Glucocorticoid synthesis and regulation US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Glucocorticoid synthesis and regulation. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Glucocorticoid synthesis and regulation US Medical PG Question 1: A scientist is trying to design a drug to modulate cellular metabolism in the treatment of obesity. Specifically, he is interested in understanding how fats are processed in adipocytes in response to different energy states. His target is a protein within these cells that catalyzes catabolism of an energy source. The products of this reaction are subsequently used in gluconeogenesis or β-oxidation. Which of the following is true of the most likely protein that is being studied by this scientist?
- A. It is stimulated by epinephrine (Correct Answer)
- B. It is inhibited by glucagon
- C. It is inhibited by acetylcholine
- D. It is inhibited by cortisol
- E. It is stimulated by insulin
Glucocorticoid synthesis and regulation Explanation: ***It is stimulated by epinephrine***
- The protein described is likely **hormone-sensitive lipase (HSL)**, which catabolizes **triglycerides** in adipocytes to **glycerol** and **fatty acids**.
- **Epinephrine** (and norepinephrine) stimulates HSL activity via a **cAMP-dependent protein kinase A (PKA)** pathway, leading to increased fatty acid release for energy.
*It is inhibited by glucagon*
- **Glucagon primarily acts on the liver** to promote gluconeogenesis and glycogenolysis, but it does **not directly inhibit HSL** in adipocytes.
- While glucagon has a lipolytic effect, it doesn't inhibit the enzyme that releases fatty acids.
*It is inhibited by acetylcholine*
- **Acetylcholine** is a neurotransmitter involved in the **parasympathetic nervous system**, which generally promotes energy storage.
- It does **not directly inhibit HSL**; its effects on lipid metabolism are indirect and typically involve other pathways.
*It is inhibited by cortisol*
- **Cortisol**, a glucocorticoid, generally **promotes lipolysis** (breakdown of fats) in certain contexts, particularly during stress to provide energy substrates.
- Therefore, it would **not inhibit HSL**; rather, it often enhances its activity or provides a permissive effect for other lipolytic hormones.
*It is stimulated by insulin*
- **Insulin** is an **anabolic hormone** that promotes energy storage, including **lipogenesis** (fat synthesis) and inhibits lipolysis.
- Insulin **inhibits HSL activity** by activating phosphodiesterase, which reduces cAMP levels, thus deactivating PKA and preventing HSL phosphorylation.
Glucocorticoid synthesis and regulation US Medical PG Question 2: A 65-year-old obese man presents to his primary care clinic feeling weak. He was in the military and stationed in Vietnam in his youth. His current weakness gradually worsened to the point that he had to call his son to help him stand to get on the ambulance. He smokes a pack of cigarettes every day and drinks a bottle of vodka a week. He has been admitted for alcohol withdrawal multiple times and has been occasionally taking thiamine, folic acid, and naltrexone. He denies taking steroids. His temperature is 98°F (36.7°C), blood pressure is 170/90 mmHg, pulse is 75/min, and respirations are 20/min. He is obese with a significant pannus. Hepatomegaly is not appreciable. Abdominal striae are present. His workup is notable for the following:
Serum:
Na+: 142 mEq/L
Cl-: 102 mEq/L
K+: 3.9 mEq/L
HCO3-: 25 mEq/L
BUN: 24 mg/dL
Glucose: 292 mg/dL
Creatinine: 1.5 mg/dL
Ca2+: 10.1 mg/dL
AST: 7 U/L
ALT: 14 U/L
24-hour urinary cortisol: 400 µg (reference range < 300 µg)
Serum cortisol: 45 pg/mL (reference range < 15 pg/mL)
A 48-hour high dose dexamethasone suppression trial shows that his serum cortisol levels partially decrease to 25 pg/mL and his adrenocorticotropin-releasing hormone (ACTH) level decreases from 10 to 6 pg/mL (reference range > 5 pg/mL). What is the best next step in management?
