Glucose homeostasis mechanisms US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Glucose homeostasis mechanisms. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Glucose homeostasis mechanisms US Medical PG Question 1: A 24-year-old man presents for an annual check-up. He is a bodybuilder and tells you he is on a protein-rich diet that only allows for minimal carbohydrate intake. His friend suggests he try exogenous glucagon to help him lose some excess weight before an upcoming competition. Which of the following effects of glucagon is he attempting to exploit?
- A. Increased glucose utilization by tissues
- B. Decreased blood cholesterol level
- C. Increased hepatic gluconeogenesis
- D. Increased lipolysis in adipose tissues (Correct Answer)
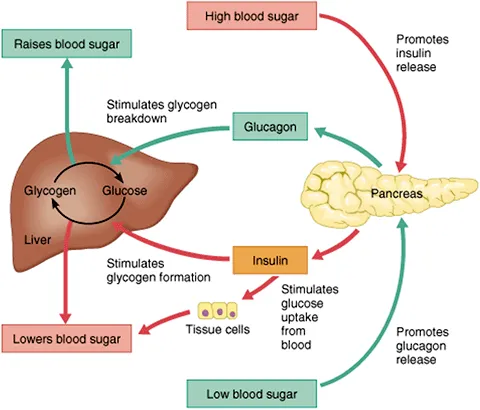
- E. Increased hepatic glycogenolysis
Glucose homeostasis mechanisms Explanation: ***Increased lipolysis in adipose tissues***
- While **glucagon's primary target is the liver**, it can have **modest lipolytic effects** on adipose tissue by opposing insulin's anti-lipolytic actions.
- Glucagon stimulates cAMP production, which can activate **hormone-sensitive lipase** to break down triglycerides into **fatty acids** and **glycerol**.
- However, **catecholamines (epinephrine/norepinephrine)** are far more potent direct stimulators of adipose tissue lipolysis than glucagon.
- The friend is attempting to exploit this lipolytic effect for fat loss, though **exogenous glucagon is not an evidence-based or safe weight-loss strategy**.
*Increased glucose utilization by tissues*
- This is **opposite** to glucagon's actual effect. **Glucagon raises blood glucose** levels; it does not promote glucose uptake by peripheral tissues.
- **Insulin** is the hormone responsible for promoting glucose uptake and utilization by muscle, adipose, and other tissues.
*Decreased blood cholesterol level*
- Glucagon does not have a direct, clinically significant effect on reducing blood cholesterol levels.
- While glucagon affects overall lipid metabolism through its catabolic actions, it is not used therapeutically for hypercholesterolemia.
*Increased hepatic gluconeogenesis*
- **Glucagon strongly stimulates hepatic gluconeogenesis**, which is the synthesis of glucose from non-carbohydrate precursors (amino acids, lactate, glycerol) in the liver.
- This action **raises blood glucose** levels and would not directly contribute to fat loss or weight reduction.
- In the context of a low-carbohydrate diet, increased gluconeogenesis would maintain blood glucose but not promote the fat loss the bodybuilder seeks.
*Increased hepatic glycogenolysis*
- **Glucagon is a potent stimulator of hepatic glycogenolysis**, the breakdown of stored liver glycogen into glucose.
- This rapidly increases blood glucose levels during fasting or hypoglycemia.
- However, this does not directly target adipose tissue for fat loss; it mobilizes glucose stores rather than fat stores, so it's not the mechanism relevant to weight loss goals.
Glucose homeostasis mechanisms US Medical PG Question 2: A 55-year-old man with alcoholic cirrhosis is admitted to the hospital for routine evaluation before liver transplantation. The physician asks the patient to stop eating 10 hours before surgery. Which of the following structures contributes directly to preventing fasting hypoglycemia by producing glucose in this patient?
- A. Adrenal cortex
- B. Skeletal muscle
- C. Red blood cells
- D. Skin
- E. Intestine (Correct Answer)
Glucose homeostasis mechanisms Explanation: ***Correct: Intestine***
- The **intestine** (particularly the small intestine) possesses the enzymatic machinery for **gluconeogenesis**, including glucose-6-phosphatase, allowing it to directly produce and release free glucose into the bloodstream.
- During prolonged fasting (>10 hours), intestinal gluconeogenesis can contribute up to **20-25% of total glucose production**, utilizing substrates like glutamine and glycerol.
