Glucagon physiology US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Glucagon physiology. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Glucagon physiology US Medical PG Question 1: A 24-year-old man presents for an annual check-up. He is a bodybuilder and tells you he is on a protein-rich diet that only allows for minimal carbohydrate intake. His friend suggests he try exogenous glucagon to help him lose some excess weight before an upcoming competition. Which of the following effects of glucagon is he attempting to exploit?
- A. Increased glucose utilization by tissues
- B. Decreased blood cholesterol level
- C. Increased hepatic gluconeogenesis
- D. Increased lipolysis in adipose tissues (Correct Answer)
- E. Increased hepatic glycogenolysis
Glucagon physiology Explanation: ***Increased lipolysis in adipose tissues***
- While **glucagon's primary target is the liver**, it can have **modest lipolytic effects** on adipose tissue by opposing insulin's anti-lipolytic actions.
- Glucagon stimulates cAMP production, which can activate **hormone-sensitive lipase** to break down triglycerides into **fatty acids** and **glycerol**.
- However, **catecholamines (epinephrine/norepinephrine)** are far more potent direct stimulators of adipose tissue lipolysis than glucagon.
- The friend is attempting to exploit this lipolytic effect for fat loss, though **exogenous glucagon is not an evidence-based or safe weight-loss strategy**.
*Increased glucose utilization by tissues*
- This is **opposite** to glucagon's actual effect. **Glucagon raises blood glucose** levels; it does not promote glucose uptake by peripheral tissues.
- **Insulin** is the hormone responsible for promoting glucose uptake and utilization by muscle, adipose, and other tissues.
*Decreased blood cholesterol level*
- Glucagon does not have a direct, clinically significant effect on reducing blood cholesterol levels.
- While glucagon affects overall lipid metabolism through its catabolic actions, it is not used therapeutically for hypercholesterolemia.
*Increased hepatic gluconeogenesis*
- **Glucagon strongly stimulates hepatic gluconeogenesis**, which is the synthesis of glucose from non-carbohydrate precursors (amino acids, lactate, glycerol) in the liver.
- This action **raises blood glucose** levels and would not directly contribute to fat loss or weight reduction.
- In the context of a low-carbohydrate diet, increased gluconeogenesis would maintain blood glucose but not promote the fat loss the bodybuilder seeks.
*Increased hepatic glycogenolysis*
- **Glucagon is a potent stimulator of hepatic glycogenolysis**, the breakdown of stored liver glycogen into glucose.
- This rapidly increases blood glucose levels during fasting or hypoglycemia.
- However, this does not directly target adipose tissue for fat loss; it mobilizes glucose stores rather than fat stores, so it's not the mechanism relevant to weight loss goals.
Glucagon physiology US Medical PG Question 2: A 32-year-old woman is found unconscious on the office floor just before lunch by her colleagues. She had previously instructed them on the location of an emergency kit in case this ever happened so they are able to successfully inject her with the substance inside. Her past medical history is significant for type 1 diabetes for which she takes long acting insulin as well as periprandial rapid acting insulin injections. She has previously been found unconscious once before when she forgot to eat breakfast. The substance inside the emergency kit most likely has which of the following properties.
- A. Promotes glucose release from skeletal muscles
- B. Promotes glycogen breakdown in the liver (Correct Answer)
- C. Promotes glycogen formation in the liver
- D. Promotes glucose uptake in muscles
- E. Inhibits activity of pancreatic alpha and beta cells
Glucagon physiology Explanation: ***Promotes glycogen breakdown in the liver***
- The woman is experiencing **hypoglycemia** due to her type 1 diabetes and missed meal, leading to unconsciousness. The emergency kit contains **glucagon**, which counteracts hypoglycemia.
- **Glucagon** primarily acts on the liver to increase blood glucose levels by promoting **glycogenolysis** (breakdown of glycogen stores into glucose), which provides rapid glucose release within minutes.
- Glucagon also stimulates **gluconeogenesis** (synthesis of new glucose from non-carbohydrate sources), though this is a slower process that takes hours and is less important for acute hypoglycemia treatment.
*Promotes glucose release from skeletal muscles*
- While skeletal muscles store glycogen, they lack the enzyme **glucose-6-phosphatase**, so they cannot directly release glucose into the bloodstream.