- A. MRI of the pituitary gland (Correct Answer)
- B. MRI of the adrenal glands
- C. Low-dose dexamethasone therapy for 3 months
- D. CT of the chest
- E. High-dose dexamethasone therapy for 3 months
Glucocorticoid synthesis and regulation Explanation: ***MRI of the pituitary gland***
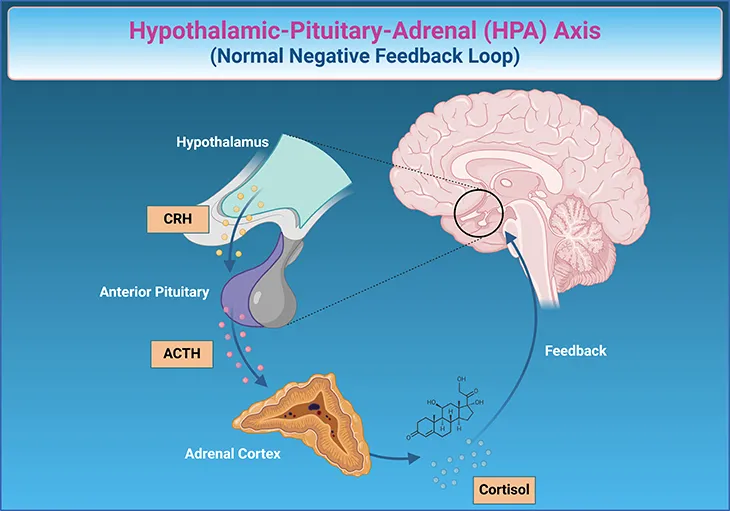
- The elevated 24-hour urinary cortisol and serum cortisol levels, along with **partial suppression on the high-dose dexamethasone suppression test** and a decrease in ACTH, strongly suggest a pituitary source of **Cushing's disease**.
- An MRI of the pituitary gland is the appropriate next step to visualize an **adenoma** responsible for the excess ACTH production.
*MRI of the adrenal glands*
- An adrenal MRI would be indicated if the ACTH levels were **low or undetectable**, suggesting an adrenal tumor as the primary cause of Cushing's syndrome.
- Since ACTH levels decreased, but remained elevated, an adrenal origin is less likely.
*Low-dose dexamethasone therapy for 3 months*
- Dexamethasone therapy is not a treatment for Cushing's syndrome; instead, it is used as a **diagnostic tool** to assess cortisol suppression.
- Long-term administration of dexamethasone would mimic iatrogenic Cushing's syndrome and **exacerbate the patient's condition**.
*CT of the chest*
- A CT of the chest would be considered if an **ectopic ACTH-producing tumor** (e.g., small cell lung cancer) was suspected, which typically presents with very high ACTH levels and no suppression with high-dose dexamethasone.
- The partial suppression and lower ACTH levels make an ectopic source less likely in this case.
*High-dose dexamethasone therapy for 3 months*
- Similar to low-dose dexamethasone therapy, high-dose dexamethasone is a **diagnostic test**, not a long-term treatment for Cushing's syndrome.
- Such therapy would worsen the patient's condition and **does not address the underlying pathology**.
Glucocorticoid synthesis and regulation US Medical PG Question 3: An investigator studying hormone synthesis and transport uses immunocytochemical techniques to localize a carrier protein in the central nervous system of an experimental animal. The investigator finds that this protein is synthesized together with a specific hormone from a composite precursor. The protein is involved in the transport of the hormone from the supraoptic and paraventricular nuclei to its destination. The hormone transported by these carrier proteins is most likely responsible for which of the following functions?
- A. Stimulation of thyroglobulin cleavage
- B. Upregulation of renal aquaporin-2 channels (Correct Answer)
- C. Hyperplasia of the adrenal zona fasciculata
- D. Increased insulin-like growth factor 1 production
- E. Maturation of primordial germ cells
Glucocorticoid synthesis and regulation Explanation: ***Upregulation of renal aquaporin-2 channels***
- The description of a hormone synthesized in the **supraoptic** and **paraventricular nuclei** and transported by a carrier protein refers to **antidiuretic hormone (ADH)**, also known as vasopressin.
- ADH's primary function in the kidney is to **increase water reabsorption** by upregulating **aquaporin-2 channels** in the principal cells of the collecting ducts.
*Stimulation of thyroglobulin cleavage*
- **Thyroglobulin cleavage** and subsequent release of thyroid hormones (T3, T4) are stimulated by **thyroid-stimulating hormone (TSH)**, which is produced by the anterior pituitary, not the hypothalamus.
- The described origin in the supraoptic and paraventricular nuclei is inconsistent with TSH.