- In patients with **alcoholic cirrhosis**, hepatic gluconeogenesis is impaired, making extrahepatic sites like the intestine and kidneys increasingly important for maintaining euglycemia.
- The intestine directly produces glucose and releases it into the portal circulation, making it a direct contributor to preventing fasting hypoglycemia.
*Incorrect: Skeletal muscle*
- **Skeletal muscle lacks glucose-6-phosphatase**, the enzyme required to convert glucose-6-phosphate to free glucose for release into the bloodstream.
- Muscle undergoes proteolysis during fasting, releasing amino acids (particularly alanine and glutamine) that serve as gluconeogenic substrates for the liver and kidneys.
- This represents an **indirect contribution** to glucose homeostasis through substrate provision, not direct glucose production.
*Incorrect: Red blood cells*
- **Red blood cells** lack mitochondria and can only perform anaerobic glycolysis, producing lactate.
- Lactate from RBCs can be recycled to glucose in the liver via the **Cori cycle**, but RBCs themselves are net glucose consumers, not producers.
- They contribute indirectly through substrate provision, not direct glucose synthesis.
*Incorrect: Skin*
- The **skin** has no significant role in glucose production or gluconeogenesis.
- Its primary functions are protection, thermoregulation, and sensation; it does not possess the enzymatic capacity for gluconeogenesis.
- Skin does not contribute to maintaining blood glucose homeostasis during fasting.
*Incorrect: Adrenal cortex*
- The **adrenal cortex** secretes hormones (cortisol, aldosterone) that regulate glucose metabolism indirectly.
- **Cortisol** promotes hepatic and renal gluconeogenesis and decreases peripheral glucose utilization, but the adrenal gland itself does not synthesize or release glucose.
- This is a regulatory role, not direct glucose production.
Glucose homeostasis mechanisms US Medical PG Question 3: A 33-year-old woman, gravida 1, para 0, at 26 weeks' gestation comes to the physician for a routine prenatal examination. Her pregnancy has been uneventful. Physical examination shows a uterus consistent in size with a 26-week gestation. She is given an oral 50-g glucose load; 1 hour later, her serum glucose concentration is 116 mg/dL. Which of the following most likely occurred immediately after the entrance of glucose into the patient's pancreatic beta-cells?
- A. Closure of membranous potassium channels
- B. Generation of adenosine triphosphate (Correct Answer)
- C. Increased expression of hexokinase I mRNA
- D. Exocytosis of insulin granules
- E. Depolarization of beta-cell membrane
Glucose homeostasis mechanisms Explanation: ***Generation of adenosine triphosphate***
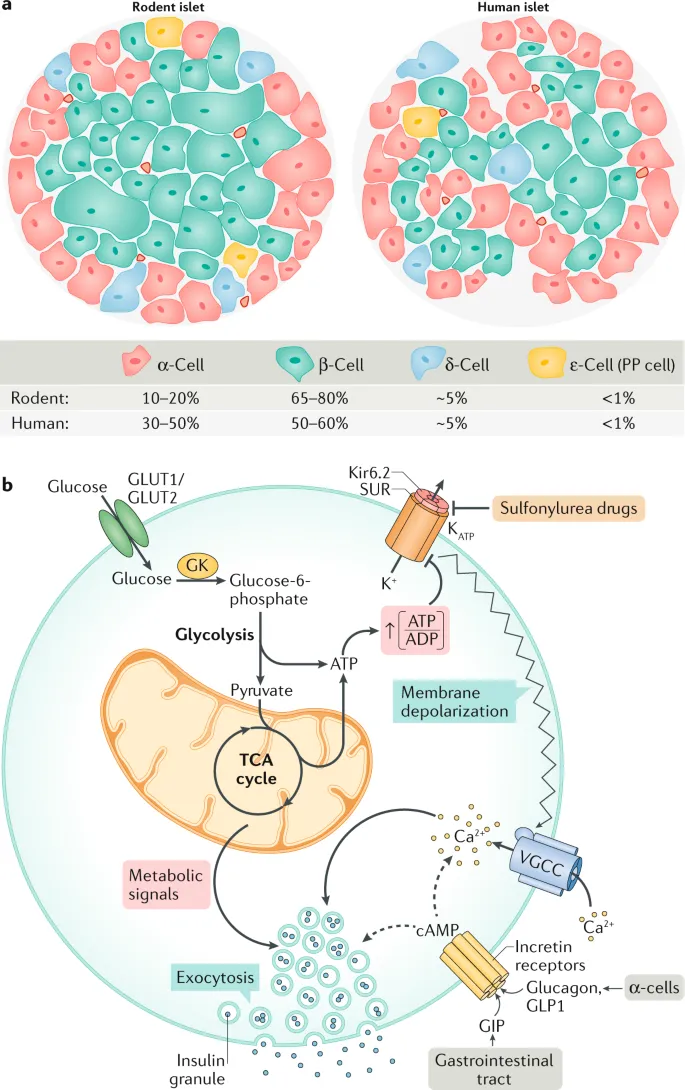
- Immediately after glucose enters pancreatic beta-cells via **GLUT2 transporters**, it is phosphorylated by **glucokinase (hexokinase IV)** to glucose-6-phosphate.