- Muscle glycogen is used only for the muscle's own energy needs, not for systemic glucose regulation.
*Promotes glycogen formation in the liver*
- **Glycogen formation** (glycogenesis) is stimulated by **insulin**, which lowers blood glucose levels.
- This action would worsen hypoglycemia, making it inappropriate treatment for an unconscious diabetic patient.
*Promotes glucose uptake in muscles*
- **Insulin** is the primary hormone that promotes **glucose uptake** into muscle cells, thereby lowering blood glucose.
- Administering a substance that promotes glucose uptake would exacerbate hypoglycemia.
*Inhibits activity of pancreatic alpha and beta cells*
- Inhibiting **alpha cells** would reduce glucagon secretion, which is counterproductive in hypoglycemia as glucagon raises blood glucose.
- Inhibiting **beta cells** would reduce insulin secretion; while this might prevent further insulin release, the primary need in acute hypoglycemia is to rapidly increase blood glucose through glycogenolysis.
Glucagon physiology US Medical PG Question 3: A 55-year-old man with alcoholic cirrhosis is admitted to the hospital for routine evaluation before liver transplantation. The physician asks the patient to stop eating 10 hours before surgery. Which of the following structures contributes directly to preventing fasting hypoglycemia by producing glucose in this patient?
- A. Adrenal cortex
- B. Skeletal muscle
- C. Red blood cells
- D. Skin
- E. Intestine (Correct Answer)
Glucagon physiology Explanation: ***Correct: Intestine***
- The **intestine** (particularly the small intestine) possesses the enzymatic machinery for **gluconeogenesis**, including glucose-6-phosphatase, allowing it to directly produce and release free glucose into the bloodstream.
- During prolonged fasting (>10 hours), intestinal gluconeogenesis can contribute up to **20-25% of total glucose production**, utilizing substrates like glutamine and glycerol.
- In patients with **alcoholic cirrhosis**, hepatic gluconeogenesis is impaired, making extrahepatic sites like the intestine and kidneys increasingly important for maintaining euglycemia.
- The intestine directly produces glucose and releases it into the portal circulation, making it a direct contributor to preventing fasting hypoglycemia.
*Incorrect: Skeletal muscle*
- **Skeletal muscle lacks glucose-6-phosphatase**, the enzyme required to convert glucose-6-phosphate to free glucose for release into the bloodstream.
- Muscle undergoes proteolysis during fasting, releasing amino acids (particularly alanine and glutamine) that serve as gluconeogenic substrates for the liver and kidneys.
- This represents an **indirect contribution** to glucose homeostasis through substrate provision, not direct glucose production.
*Incorrect: Red blood cells*
- **Red blood cells** lack mitochondria and can only perform anaerobic glycolysis, producing lactate.
- Lactate from RBCs can be recycled to glucose in the liver via the **Cori cycle**, but RBCs themselves are net glucose consumers, not producers.
- They contribute indirectly through substrate provision, not direct glucose synthesis.
*Incorrect: Skin*
- The **skin** has no significant role in glucose production or gluconeogenesis.
- Its primary functions are protection, thermoregulation, and sensation; it does not possess the enzymatic capacity for gluconeogenesis.
- Skin does not contribute to maintaining blood glucose homeostasis during fasting.
*Incorrect: Adrenal cortex*
- The **adrenal cortex** secretes hormones (cortisol, aldosterone) that regulate glucose metabolism indirectly.
- **Cortisol** promotes hepatic and renal gluconeogenesis and decreases peripheral glucose utilization, but the adrenal gland itself does not synthesize or release glucose.
- This is a regulatory role, not direct glucose production.