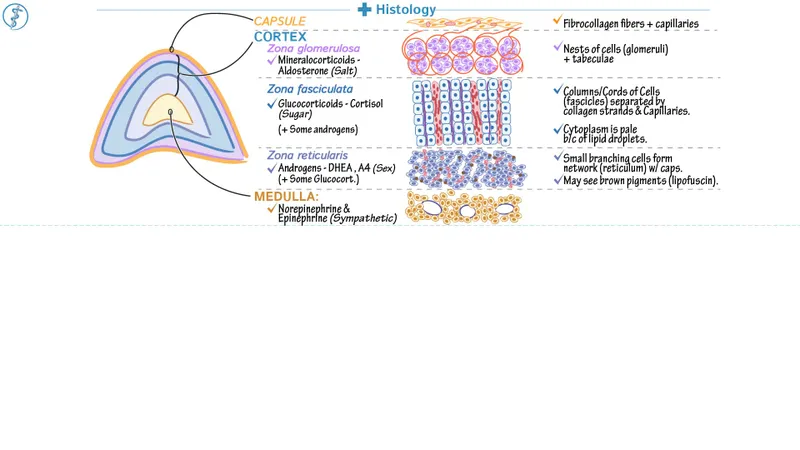
*Hyperplasia of the adrenal zona fasciculata*
- **Adrenocorticotropic hormone (ACTH)** from the anterior pituitary stimulates the adrenal cortex, including the zona fasciculata, to produce cortisol.
- The hormone described here originates in the hypothalamus and is transported to the posterior pituitary, not stimulating adrenal hyperplasia.
*Increased insulin-like growth factor 1 production*
- **Insulin-like growth factor 1 (IGF-1)** production is stimulated primarily by **growth hormone (GH)**, which is secreted by the anterior pituitary.
- This function is not associated with hormones produced in the supraoptic and paraventricular nuclei.
*Maturation of primordial germ cells*
- The maturation of **primordial germ cells** is regulated by **gonadotropins (FSH and LH)**, which are secreted by the anterior pituitary, and sex steroids.
- This process is not directly controlled by hormones originating from the supraoptic and paraventricular nuclei.
Glucocorticoid synthesis and regulation US Medical PG Question 4: A 57-year-old presents to your clinic complaining of baldness. He is overweight, has been diagnosed with BPH, and is currently taking atorvastatin for hyperlipidemia. The patient has tried several over-the-counter products for hair-loss; however, none have been effective. After discussing several options, the patient is prescribed a medication to treat his baldness that has the additional benefit of treating symptoms of BPH as well. Synthesis of which of the following compounds would be expected to decrease in response to this therapy?
- A. Testosterone
- B. FSH
- C. LH
- D. GnRH
- E. DHT (Correct Answer)
Glucocorticoid synthesis and regulation Explanation: ***DHT***
- The medication described is likely **finasteride**, a **5-alpha-reductase inhibitor**. This enzyme converts **testosterone** to **dihydrotestosterone (DHT)**.
- Decreased DHT levels are beneficial for treating both **androgenetic alopecia (baldness)** and **benign prostatic hyperplasia (BPH)** due to its potent androgenic effects on hair follicles and prostate growth.
*Testosterone*
- While finasteride inhibits the conversion of testosterone to DHT, it does not directly decrease testosterone synthesis. In fact, **testosterone levels may slightly increase** as its conversion to DHT is blocked.
- Testosterone itself is not the primary androgen responsible for male pattern baldness or BPH; it's its more potent metabolite, DHT.
*FSH*
- **Follicle-stimulating hormone (FSH)** is a gonadotropin released from the anterior pituitary that stimulates sperm production and ovarian follicle development.
- The medication prescribed does not directly affect FSH synthesis or release; its action is peripheral, affecting androgen metabolism.
*LH*
- **Luteinizing hormone (LH)** is another gonadotropin that stimulates testosterone production in Leydig cells.
- The drug's mechanism of action is local inhibition of an enzyme, not a central regulation of pituitary hormones like LH.
*GnRH*
- **Gonadotropin-releasing hormone (GnRH)** is released from the hypothalamus and stimulates the anterior pituitary to release FSH and LH.
- This therapy specifically targets the conversion of an androgen and does not impact the hypothalamic-pituitary-gonadal axis at the level of GnRH.
