorylation of tyrosine residues on target proteins.
- Glucagon's receptor is a **G protein-coupled receptor**, not a receptor tyrosine kinase, and its actions are mediated through serine/threonine phosphorylation via PKA.
*Direct nuclear receptor binding*
- This mechanism is typical for **steroid hormones** and **thyroid hormones**, which are lipid-soluble and bind to receptors in the nucleus to directly influence gene transcription.
- As a peptide hormone, glucagon binds to cell surface receptors and does not directly interact with nuclear receptors.
Insulin secretion and action US Medical PG Question 8: You have been asked to deliver a lecture to medical students about the effects of various body hormones and neurotransmitters on the metabolism of glucose. Which of the following statements best describes the effects of sympathetic stimulation on glucose metabolism?
- A. Norepinephrine causes increased glucose absorption within the intestines.
- B. Without epinephrine, insulin cannot act on the liver.
- C. Peripheral tissues require epinephrine to take up glucose.
- D. Epinephrine increases liver glycogenolysis. (Correct Answer)
- E. Sympathetic stimulation to alpha receptors of the pancreas increases insulin release.
Insulin secretion and action Explanation: ***Epinephrine increases liver glycogenolysis.***
- **Epinephrine**, released during sympathetic stimulation, primarily acts to increase **glucose availability** for immediate energy.
- It achieves this by stimulating **glycogenolysis** (breakdown of glycogen into glucose) in the liver via **beta-adrenergic receptors**.
*Norepinephrine causes increased glucose absorption within the intestines.*
- **Norepinephrine** primarily causes **vasoconstriction** and can *decrease* **intestinal motility** and nutrient absorption due to shunting blood away from the digestive tract during stress.
- Glucose absorption is mainly regulated by digestive enzymes and transport proteins, not directly increased by norepinephrine.
*Without epinephrine, insulin cannot act on the liver.*
- **Insulin** acts on the liver independent of epinephrine to promote **glucose uptake**, **glycogenesis**, and **lipid synthesis**.
- Epinephrine and insulin have **antagonistic effects** on liver glucose metabolism; epinephrine increases glucose output, while insulin decreases it.
*Peripheral tissues require epinephrine to take up glucose.*
- **Insulin** is the primary hormone required for **glucose uptake** by most peripheral tissues, especially **muscle** and **adipose tissue**, via **GLUT4 transporters**.
- Epinephrine generally *reduces* glucose uptake by peripheral tissues to preserve glucose for the brain during stress.
*Sympathetic stimulation to alpha receptors of the pancreas increases insulin release.*
- Sympathetic stimulation, primarily acting through **alpha-2 adrenergic receptors** on pancreatic beta cells, actually **inhibits** **insulin secretion**.
- This inhibition helps to increase blood glucose levels by reducing insulin's glucose-lowering effects.
Insulin secretion and action US Medical PG Question 9: A neurophysiology expert is teaching his students the physiology of the neuromuscular junction. While describing the sequence of events that takes place at the neuromuscular junction, he mentions that as the action potential travels down the motor neuron, it causes depolarization of the presynaptic membrane. This results in the opening of voltage-gated calcium channels, which leads to an influx of calcium into the synapse of the motor neuron. Consequently, the cytosolic concentration of Ca2+ ions increases. Which of the following occurs at the neuromuscular junction as a result of this increase in cytosolic Ca2+?
- A. Generation of an end plate potential
- B. Exocytosis of acetylcholine from the synaptic vesicles (Correct Answer)
- C. Increased Na+ and K+ conductance of the motor end plate
- D. Binding of Ca2+ ions to NM receptors
- E. Release of Ca2+ ions into the synaptic cleft
Insulin secretion and action Explanation: ***Exocytosis of acetylcholine from the synaptic vesicles***
- The increase in **cytosolic Ca2+** within the presynaptic terminal is the primary trigger for the fusion of **synaptic vesicles** filled with **acetylcholine (ACh)** with the presynaptic membrane.
- This fusion process, known as **exocytosis**, releases ACh into the **synaptic cleft**, initiating synaptic transmission.