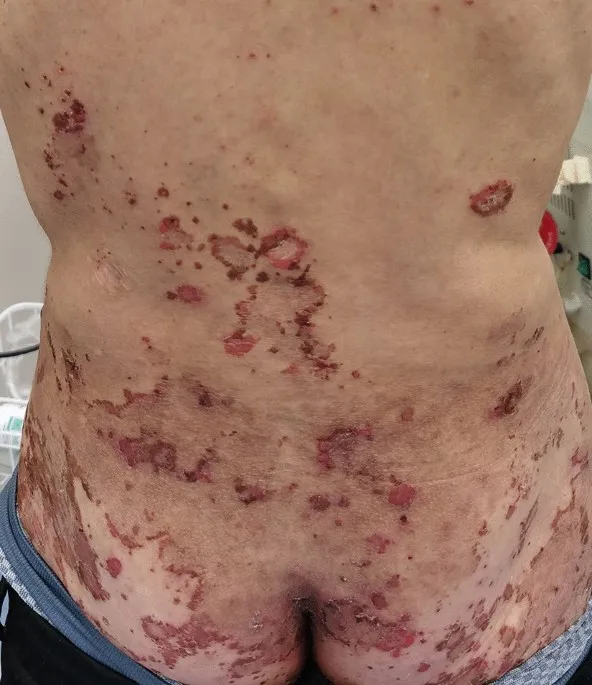
Glucagon physiology US Medical PG Question 4: A 53-year-old man comes to the physician because of fatigue, recurrent diarrhea, and an 8-kg (17.6-lb) weight loss over the past 6 months. He has a 4-month history of recurrent blistering rashes on different parts of his body that grow and develop into pruritic, crusty lesions before resolving spontaneously. Physical examination shows scaly lesions in different phases of healing with central, bronze-colored induration around the mouth, perineum, and lower extremities. Laboratory studies show:
Hemoglobin 10.1 mg/dL
Mean corpuscular volume 85 μm3
Mean corpuscular hemoglobin 30.0 pg/cell
Serum
Glucose 236 mg/dL
Abdominal ultrasonography shows a 3-cm, solid mass located in the upper abdomen. This patient's mass is most likely derived from which of the following types of cells?
- A. Gastrointestinal enterochromaffin cells
- B. Pancreatic β-cells
- C. Pancreatic δ-cells
- D. Pancreatic α-cells (Correct Answer)
- E. Gastric G-cells
Glucagon physiology Explanation: ***Pancreatic α-cells***
- The patient's symptoms of **fatigue, recurrent diarrhea, weight loss, blistering rash (necrolytic migratory erythema)**, and **hyperglycemia** are classic features of a **glucagonoma**.
- A **glucagonoma** is a tumor of the pancreatic α-cells that **secretes excessive glucagon**, leading to these characteristic signs and symptoms, supported by the presence of an **upper abdominal mass**.
*Gastrointestinal enterochromaffin cells*
- Tumors of gastrointestinal enterochromaffin cells (carcinoid tumors) typically produce **serotonin** and present with flushing, diarrhea, bronchospasm, and valvular heart disease, not the skin rash or hyperglycemia seen here.
- While carcinoid tumors can cause diarrhea, the additional symptoms of **necrolytic migratory erythema** and **diabetes** are not characteristic.
*Pancreatic β-cells*
- Tumors of pancreatic β-cells (**insulinomas**) produce excessive insulin, leading to **hypoglycemia**, not the hyperglycemia observed in this patient.
- Insulinomas cause symptoms like sweating, tremors, confusion, and palpitations, which are inconsistent with the patient's presentation.
*Pancreatic δ-cells*
- Pancreatic δ-cell tumors (**somatostatinomas**) secrete **somatostatin**, which can cause **diabetes mellitus**, steatorrhea, and gallstones.
- While diabetes is present, the characteristic **necrolytic migratory erythema** and severe diarrhea are less common with somatostatinomas.
*Gastric G-cells*
- Tumors of gastric G-cells (**gastrinomas**) secrete **gastrin**, leading to **Zollinger-Ellison syndrome**, characterized by severe peptic ulcers, abdominal pain, and chronic diarrhea.
- Gastrinomas do not typically cause **necrolytic migratory erythema** or significant hyperglycemia.
Glucagon physiology US Medical PG Question 5: A 45-year-old man comes to the physician for evaluation of a recurrent rash. He has multiple skin lesions on his legs, buttocks, and around his mouth. The rash first appeared a year ago and tends to resolve spontaneously in one location before reappearing in another location a few days later. It begins with painless, reddish spots that gradually increase in size and then develop into painful and itchy blisters. The patient also reports having repeated bouts of diarrhea and has lost 10 kg (22 lb) over the past year. One year ago, the patient was diagnosed with major depressive syndrome and was started on fluoxetine. Vital signs are within normal limits. Physical examination shows multiple crusty patches with central areas of bronze-colored induration, as well as tender eruptive lesions with irregular borders and on his legs, buttocks, and around his lips. The Nikolsky sign is negative. His hemoglobin concentration is 10.2 g/dL, mean corpuscular volume is 88 μm3, and serum glucose is 210 mg/dL. A skin biopsy of the lesion shows epidermal necrosis. Which of the following additional findings is most likely to be found in this patient?