Glucocorticoid synthesis and regulation US Medical PG Question 5: A newborn female is found to have ambiguous genitalia and hypotension. Laboratory workup reveals hyperkalemia, hyperreninemia, and elevated levels of 17-hydroxyprogesterone in the patient's urine. Which of the following enzymes would you expect to be deficient in this patient?
- A. 11-hydroxylase
- B. 3β-hydroxysteroid dehydrogenase
- C. 11β-hydroxysteroid dehydrogenase
- D. 21-hydroxylase (Correct Answer)
- E. 17-hydroxylase
Glucocorticoid synthesis and regulation Explanation: ***21-hydroxylase***
- **21-hydroxylase deficiency** is the most common cause of **congenital adrenal hyperplasia (CAH)**, leading to a build-up of **17-hydroxyprogesterone** and its metabolites, which are shunted into androgen pathways.
- The deficiency in cortisol and aldosterone synthesis results in **hyponatremia**, **hyperkalemia**, and **hypotension** (due to salt-wasting) and **ambiguous genitalia** in females due to excess androgens.
*11-hydroxylase*
- **11-hydroxylase deficiency** also causes **CAH** and leads to ambiguous genitalia in females, but it is typically associated with **hypertension** due to the accumulation of 11-deoxycorticosterone, a mineralocorticoid, not hypotension.
- While 17-hydroxyprogesterone might be elevated, the defining feature of this deficiency is elevated levels of **11-deoxycorticosterone** and **11-deoxycortisol**.
*3β-hydroxysteroid dehydrogenase*
- **3β-hydroxysteroid dehydrogenase deficiency** leads to impaired synthesis of all adrenal steroids (glucocorticoids, mineralocorticoids, and androgens), resulting in severe **salt-wasting** and **hypotension**.
- However, females with this deficiency would typically present with **undervirilized** or normal genitalia, rather than ambiguous genitalia, due to reduced androgen synthesis.
*11β-hydroxysteroid dehydrogenase*
- **11β-hydroxysteroid dehydrogenase deficiency** is typically associated with **apparent mineralocorticoid excess syndrome**, leading to **hypertension** and **hypokalemia**, not hyperkalemia or ambiguous genitalia.
- This enzyme is responsible for converting cortisol to cortisone, preventing cortisol from activating mineralocorticoid receptors.
*17-hydroxylase*
- **17-hydroxylase deficiency** impairs the synthesis of sex hormones and cortisol, leading to **hypertension** and **hypokalemia** due to increased mineralocorticoid production (e.g., corticosterone, 11-deoxycorticosterone).
- Females would typically have **normal female external genitalia** but lack pubertal development, and males would present with **undervirilized external genitalia**.
Glucocorticoid synthesis and regulation US Medical PG Question 6: A 55-year-old man presents to his primary care physician for a new patient appointment. The patient states that he feels well and has no concerns at this time. The patient has a past medical history of hypertension, an elevated fasting blood glucose, and is not currently taking any medications. His blood pressure is 177/118 mmHg, pulse is 90/min, respirations are 16/min, and oxygen saturation is 97% on room air. Physical exam is notable for an obese man with atrophy of his limbs and striae on his abdomen. Laboratory values are notable for a blood glucose of 175 mg/dL. Which of the following is the best next step in evaluation?
- A. Hydrochlorothiazide
- B. MRI of the head
- C. Metformin
- D. Weight loss
- E. Dexamethasone suppression test (Correct Answer)
Glucocorticoid synthesis and regulation Explanation: ***Dexamethasone suppression test***
- The patient presents with **atrophy of the limbs** with concurrent **striae on the abdomen**, uncontrolled hypertension, and elevated blood glucose, which are all classic signs of **Cushing's syndrome**.
- A **dexamethasone suppression test** is the best initial diagnostic step to confirm Cushing's syndrome by assessing the body's cortisol regulation.
*Hydrochlorothiazide*
- While the patient has **hypertension**, treating the symptom without addressing the underlying cause (Cushing's syndrome) would be insufficient and potentially delay proper diagnosis.
- **Hydrochlorothiazide** is an antihypertensive, but without addressing the likely cortisol excess, blood pressure control will be challenging.
*MRI of the head*
- An **MRI of the head** (specifically the pituitary) would be considered after biochemical confirmation of Cushing's syndrome to localize a potential tumor, but it is not the initial diagnostic step.