- This glucose is then metabolized through **glycolysis** and the **Krebs cycle**, leading to the generation of **ATP**.
- This increase in intracellular **ATP/ADP ratio** is the **primary signal** that links glucose metabolism to insulin secretion.
- Among the listed options, ATP generation is the **earliest event** that occurs.
*Closure of membranous potassium channels*
- The elevated **ATP** levels from glucose metabolism lead to the closure of **ATP-sensitive potassium (K-ATP) channels**.
- This closure is a subsequent event that depends on the increased ATP/ADP ratio, not an immediate consequence of glucose entry.
*Increased expression of hexokinase I mRNA*
- While **glucokinase (hexokinase IV)** activity is crucial for glucose phosphorylation in beta-cells, increased mRNA expression is a **long-term adaptive response** requiring transcription and translation.
- The immediate response involves the existing enzyme converting glucose to **glucose-6-phosphate**, followed by ATP generation.
*Exocytosis of insulin granules*
- **Insulin granule exocytosis** is the final step in insulin release, occurring after a cascade of events: ATP generation → K-ATP channel closure → membrane depolarization → calcium influx.
- This event is a *downstream consequence*, not an immediate result of glucose entering the cell.
*Depolarization of beta-cell membrane*
- **Membrane depolarization** follows the closure of ATP-sensitive potassium channels, which then leads to the opening of **voltage-gated calcium channels**.
- This is a subsequent event that depends on the initial ATP generation and K-ATP channel closure.
Glucose homeostasis mechanisms US Medical PG Question 4: Researchers are experimenting with hormone levels in mice in fasting and fed states. To test hormone levels in the fed state, the mice are given an oral glucose load and various hormones are measured in a blood sample. Researchers are most interested in the hormone whose blood levels track evenly with C-peptide levels. The hormone the researchers are most interested in is responsible for which of the following actions in the body?
- A. Protein catabolism
- B. Fatty acid breakdown
- C. Fatty acid synthesis (Correct Answer)
- D. Ketogenesis
- E. Lipolysis
Glucose homeostasis mechanisms Explanation: ***Fatty acid synthesis***
- The hormone whose blood levels track evenly with **C-peptide** levels after a glucose load is **insulin**.
- Insulin is a key anabolic hormone that promotes **fatty acid synthesis** from excess glucose in the fed state, particularly in the liver and adipose tissue.
*Protein catabolism*
- **Insulin** is an anabolic hormone that generally **inhibits protein catabolism** and promotes protein synthesis.
- Conditions like **glucagon excess** or **cortisol excess** promote protein catabolism, not insulin.
*Fatty acid breakdown*
- **Insulin inhibits fatty acid breakdown** (beta-oxidation) by suppressing hormone-sensitive lipase.
- **Glucagon** and **epinephrine** promote fatty acid breakdown, especially during fasting.
*Ketogenesis*
- **Insulin inhibits ketogenesis** by reducing the supply of fatty acids to the liver and inhibiting the enzymes involved in ketone body formation.
- **Glucagon** and **low insulin levels** (as in uncontrolled diabetes or prolonged fasting) promote ketogenesis.
*Lipolysis*
- **Insulin is a potent inhibitor of lipolysis** (breakdown of triglycerides into fatty acids and glycerol) in adipose tissue.
- **Glucagon**, **catecholamines**, and **growth hormone** stimulate lipolysis.
Glucose homeostasis mechanisms US Medical PG Question 5: A 20-year-old male is brought by ambulance to the emergency room in extremis. He is minimally conscious, hypotensive, and tachypneic, and his breath gives off a "fruity" odor. An arterial blood gas and metabolic panel show anion gap metabolic acidosis. This patient is most likely deficient in which of the following metabolic actions?