- A. Increased serum vasoactive intestinal polypeptide level
- B. Antibodies against desmoglein 1 and 3
- C. Increased fasting serum glucagon level (Correct Answer)
- D. Antibodies against glutamic acid decarboxylase
- E. Antibodies against hemidesmosomes
Glucagon physiology Explanation: ***Increased fasting serum glucagon level***
- The constellation of **migratory necrolytic erythema** (the described rash), **weight loss**, **diarrhea**, and **hyperglycemia** is highly characteristic of a **glucagonoma**.
- A glucagonoma is a **pancreatic neuroendocrine tumor** that overproduces **glucagon**, leading to these systemic manifestations.
*Increased serum vasoactive intestinal polypeptide level*
- Elevated **VIP (vasoactive intestinal polypeptide)** levels are associated with **VIPomas**, which typically present with **watery diarrhea**, hypokalemia, and achlorhydria (WDHA syndrome).
- While diarrhea is present in this patient, the prominent skin rash, weight loss, and hyperglycemia point away from a VIPoma as the primary diagnosis.
*Antibodies against desmoglein 1 and 3*
- These antibodies are characteristic of **pemphigus vulgaris**, an autoimmune bullous disease that causes **flaccid blisters** and a positive Nikolsky sign.
- The patient's presentation of crusty patches with central induration, a negative Nikolsky sign, and epidermal necrosis on biopsy are inconsistent with pemphigus vulgaris.
*Antibodies against glutamic acid decarboxylase*
- **GAD antibodies** are strongly associated with **Type 1 diabetes mellitus** and **Stiff-person syndrome**.
- While the patient has hyperglycemia, the rash and other systemic symptoms are not typical features of Type 1 diabetes, and there are no signs of neurological stiffness.
*Antibodies against hemidesmosomes*
- Antibodies against hemidesmosomes (specifically BP180 and BP230) are found in **bullous pemphigoid**, an autoimmune blistering disease.
- Bullous pemphigoid typically presents with **tense blisters** on an erythematous base and usually affects older individuals, but the distinctive migratory rash, weight loss, and diarrhea are not consistent with this diagnosis.
Glucagon physiology US Medical PG Question 6: A 16-year-old girl is brought to the emergency department unresponsive. A witness reports that she became anxious, lightheaded, and began sweating and trembling a few minutes before she lost consciousness. Her vitals are as follows: blood pressure 95/60 mm Hg, heart rate 110/min, respiratory rate 21/min, and temperature 35.5°C (95.5°F). She becomes responsive but is still somnolent. She complains of dizziness and weakness. A more detailed history reveals that she has drastically restricted her diet to lose weight for the past 18 hours, and has not eaten today. Her skin is pale, wet, and cold. The rest of the physical examination is unremarkable. Blood testing shows a plasma glucose level of 2.8 mmol/L (50.5 mg/dL). Which of the following statements is true?
- A. Hypoglycemia in this patient is being compensated with an increased glycogenolysis rate. (Correct Answer)
- B. Epinephrine-induced gluconeogenesis is the main process that allows for the compensation of a decreased glucose level.
- C. There is an increase in the glycogen synthesis rate in this patient’s hepatocytes.
- D. The patient’s symptoms are most likely the consequence of increased insulin secretion from the pancreatic islets.
- E. The patient’s hypoglycemia inhibits glucagon release from pancreatic alpha cells.
Glucagon physiology Explanation: ***Hypoglycemia in this patient is being compensated with an increased glycogenolysis rate.***
- The patient's symptoms (anxiety, sweating, trembling, dizziness, weakness) and **low blood glucose (2.8 mmol/L)** confirm hypoglycemia. The immediate physiological response to hypoglycemia is the release of counter-regulatory hormones (glucagon, epinephrine, cortisol, growth hormone) which stimulate **glycogenolysis** (breakdown of glycogen to glucose) in the liver to maintain blood glucose, especially in the initial hours of fasting.