- Imaging is performed *after* biochemical tests indicate cortisol excess, to differentiate between pituitary, adrenal, or ectopic causes.
*Metformin*
- The patient has **elevated blood glucose**, but initiating an antidiabetic medication like **metformin** before evaluating for Cushing's syndrome would be treating a symptom without identifying the root cause.
- Diabetes in this context is likely secondary to excess cortisol, so managing it effectively requires addressing the underlying endocrine disorder.
*Weight loss*
- While **weight loss** is generally beneficial for hypertension and diabetes, in the context of Cushing's syndrome with **limb atrophy** and **central obesity**, focusing solely on weight loss without addressing the hormonal imbalance would be ineffective.
- The characteristic fat redistribution in Cushing's syndrome makes simple weight loss difficult and less impactful until cortisol levels are managed.
Glucocorticoid synthesis and regulation US Medical PG Question 7: A 45-year-old woman presents to her physician with a four-month history of headache. Her headache is nonfocal but persistent throughout the day without any obvious trigger. She was told that it was a migraine but has never responded to sumatriptan, oxygen, or antiemetics. She takes amlodipine for hypertension. She does not smoke. She denies any recent weight loss or constitutional symptoms. Her temperature is 98°F (36.7°C), blood pressure is 180/100 mmHg, pulse is 70/min, and respirations are 15/min. She is obese with posterior cervical fat pads and central abdominal girth. Her neurological exam is unremarkable. In her initial laboratory workup, her fasting blood glucose level is 200 mg/dL. The following additional lab work is obtained and is as follows:
Serum:
Na+: 142 mEq/L
Cl-: 102 mEq/L
K+: 4.1 mEq/L
HCO3-: 24 mEq/L
BUN: 20 mg/dL
Glucose: 135 mg/dL
Creatinine: 1.3 mg/dL
Ca2+: 10.0 mg/dL
AST: 8 U/L
ALT: 8 U/L
24-hour urinary cortisol: 500 µg (reference range < 300 µg)
Serum cortisol: 25 µg/mL (reference range 5-23 µg/dL)
24-hour low dose dexamethasone suppression test: Not responsive
High dose dexamethasone suppression test: Responsive
Adrenocorticotropin-releasing hormone (ACTH): 20 pg/mL (5-15 pg/mL)
Imaging reveals a 0.5 cm calcified pulmonary nodule in the right middle lobe that has been present for 5 years but an otherwise unremarkable pituitary gland, mediastinum, and adrenal glands. What is the best next step in management?
- A. Pituitary resection
- B. CT-guided biopsy of the pulmonary nodule
- C. Inferior petrosal sinus sampling (Correct Answer)
- D. Pulmonary nodule resection
- E. Repeat high dose dexamethasone suppression test
Glucocorticoid synthesis and regulation Explanation: ***Inferior petrosal sinus sampling***
- The patient exhibits clear signs of **Cushing's syndrome** (hypertension, obesity with central fat distribution, hyperglycemia, elevated cortisol, lack of suppression with low-dose dexamethasone).
- The elevated ACTH and suppression with high-dose dexamethasone point towards **Cushing's disease** (pituitary ACTH overproduction). However, with an unremarkable pituitary MRI, **inferior petrosal sinus sampling (IPSS)** is crucial to differentiate ectopic ACTH production (e.g., from a bronchial carcinoid, lung nodule) from pituitary disease.
*Pituitary resection*
- This is a treatment for **Cushing's disease** (pituitary adenoma), but it should only be performed after definitive localization of the ACTH-producing tumor.
- Since the pituitary gland appears unremarkable on imaging and the patient has a lung nodule, **IPSS** is needed to confirm the source of ACTH overexpression before surgery.
*CT-guided biopsy of the pulmonary nodule*
- While the patient has a calcified pulmonary nodule, it has been stable for 5 years and calcified, suggesting it is likely **benign**.
- Without evidence that this nodule is the source of **ectopic ACTH production** (which IPSS would help determine), a biopsy is premature and may not yield a definitive answer for the Cushing's presentation.
*Pulmonary nodule resection*
- Resection is a treatment for **ectopic ACTH-producing tumors**, typically **carcinoid tumors** in the lung.