- A. Glucagon production
- B. Cortisol secretion
- C. Formation of ketone bodies
- D. Glucose production
- E. Cellular uptake of glucose (Correct Answer)
Glucose homeostasis mechanisms Explanation: ***Cellular uptake of glucose***
- The patient's symptoms, including **fruity odor breath**, **anion gap metabolic acidosis**, and being found in extremis, are classic signs of **diabetic ketoacidosis (DKA)**.
- DKA results from a profound lack of **insulin**, which is essential for cells (especially muscle and adipose tissue) to take up glucose from the bloodstream, leading to hyperglycemia and a shift to fat metabolism.
*Glucagon production*
- **Glucagon** is a counter-regulatory hormone that *raises* blood glucose levels, and its production is often *increased* in DKA as the body attempts to provide fuel to cells in the absence of insulin's effect.
- A deficiency in glucagon production would more likely lead to **hypoglycemia**, not the profound hyperglycemia seen in DKA.
*Cortisol secretion*
- **Cortisol** is another counter-regulatory hormone that *increases* blood glucose, and its secretion is typically *elevated* in stress states like DKA.
- A deficiency in cortisol (e.g., in adrenal insufficiency) would present with different symptoms such as **hypoglycemia**, **hyponatremia**, and **hyperkalemia**, without the classic DKA picture.
*Formation of ketone bodies*
- The patient's **fruity odor breath** and **anion gap metabolic acidosis** are direct consequences of the *overproduction* of **ketone bodies**.
- This overproduction occurs when the body, lacking glucose for fuel due to insulin deficiency, switches to **fat metabolism**, leading to excessive formation of acetoacetate, beta-hydroxybutyrate, and acetone.
*Glucose production*
- **Glucose production** (gluconeogenesis and glycogenolysis) is typically *increased* in DKA as the liver tries to supply glucose to the body due to perceived cellular starvation (despite high blood glucose).
- A deficiency in glucose production, such as in certain glycogen storage diseases or severe liver failure, would lead to **hypoglycemia**, not the hyperglycemia characteristic of DKA.
Glucose homeostasis mechanisms US Medical PG Question 6: You have been asked to deliver a lecture to medical students about the effects of various body hormones and neurotransmitters on the metabolism of glucose. Which of the following statements best describes the effects of sympathetic stimulation on glucose metabolism?
- A. Norepinephrine causes increased glucose absorption within the intestines.
- B. Without epinephrine, insulin cannot act on the liver.
- C. Peripheral tissues require epinephrine to take up glucose.
- D. Epinephrine increases liver glycogenolysis. (Correct Answer)
- E. Sympathetic stimulation to alpha receptors of the pancreas increases insulin release.
Glucose homeostasis mechanisms Explanation: ***Epinephrine increases liver glycogenolysis.***
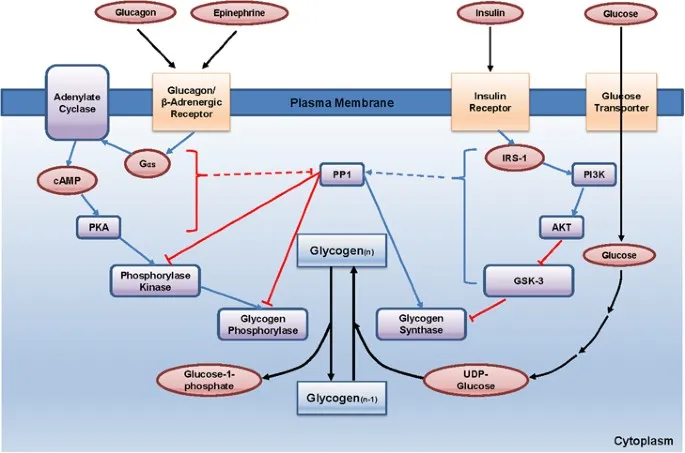
- **Epinephrine**, released during sympathetic stimulation, primarily acts to increase **glucose availability** for immediate energy.
- It achieves this by stimulating **glycogenolysis** (breakdown of glycogen into glucose) in the liver via **beta-adrenergic receptors**.
*Norepinephrine causes increased glucose absorption within the intestines.*
- **Norepinephrine** primarily causes **vasoconstriction** and can *decrease* **intestinal motility** and nutrient absorption due to shunting blood away from the digestive tract during stress.