- Given that she has only fasted for 18 hours, her **hepatic glycogen stores** would still be recruited to provide glucose, making increased glycogenolysis a primary compensatory mechanism before gluconeogenesis becomes dominant.
*Epinephrine-induced gluconeogenesis is the main process that allows for the compensation of a decreased glucose level.*
- While epinephrine promotes **gluconeogenesis**, it is not the *main* compensatory process in the *initial* stages of fasting (0-24 hours). **Glycogenolysis** is the primary response in the first few hours.
- Gluconeogenesis becomes the predominant source of glucose after glycogen stores are significantly depleted, typically after 24 hours of fasting or longer.
*There is an increase in the glycogen synthesis rate in this patient’s hepatocytes.*
- **Glycogen synthesis (glycogenesis)** occurs when blood glucose levels are high, typically after a meal, to store excess glucose as glycogen.
- In a state of hypoglycemia, the liver's priority is to *release* glucose, meaning **glycogenolysis** is increased, and glycogen synthesis is inhibited.
*The patient’s symptoms are most likely the consequence of increased insulin secretion from the pancreatic islets.*
- **Increased insulin secretion** would *cause* hypoglycemia, not be a consequence. In response to hypoglycemia, insulin secretion is *reduced* to prevent further lowering of blood glucose.
- The symptoms described (anxiety, sweating, trembling) are characteristic of the **adrenergic response** to hypoglycemia, mediated by epinephrine and norepinephrine, which are counter-regulatory hormones.
*The patient’s hypoglycemia inhibits glucagon release from pancreatic alpha cells.*
- **Hypoglycemia** is a strong stimulant for **glucagon release** from pancreatic alpha cells. Glucagon's primary role is to raise blood glucose levels by promoting hepatic glycogenolysis and gluconeogenesis.
- Therefore, glucagon release would be *stimulated*, not inhibited, in this patient's condition.
Glucagon physiology US Medical PG Question 7: Which hormone most strongly stimulates gluconeogenesis during prolonged fasting?
- A. Insulin
- B. Epinephrine
- C. Cortisol
- D. Glucagon (Correct Answer)
Glucagon physiology Explanation: ***Glucagon***
- **Glucagon** is the primary hormone that promotes **gluconeogenesis** and glycogenolysis to maintain blood glucose during fasting.
- Its secretion is strongly stimulated by **low blood glucose levels**, making it critical throughout fasting states.
- Glucagon directly stimulates hepatic gluconeogenic enzymes and increases the availability of gluconeogenic substrates.
*Insulin*
- **Insulin** is an **anabolic hormone** that promotes glucose uptake and storage, thereby decreasing blood glucose levels.
- Its levels decrease during fasting, *suppressing* rather than stimulating gluconeogenesis.
- Insulin inhibits gluconeogenic enzyme expression and promotes glycolysis instead.
*Epinephrine*
- **Epinephrine** (adrenaline) is a stress hormone that rapidly increases blood glucose through both **glycogenolysis** and gluconeogenesis.
- Its effects are more prominent during **acute stress** or immediate energy demands (fight-or-flight response), rather than sustained fasting.
- Its action is rapid but transient compared to glucagon's sustained effect during fasting.
*Cortisol*
- **Cortisol** is a glucocorticoid that promotes **gluconeogenesis** by providing amino acid substrates through protein catabolysis and inducing gluconeogenic enzymes.
- While cortisol becomes increasingly important in **prolonged fasting** (>24-48 hours), **glucagon remains the primary and most potent direct stimulator** of hepatic gluconeogenesis throughout all phases of fasting.
- Cortisol's effects are slower in onset but more sustained, working synergistically with glucagon during extended fasting periods.
Glucagon physiology US Medical PG Question 8: A 60-year-old man with type 2 diabetes on metformin and insulin presents with 3 days of nausea, vomiting, and diffuse abdominal pain. He appears ill and confused. Vital signs: BP 95/60 mmHg, HR 115/min, RR 28/min, T 37.2°C. Labs show glucose 380 mg/dL, pH 7.28, HCO3 18 mEq/L, anion gap 24, serum osmolality 310 mOsm/kg, negative urine ketones, creatinine 2.8 mg/dL (baseline 1.1), lactate 8.2 mmol/L. Apply physiological principles to determine the primary acid-base and metabolic disturbance.