- However, the nodule is calcified and stable, making it unlikely to be the cause of Cushing's syndrome, and further, the diagnosis of ectopic ACTH needs to be confirmed with **IPSS** before considering such an invasive procedure.
*Repeat high dose dexamethasone suppression test*
- The results already indicate responsiveness to the high-dose dexamethasone suppression test, suggesting a **pituitary source** of ACTH.
- Repeating the test would not add more diagnostic value and would only delay the necessary localization studies like **IPSS** or imaging.
Glucocorticoid synthesis and regulation US Medical PG Question 8: A 5-year-old male visits his pediatrician for a check-up. His height corresponds to the 99th percentile for his age, and pubic hair is present upon physical examination. Serum renin and potassium levels are high, as is 17-hydroxyprogesterone. Which of the following is likely deficient in this patient?
- A. 11ß-hydroxylase
- B. 21-hydroxylase (Correct Answer)
- C. Aromatase
- D. 5a-reductase
- E. 17a-hydroxylase
Glucocorticoid synthesis and regulation Explanation: ***21-hydroxylase***
- A deficiency in **21-hydroxylase** leads to the accumulation of **17-hydroxyprogesterone**, as conversion to 11-deoxycorticosterone and 11-deoxycortisol is blocked, which aligns with the high levels observed in the patient.
- The shunting of precursors towards **androgen synthesis** due to the block explains the **precocious puberty** (pubic hair, advanced height for age).
- **Mineralocorticoid deficiency** (low aldosterone) causes **salt-wasting** with sodium loss and potassium retention (hyperkalemia), which stimulates compensatory **renin elevation**, explaining the high renin and potassium levels.
*11ß-hydroxylase*
- A deficiency would cause an accumulation of **11-deoxycorticosterone** and **11-deoxycortisol**, not primarily 17-hydroxyprogesterone.
- This deficiency typically presents with **hypertension** and **virilization** due to elevated 11-deoxycorticosterone (has mineralocorticoid activity) and androgens, but mineralocorticoid excess would **suppress renin**, which contradicts the high renin observed.
*Aromatase*
- **Aromatase** is responsible for converting androgens to estrogens. Its deficiency in males would typically result in **tall stature** due to delayed epiphyseal fusion but would not cause precocious puberty or the specific hormonal imbalance seen (high 17-hydroxyprogesterone, high renin/potassium).
- The absence of estrogen conversion would lead to **continued growth** and delayed bone maturation rather than early virilization with adrenal androgen excess.
*5a-reductase*
- **5a-reductase** converts testosterone to the more potent dihydrotestosterone (DHT). A deficiency in males would cause **undervirilization** at birth (ambiguous genitalia) and incomplete masculinization at puberty.
- This scenario contradicts the observed signs of **precocious puberty** and virilization in a 5-year-old male.
*17a-hydroxylase*
- A **17a-hydroxylase deficiency** would block the synthesis of cortisol and sex steroids, leading to increased production of mineralocorticoids like **corticosterone** and **11-deoxycorticosterone**.
- This typically results in **hypertension**, **hypokalemia** (mineralocorticoid excess), and **absent or delayed puberty** (lack of sex steroids), which are contrary to the symptoms presented in this patient (high potassium, precocious puberty).
Glucocorticoid synthesis and regulation US Medical PG Question 9: You have been asked to deliver a lecture to medical students about the effects of various body hormones and neurotransmitters on the metabolism of glucose. Which of the following statements best describes the effects of sympathetic stimulation on glucose metabolism?
- A. Norepinephrine causes increased glucose absorption within the intestines.
- B. Without epinephrine, insulin cannot act on the liver.
- C. Peripheral tissues require epinephrine to take up glucose.
- D. Epinephrine increases liver glycogenolysis. (Correct Answer)
- E. Sympathetic stimulation to alpha receptors of the pancreas increases insulin release.
Glucocorticoid synthesis and regulation Explanation: ***Epinephrine increases liver glycogenolysis.***
- **Epinephrine**, released during sympathetic stimulation, primarily acts to increase **glucose availability** for immediate energy.
- It achieves this by stimulating **glycogenolysis** (breakdown of glycogen into glucose) in the liver via **beta-adrenergic receptors**.