*Generation of an end plate potential*
- The **end plate potential (EPP)** is generated *after* acetylcholine (ACh) is released into the synaptic cleft and binds to receptors on the motor end plate.
- This event occurs *following* the Ca2+-induced release of neurotransmitter, not as a direct result of the Ca2+ increase itself.
*Increased Na+ and K+ conductance of the motor end plate*
- Increased **Na+ and K+ conductance** across the motor end plate membrane is a direct consequence of acetylcholine binding to its receptors, which are **ligand-gated ion channels**.
- This change in conductance *generates the end plate potential*, occurring after ACh release.
*Binding of Ca2+ ions to NM receptors*
- **NM receptors** (nicotinic muscle receptors) are located on the **postsynaptic membrane** (motor end plate) and bind to **acetylcholine (ACh)**, not Ca2+ ions.
- Calcium's primary role in this context is presynaptic: triggering ACh release.
*Release of Ca2+ ions into the synaptic cleft*
- Calcium ions enter the **presynaptic terminal** from the synaptic cleft, and their increased cytosolic concentration within the presynaptic terminal drives neurotransmitter release.
- Calcium itself is not released *into* the synaptic cleft in this process; rather, it enters the presynaptic neuron from the cleft.
Insulin secretion and action US Medical PG Question 10: A 60-year-old man with type 2 diabetes on metformin and insulin presents with 3 days of nausea, vomiting, and diffuse abdominal pain. He appears ill and confused. Vital signs: BP 95/60 mmHg, HR 115/min, RR 28/min, T 37.2°C. Labs show glucose 380 mg/dL, pH 7.28, HCO3 18 mEq/L, anion gap 24, serum osmolality 310 mOsm/kg, negative urine ketones, creatinine 2.8 mg/dL (baseline 1.1), lactate 8.2 mmol/L. Apply physiological principles to determine the primary acid-base and metabolic disturbance.
- A. Sepsis-induced lactic acidosis with stress hyperglycemia
- B. Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin (Correct Answer)
- C. Alcoholic ketoacidosis with concurrent diabetic emergency
- D. Diabetic ketoacidosis with renal failure from volume depletion
- E. Mixed metabolic acidosis from uremia and starvation ketosis
Insulin secretion and action Explanation: ***Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin***
- The patient exhibits features of **Hyperosmolar Hyperglycemic State (HHS)**, including significant hyperglycemia and confusion, but the **negative urine ketones** effectively rule out DKA.
- The severe **high anion gap metabolic acidosis** is driven by a **lactate of 8.2 mmol/L**, likely due to **Metformin-Associated Lactic Acidosis (MALA)** triggered by **acute kidney injury** (creatinine 2.8).
*Sepsis-induced lactic acidosis with stress hyperglycemia*
- While the patient is hypotensive and tachycardic, the **serum glucose of 380 mg/dL** and history of insulin use point primarily to a diabetic emergency rather than simple stress hyperglycemia.
- **Lactic acidosis** in sepsis usually occurs alongside clinical signs of infection, which are not the focus of this metformin-using patient's profile.
*Alcoholic ketoacidosis with concurrent diabetic emergency*
- **Alcoholic ketoacidosis** would typically present with a history of alcohol abuse and **positive ketones** (specifically beta-hydroxybutyrate), which contradicts the **negative urine ketones** found here.
- The primary source of acidosis in this patient is clearly identified as **lactate (8.2 mmol/L)**, not ketoacids.
*Diabetic ketoacidosis with renal failure from volume depletion*
- **Diabetic Ketoacidosis (DKA)** is unlikely given the **negative urine ketones** and a pH/bicarbonate profile that is less severe than typically seen in profound ketoacidosis.
- DKA usually presents with a lower glucose level (often <250-300 mg/dL) compared to the hyperosmolar states seen in **Type 2 Diabetes** patients.
*Mixed metabolic acidosis from uremia and starvation ketosis*
- While **uremia** contributes to the anion gap when creatinine is elevated (2.8 mg/dL), it is rarely the primary cause of an **anion gap of 24** without more advanced renal failure.
- **Starvation ketosis** would result in positive ketones and a much milder acidosis than the profound **lactic acidosis** (8.2 mmol/L) observed in this case.
More Insulin secretion and action US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.