- A. Sepsis-induced lactic acidosis with stress hyperglycemia
- B. Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin (Correct Answer)
- C. Alcoholic ketoacidosis with concurrent diabetic emergency
- D. Diabetic ketoacidosis with renal failure from volume depletion
- E. Mixed metabolic acidosis from uremia and starvation ketosis
Glucagon physiology Explanation: ***Hyperosmolar hyperglycemic state complicated by lactic acidosis from metformin***
- The patient exhibits features of **Hyperosmolar Hyperglycemic State (HHS)**, including significant hyperglycemia and confusion, but the **negative urine ketones** effectively rule out DKA.
- The severe **high anion gap metabolic acidosis** is driven by a **lactate of 8.2 mmol/L**, likely due to **Metformin-Associated Lactic Acidosis (MALA)** triggered by **acute kidney injury** (creatinine 2.8).
*Sepsis-induced lactic acidosis with stress hyperglycemia*
- While the patient is hypotensive and tachycardic, the **serum glucose of 380 mg/dL** and history of insulin use point primarily to a diabetic emergency rather than simple stress hyperglycemia.
- **Lactic acidosis** in sepsis usually occurs alongside clinical signs of infection, which are not the focus of this metformin-using patient's profile.
*Alcoholic ketoacidosis with concurrent diabetic emergency*
- **Alcoholic ketoacidosis** would typically present with a history of alcohol abuse and **positive ketones** (specifically beta-hydroxybutyrate), which contradicts the **negative urine ketones** found here.
- The primary source of acidosis in this patient is clearly identified as **lactate (8.2 mmol/L)**, not ketoacids.
*Diabetic ketoacidosis with renal failure from volume depletion*
- **Diabetic Ketoacidosis (DKA)** is unlikely given the **negative urine ketones** and a pH/bicarbonate profile that is less severe than typically seen in profound ketoacidosis.
- DKA usually presents with a lower glucose level (often <250-300 mg/dL) compared to the hyperosmolar states seen in **Type 2 Diabetes** patients.
*Mixed metabolic acidosis from uremia and starvation ketosis*
- While **uremia** contributes to the anion gap when creatinine is elevated (2.8 mg/dL), it is rarely the primary cause of an **anion gap of 24** without more advanced renal failure.
- **Starvation ketosis** would result in positive ketones and a much milder acidosis than the profound **lactic acidosis** (8.2 mmol/L) observed in this case.
Glucagon physiology US Medical PG Question 9: A 38-year-old woman presents with hypertension (170/105 mmHg), hypokalemia (2.9 mEq/L), and metabolic alkalosis. Plasma aldosterone is elevated at 35 ng/dL (normal 4-31) and plasma renin activity is suppressed at 0.2 ng/mL/hr (normal 0.5-3.5). CT scan shows a 2.5 cm left adrenal mass. She also reports recent diagnosis of hyperthyroidism and is being evaluated for a neck mass. Synthesize these findings to evaluate for an underlying unifying diagnosis requiring modified treatment approach.
- A. Ectopic ACTH syndrome from thyroid carcinoma causing bilateral adrenal hyperplasia
- B. Multiple endocrine neoplasia type 2 requiring RET proto-oncogene testing and comprehensive screening (Correct Answer)
- C. Carney complex requiring cardiac myxoma screening before adrenal surgery
- D. Isolated aldosterone-producing adenoma requiring unilateral adrenalectomy only
- E. Coincidental adrenal adenoma and Graves' disease requiring separate standard treatments
Glucagon physiology Explanation: ***Multiple endocrine neoplasia type 2 requiring RET proto-oncogene testing and comprehensive screening***
- The presence of an **adrenal mass** and a **neck mass** in a relatively young patient with hypertension points toward **Multiple Endocrine Neoplasia type 2 (MEN 2)**, specifically medullary thyroid cancer and potential pheochromocytoma.
- While the labs mimic **primary hyperaldosteronism**, the high-risk combination requires **RET proto-oncogene** testing to identify a syndromic association and prevent surgical catastrophes.
*Ectopic ACTH syndrome from thyroid carcinoma causing bilateral adrenal hyperplasia*
- Ectopic ACTH typically results from **small cell lung cancer** or bronchial carcinoids and presents with **hypercortisolism** (Cushing syndrome), not primary hyperaldosteronism.