*Norepinephrine causes increased glucose absorption within the intestines.*
- **Norepinephrine** primarily causes **vasoconstriction** and can *decrease* **intestinal motility** and nutrient absorption due to shunting blood away from the digestive tract during stress.
- Glucose absorption is mainly regulated by digestive enzymes and transport proteins, not directly increased by norepinephrine.
*Without epinephrine, insulin cannot act on the liver.*
- **Insulin** acts on the liver independent of epinephrine to promote **glucose uptake**, **glycogenesis**, and **lipid synthesis**.
- Epinephrine and insulin have **antagonistic effects** on liver glucose metabolism; epinephrine increases glucose output, while insulin decreases it.
*Peripheral tissues require epinephrine to take up glucose.*
- **Insulin** is the primary hormone required for **glucose uptake** by most peripheral tissues, especially **muscle** and **adipose tissue**, via **GLUT4 transporters**.
- Epinephrine generally *reduces* glucose uptake by peripheral tissues to preserve glucose for the brain during stress.
*Sympathetic stimulation to alpha receptors of the pancreas increases insulin release.*
- Sympathetic stimulation, primarily acting through **alpha-2 adrenergic receptors** on pancreatic beta cells, actually **inhibits** **insulin secretion**.
- This inhibition helps to increase blood glucose levels by reducing insulin's glucose-lowering effects.
Glucocorticoid synthesis and regulation US Medical PG Question 10: A 60-year-old man with type 2 diabetes on metformin and insulin presents with 3 days of nausea, vomiting, and diffuse abdominal pain. He appears ill and confused. Vital signs: BP 95/60 mmHg, HR 115/min, RR 28/min, T 37.2°C. Labs show glucose 380 mg/dL, pH 7.28, HCO3 18 mEq/L, anion gap 24, serum osmolality 310 mOsm/kg, negative urine ketones, creatinine 2.8 mg/dL (baseline 1.1), lactate 8.2 mmol/L. Apply physiological principles to determine the primary acid-base and metabolic disturbance.
- A. Sepsis-induced lactic acidosis with stress hyperglycemia
- B. Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin (Correct Answer)
- C. Alcoholic ketoacidosis with concurrent diabetic emergency
- D. Diabetic ketoacidosis with renal failure from volume depletion
- E. Mixed metabolic acidosis from uremia and starvation ketosis
Glucocorticoid synthesis and regulation Explanation: ***Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin***
- The patient exhibits features of **Hyperosmolar Hyperglycemic State (HHS)**, including significant hyperglycemia and confusion, but the **negative urine ketones** effectively rule out DKA.
- The severe **high anion gap metabolic acidosis** is driven by a **lactate of 8.2 mmol/L**, likely due to **Metformin-Associated Lactic Acidosis (MALA)** triggered by **acute kidney injury** (creatinine 2.8).
*Sepsis-induced lactic acidosis with stress hyperglycemia*
- While the patient is hypotensive and tachycardic, the **serum glucose of 380 mg/dL** and history of insulin use point primarily to a diabetic emergency rather than simple stress hyperglycemia.
- **Lactic acidosis** in sepsis usually occurs alongside clinical signs of infection, which are not the focus of this metformin-using patient's profile.
*Alcoholic ketoacidosis with concurrent diabetic emergency*
- **Alcoholic ketoacidosis** would typically present with a history of alcohol abuse and **positive ketones** (specifically beta-hydroxybutyrate), which contradicts the **negative urine ketones** found here.
- The primary source of acidosis in this patient is clearly identified as **lactate (8.2 mmol/L)**, not ketoacids.
*Diabetic ketoacidosis with renal failure from volume depletion*
- **Diabetic Ketoacidosis (DKA)** is unlikely given the **negative urine ketones** and a pH/bicarbonate profile that is less severe than typically seen in profound ketoacidosis.
- DKA usually presents with a lower glucose level (often <250-300 mg/dL) compared to the hyperosmolar states seen in **Type 2 Diabetes** patients.
*Mixed metabolic acidosis from uremia and starvation ketosis*
- While **uremia** contributes to the anion gap when creatinine is elevated (2.8 mg/dL), it is rarely the primary cause of an **anion gap of 24** without more advanced renal failure.
- **Starvation ketosis** would result in positive ketones and a much milder acidosis than the profound **lactic acidosis** (8.2 mmol/L) observed in this case.
More Glucocorticoid synthesis and regulation US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.