- Glucose absorption is mainly regulated by digestive enzymes and transport proteins, not directly increased by norepinephrine.
*Without epinephrine, insulin cannot act on the liver.*
- **Insulin** acts on the liver independent of epinephrine to promote **glucose uptake**, **glycogenesis**, and **lipid synthesis**.
- Epinephrine and insulin have **antagonistic effects** on liver glucose metabolism; epinephrine increases glucose output, while insulin decreases it.
*Peripheral tissues require epinephrine to take up glucose.*
- **Insulin** is the primary hormone required for **glucose uptake** by most peripheral tissues, especially **muscle** and **adipose tissue**, via **GLUT4 transporters**.
- Epinephrine generally *reduces* glucose uptake by peripheral tissues to preserve glucose for the brain during stress.
*Sympathetic stimulation to alpha receptors of the pancreas increases insulin release.*
- Sympathetic stimulation, primarily acting through **alpha-2 adrenergic receptors** on pancreatic beta cells, actually **inhibits** **insulin secretion**.
- This inhibition helps to increase blood glucose levels by reducing insulin's glucose-lowering effects.
Glucose homeostasis mechanisms US Medical PG Question 7: Certain glucose transporters that are expressed predominantly on skeletal muscle cells and adipocytes are unique compared to those transporters found on other cell types within the body. Without directly affecting glucose transport in other cell types, which of the following would be most likely to selectively increase glucose uptake in skeletal muscle cells and adipocytes?
- A. Increased plasma glucose concentration
- B. It is physiologically impossible to selectively increase glucose uptake in specific cells
- C. Increased levels of circulating insulin (Correct Answer)
- D. Decreased plasma glucose concentration
- E. Decreased levels of circulating insulin
Glucose homeostasis mechanisms Explanation: ***Increased levels of circulating insulin***
- Insulin stimulates the translocation of **GLUT4 transporters** from intracellular vesicles to the cell membrane in **skeletal muscle** and **adipocytes**, thereby increasing glucose uptake.
- This mechanism is **selective** because other cell types (e.g., brain, liver) primarily use insulin-independent glucose transporters (e.g., GLUT1, GLUT2, GLUT3) that are constitutively active or respond to different signals.
*Increased plasma glucose concentration*
- While increased glucose concentration would drive glucose uptake in many cells, it is not **selective** for skeletal muscle and adipocytes since other cells also take up glucose.
- Insulin-independent tissues would also increase glucose uptake, making this a non-specific effect.
*It is physiologically impossible to selectively increase glucose uptake in specific cells*
- This statement is incorrect because the body has mechanisms, such as **insulin-mediated GLUT4 translocation**, that specifically regulate glucose uptake in certain cell types like skeletal muscle and adipocytes.
- This regulatory specificity is crucial for maintaining **glucose homeostasis**.
*Decreased plasma glucose concentration*
- A decrease in plasma glucose would generally **reduce** glucose uptake across all cell types, including skeletal muscle and adipocytes.
- It would not selectively increase uptake in any specific cell population.
*Decreased levels of circulating insulin*
- Decreased insulin levels would lead to **reduced** glucose uptake in insulin-sensitive tissues like skeletal muscle and adipocytes, as GLUT4 transporters would remain sequestered intracellularly.
- This would result in higher circulating glucose levels rather than increased uptake.
Glucose homeostasis mechanisms US Medical PG Question 8: A 33-year-old woman presents to the physician because of abdominal discomfort, weakness, and fever. She has had a significant weight loss of 15 kg (33.1 lb) over the past 2 months. She has no history of medical illness and is not on any medications. Her pulse is 96/min, the blood pressure is 167/92 mm Hg, the respiratory rate is 20/min, and the temperature is 37.7°C (99.8°F). Her weight is 67 kg (147.71 lb), height is 160 cm (5 ft 3 in), and BMI is 26.17 kg/m2. Abdominal examination shows purple striae and a vaguely palpable mass in the left upper quadrant of the abdomen, which does not move with respirations. She has coarse facial hair and a buffalo hump along with central obesity. Her extremities have poor muscle bulk, and muscle weakness is noted on examination. An ultrasound of the abdomen demonstrates an adrenal mass with para-aortic lymphadenopathy. Which of the following is the most likely laboratory profile in this patient?