- The CT scan specifically identified a **unilateral 2.5 cm mass**, which is inconsistent with the **bilateral adrenal hyperplasia** seen in ACTH-secreting tumors.
*Carney complex requiring cardiac myxoma screening before adrenal surgery*
- Carney complex usually involves **Primary Pigmented Nodular Adrenocortical Disease (PPNAD)**, which presents with Cushing syndrome, not the mineralocorticoid excess seen here.
- This syndrome is characterized by **skin lentigines**, **blue nevi**, and **atrial myxomas**, which are not reported in this patient's clinical presentation.
*Isolated aldosterone-producing adenoma requiring unilateral adrenalectomy only*
- While the **high aldosterone** and **low renin** (ARR > 30) suggest an **aldosteronoma (Conn syndrome)**, this diagnosis does not account for the concurrent thyroid and neck masses.
- Proceeding with surgery based on an isolated diagnosis would be dangerous if the mass is actually a **pheochromocytoma** (common in MEN 2) disguised by confounding labs.
*Coincidental adrenal adenoma and Graves' disease requiring separate standard treatments*
- **Graves' disease** typically presents with a diffuse goiter and ophthalmopathy rather than a discrete **neck mass**, which is more indicative of a thyroid nodule or carcinoma.
- In medical examinations, clusters of endocrine findings are rarely coincidental; assuming they are unrelated misses the opportunity to screen for **hereditary syndromes**.
Glucagon physiology US Medical PG Question 10: A 32-year-old pregnant woman at 28 weeks gestation with type 1 diabetes presents with recurrent severe hypoglycemia despite reducing her insulin dose. Her insulin requirements have decreased by 40% over the past week. She reports decreased fetal movement. Fetal ultrasound shows intrauterine fetal demise. Evaluate the physiological mechanism explaining her changing insulin requirements in the context of pregnancy loss.
- A. Increased maternal growth hormone from pituitary compensation for fetal loss
- B. Maternal thyroid hormone surge causing enhanced glucose utilization
- C. Loss of placental lactogen and other diabetogenic hormones that normally increase insulin resistance (Correct Answer)
- D. Increased maternal cortisol from stress of fetal loss improving insulin sensitivity
- E. Placental glucose consumption cessation leading to maternal hyperglycemia compensation
Glucagon physiology Explanation: ***Loss of placental lactogen and other diabetogenic hormones that normally increase insulin resistance***
- Sudden **intrauterine fetal demise** leads to the cessation of placental function and a rapid drop in **human placental lactogen (hPL)**, which normally promotes maternal **insulin resistance**.
- Without these antagonizing hormones, the patient's **insulin sensitivity** returns to pre-pregnancy levels, causing a dramatic decrease in insulin requirements and severe **hypoglycemia**.
*Increased maternal growth hormone from pituitary compensation for fetal loss*
- Maternal **pituitary growth hormone** is actually suppressed during pregnancy as placental growth hormone takes over; there is no compensatory surge upon fetal loss.
- Even if growth hormone were to increase, it is a **diabetogenic hormone** that would increase insulin resistance rather than cause hypoglycemia.
*Maternal thyroid hormone surge causing enhanced glucose utilization*
- Pregnancy loss does not trigger a maternal **thyroid hormone surge**; thyroid levels typically stabilize or decrease following placental dysfunction.
- While hyperthyroidism can affect metabolism, it more commonly causes **glucose intolerance** rather than a 40% reduction in insulin needs.
*Increased maternal cortisol from stress of fetal loss improving insulin sensitivity*
- **Cortisol** is a stress hormone that increases gluconeogenesis and **decreases insulin sensitivity**, which would lead to hyperglycemia.
- Normal pregnancy is already a state of **physiologic hypercortisolism**; the loss of placental function actually reduces the contributions of placental CRH and cortisol.
*Placental glucose consumption cessation leading to maternal hyperglycemia compensation*
- While the fetus and placenta stop consuming glucose after demise, the loss of **anti-insulin hormones** has a much larger impact on the maternal metabolic state.
- The clinical presentation clearly shows **hypoglycemia**, which contradicts a compensatory mechanism for maternal hyperglycemia.
More Glucagon physiology US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.