- A. Impaired glucose tolerance, elevated serum cortisol, elevated 24-h urinary free cortisol, and high plasma ACTH
- B. Normal glucose tolerance, elevated serum cortisol, normal 24-h urinary free cortisol, and normal plasma adrenocorticotropic hormone (ACTH)
- C. Impaired glucose tolerance, elevated serum cortisol, elevated 24-h urinary free cortisol, and low plasma ACTH (Correct Answer)
- D. Impaired glucose tolerance, reduced serum cortisol, normal 24-h urinary free cortisol, and low plasma ACTH
- E. Impaired glucose tolerance, elevated serum cortisol, normal 24-h urinary free cortisol, and normal plasma ACTH
Glucose homeostasis mechanisms Explanation: ***Impaired glucose tolerance, elevated serum cortisol, elevated 24-h urinary free cortisol, and low plasma ACTH***
- The clinical picture of **Cushing's syndrome** is evident from purple striae, coarse facial hair, buffalo hump, central obesity, muscle weakness, hypertension, and abdominal mass. The adrenal mass with para-aortic lymphadenopathy points to an **adrenocortical carcinoma**, which independently produces cortisol.
- In cases of **adrenal tumors** producing cortisol, the **exogenous cortisol suppresses ACTH production** from the pituitary, leading to low plasma ACTH levels. Elevated cortisol leads to **insulin resistance** and impaired glucose tolerance.
*Impaired glucose tolerance, elevated serum cortisol, elevated 24-h urinary free cortisol, and high plasma ACTH*
- While significant **hypercortisolism** would cause impaired glucose tolerance, elevated serum and urinary free cortisol, **high plasma ACTH** is characteristic of **Cushing's disease** (pituitary ACTH overproduction), not an adrenal tumor.
- An adrenal tumor directly secretes cortisol, thereby **suppressing ACTH** via negative feedback.
*Normal glucose tolerance, elevated serum cortisol, normal 24-h urinary free cortisol, and normal plasma adrenocorticotropic hormone (ACTH)*
- With the strong clinical signs of Cushing's syndrome and an adrenal mass, **elevated serum cortisol** and **elevated 24-h urinary free cortisol** are highly expected, making "normal" results for these parameters incorrect.
- **Impaired glucose tolerance** is a common consequence of chronic hypercortisolism, so normal glucose tolerance would be unlikely.
*Impaired glucose tolerance, reduced serum cortisol, normal 24-h urinary free cortisol, and low plasma ACTH*
- The clinical presentation clearly indicates **hypercortisolism** (Cushing's syndrome), making **reduced serum cortisol** and normal 24-h urinary free cortisol inconsistent with the diagnosis.
- Low plasma ACTH would be appropriate for an adrenal tumor, but the cortisol levels contradict the clinical picture.
*Impaired glucose tolerance, elevated serum cortisol, normal 24-h urinary free cortisol, and normal plasma ACTH*
- While **impaired glucose tolerance** and **elevated serum cortisol** are consistent with Cushing's syndrome, a **normal 24-h urinary free cortisol** would be highly unlikely given the other clinical signs and the presence of an adrenal mass secreting cortisol.
- **Normal plasma ACTH** is also incorrect; it should be suppressed in cases of primary adrenal hypercortisolism.
Glucose homeostasis mechanisms US Medical PG Question 9: A neurophysiology expert is teaching his students the physiology of the neuromuscular junction. While describing the sequence of events that takes place at the neuromuscular junction, he mentions that as the action potential travels down the motor neuron, it causes depolarization of the presynaptic membrane. This results in the opening of voltage-gated calcium channels, which leads to an influx of calcium into the synapse of the motor neuron. Consequently, the cytosolic concentration of Ca2+ ions increases. Which of the following occurs at the neuromuscular junction as a result of this increase in cytosolic Ca2+?
- A. Generation of an end plate potential
- B. Exocytosis of acetylcholine from the synaptic vesicles (Correct Answer)
- C. Increased Na+ and K+ conductance of the motor end plate
- D. Binding of Ca2+ ions to NM receptors
- E. Release of Ca2+ ions into the synaptic cleft
Glucose homeostasis mechanisms Explanation: ***Exocytosis of acetylcholine from the synaptic vesicles***
- The increase in **cytosolic Ca2+** within the presynaptic terminal is the primary trigger for the fusion of **synaptic vesicles** filled with **acetylcholine (ACh)** with the presynaptic membrane.
- This fusion process, known as **exocytosis**, releases ACh into the **synaptic cleft**, initiating synaptic transmission.
*Generation of an end plate potential*
- The **end plate potential (EPP)** is generated *after* acetylcholine (ACh) is released into the synaptic cleft and binds to receptors on the motor end plate.
- This event occurs *following* the Ca2+-induced release of neurotransmitter, not as a direct result of the Ca2+ increase itself.
*Increased Na+ and K+ conductance of the motor end plate*
- Increased **Na+ and K+ conductance** across the motor end plate membrane is a direct consequence of acetylcholine binding to its receptors, which are **ligand-gated ion channels**.
- This change in conductance *generates the end plate potential*, occurring after ACh release.
*Binding of Ca2+ ions to NM receptors*
- **NM receptors** (nicotinic muscle receptors) are located on the **postsynaptic membrane** (motor end plate) and bind to **acetylcholine (ACh)**, not Ca2+ ions.
- Calcium's primary role in this context is presynaptic: triggering ACh release.
*Release of Ca2+ ions into the synaptic cleft*
- Calcium ions enter the **presynaptic terminal** from the synaptic cleft, and their increased cytosolic concentration within the presynaptic terminal drives neurotransmitter release.
- Calcium itself is not released *into* the synaptic cleft in this process; rather, it enters the presynaptic neuron from the cleft.
Glucose homeostasis mechanisms US Medical PG Question 10: A 60-year-old man with type 2 diabetes on metformin and insulin presents with 3 days of nausea, vomiting, and diffuse abdominal pain. He appears ill and confused. Vital signs: BP 95/60 mmHg, HR 115/min, RR 28/min, T 37.2°C. Labs show glucose 380 mg/dL, pH 7.28, HCO3 18 mEq/L, anion gap 24, serum osmolality 310 mOsm/kg, negative urine ketones, creatinine 2.8 mg/dL (baseline 1.1), lactate 8.2 mmol/L. Apply physiological principles to determine the primary acid-base and metabolic disturbance.
- A. Sepsis-induced lactic acidosis with stress hyperglycemia
- B. Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin (Correct Answer)
- C. Alcoholic ketoacidosis with concurrent diabetic emergency
- D. Diabetic ketoacidosis with renal failure from volume depletion
- E. Mixed metabolic acidosis from uremia and starvation ketosis
Glucose homeostasis mechanisms Explanation: ***Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin***
- The patient exhibits features of **Hyperosmolar Hyperglycemic State (HHS)**, including significant hyperglycemia and confusion, but the **negative urine ketones** effectively rule out DKA.
- The severe **high anion gap metabolic acidosis** is driven by a **lactate of 8.2 mmol/L**, likely due to **Metformin-Associated Lactic Acidosis (MALA)** triggered by **acute kidney injury** (creatinine 2.8).
*Sepsis-induced lactic acidosis with stress hyperglycemia*
- While the patient is hypotensive and tachycardic, the **serum glucose of 380 mg/dL** and history of insulin use point primarily to a diabetic emergency rather than simple stress hyperglycemia.
- **Lactic acidosis** in sepsis usually occurs alongside clinical signs of infection, which are not the focus of this metformin-using patient's profile.
*Alcoholic ketoacidosis with concurrent diabetic emergency*
- **Alcoholic ketoacidosis** would typically present with a history of alcohol abuse and **positive ketones** (specifically beta-hydroxybutyrate), which contradicts the **negative urine ketones** found here.
- The primary source of acidosis in this patient is clearly identified as **lactate (8.2 mmol/L)**, not ketoacids.
*Diabetic ketoacidosis with renal failure from volume depletion*
- **Diabetic Ketoacidosis (DKA)** is unlikely given the **negative urine ketones** and a pH/bicarbonate profile that is less severe than typically seen in profound ketoacidosis.
- DKA usually presents with a lower glucose level (often <250-300 mg/dL) compared to the hyperosmolar states seen in **Type 2 Diabetes** patients.
*Mixed metabolic acidosis from uremia and starvation ketosis*
- While **uremia** contributes to the anion gap when creatinine is elevated (2.8 mg/dL), it is rarely the primary cause of an **anion gap of 24** without more advanced renal failure.
- **Starvation ketosis** would result in positive ketones and a much milder acidosis than the profound **lactic acidosis** (8.2 mmol/L) observed in this case.
More Glucose homeostasis mechanisms US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.