Electrical-mechanical coupling US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Electrical-mechanical coupling. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Electrical-mechanical coupling US Medical PG Question 1: A molecular biologist is studying the roles of different types of ion channels regulating cardiac excitation. He identifies a voltage-gated calcium channel in the sinoatrial node, which is also present throughout the myocardium. The channel is activated at ~ -40 mV of membrane potential, undergoes voltage-dependent inactivation, and is highly sensitive to nifedipine. Which of the following phases of the action potential in the sinoatrial node is primarily mediated by ion currents through the channel that the molecular biologist is studying?
- A. Phase 2
- B. Phase 3
- C. Phase 1
- D. Phase 4
- E. Phase 0 (Correct Answer)
Electrical-mechanical coupling Explanation: ***Phase 0***
- The description of the channel (**activated at -40 mV**, **voltage-dependent inactivation**, sensitive to **nifedipine**) points to an **L-type calcium channel**.
- In the **sinoatrial node**, **L-type calcium channels** are primarily responsible for the **Phase 0 depolarization** (upstroke) of the action potential.
*Phase 2*
- In **myocardial cells**, **Phase 2** (plateau phase) is primarily mediated by **L-type calcium channels**, but the question refers to the **sinoatrial node action potential**.
- **Sinoatrial node cells** typically lack a distinct **Phase 2** plateau, distinguishing them from ventricular myocytes.
*Phase 3*
- **Phase 3** (repolarization) in the **sinoatrial node** is primarily mediated by the **efflux of potassium ions** through various **potassium channels**.
- The described channel, being a **calcium channel**, would contribute to depolarization rather than repolarization.
*Phase 1*
- **Phase 1** (initial repolarization) is characteristic of **ventricular myocytes** and is mediated by a transient outward **potassium current (Ito)**.
- The **sinoatrial node** action potential typically lacks a distinct **Phase 1**, as it does not have this rapid initial repolarization.
*Phase 4*
- **Phase 4** (spontaneous depolarization) in the **sinoatrial node** is primarily driven by the "funny" current (**If**, carried by **HCN channels**) and a gradually increasing **calcium current** (mainly through **T-type calcium channels**), leading to the threshold for **Phase 0**.
- While L-type channels contribute to reaching the threshold, their primary role is the rapid depolarization of **Phase 0**.
Electrical-mechanical coupling US Medical PG Question 2: An investigator is examining tissue samples from various muscle tissue throughout the body. She notices that biopsies collected from a specific site have a high concentration of sarcoplasmic reticulum, mitochondria, and myoglobin; they also stain poorly for ATPase. Additionally, the cell surface membranes of the myocytes in the specimen lack voltage-gated calcium channels. These myocytes are found in the greatest concentration at which of the following sites?
- A. Ventricular myocardium
- B. Tunica media
- C. Lateral rectus muscle
- D. Glandular myoepithelium
- E. Semispinalis muscle (Correct Answer)
Electrical-mechanical coupling Explanation: ***Semispinalis muscle***
- The described characteristics—**high concentration of sarcoplasmic reticulum, mitochondria, and myoglobin** with **poor ATPase staining**—are hallmarks of **Type I (slow-twitch oxidative) skeletal muscle fibers**.
- Postural muscles like the **semispinalis** (part of the erector spinae group) are predominantly composed of Type I fibers adapted for sustained, aerobic contraction to maintain posture.
- These fibers appear **red** due to high myoglobin content, have abundant mitochondria for aerobic metabolism, and stain **poorly for ATPase** (distinguishing them from Type II fast-twitch fibers).
- While all skeletal muscle does possess voltage-gated calcium channels for excitation-contraction coupling, the overall profile best matches slow-twitch postural muscles.
*Ventricular myocardium*
- While cardiac muscle has high mitochondria, myoglobin, and sarcoplasmic reticulum, it **does possess L-type voltage-gated calcium channels** on the sarcolemma, which are essential for cardiac excitation-contraction coupling.
- Cardiac muscle relies on **both** extracellular Ca²⁺ influx through these channels and calcium-induced calcium release from the SR.
- Cardiac muscle typically stains **strongly for ATPase**, not poorly.
*Tunica media*
- Composed of **vascular smooth muscle** with poorly developed sarcoplasmic reticulum and relatively few mitochondria compared to skeletal or cardiac muscle.
- Smooth muscle relies heavily on **extracellular calcium influx** and the calmodulin pathway for contraction.
- Not characterized by high myoglobin content.
*Lateral rectus muscle*
- This extraocular muscle contains predominantly **Type IIb fast-twitch glycolytic fibers** adapted for rapid, precise eye movements.
- These fibers have **low myoglobin** (white muscle), fewer mitochondria, and stain **strongly for ATPase**.
- Opposite profile from the described tissue.
*Glandular myoepithelium*
- Myoepithelial cells are specialized contractile cells in secretory glands with minimal sarcoplasmic reticulum and mitochondria.
- Function is brief contraction for secretion expulsion, not sustained aerobic work.
- Do not exhibit the high oxidative capacity described.
Electrical-mechanical coupling US Medical PG Question 3: An investigator is studying the crossbridge cycle of muscle contraction. Tissue from the biceps brachii muscle is obtained at the autopsy of an 87-year-old man. Investigation of the muscle tissue shows myosin heads attached to actin filaments. Binding of myosin heads to which of the following elements would most likely cause detachment of myosin from actin filaments?
- A. ATP (Correct Answer)
- B. Troponin C
- C. Tropomyosin
- D. ADP
- E. cGMP
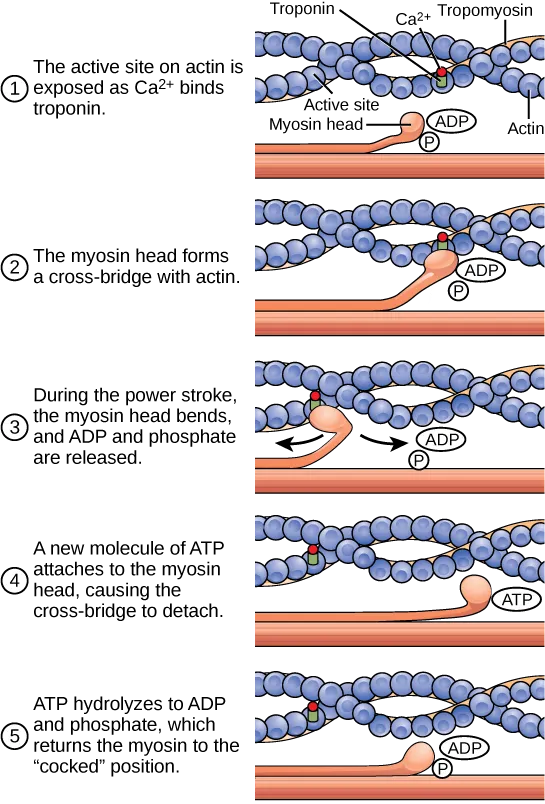
Electrical-mechanical coupling Explanation: ***ATP***
- The binding of **ATP** to the **myosin head** causes a conformational change that reduces its affinity for actin, leading to detachment.
- This step is crucial for the muscle to relax and for the subsequent power stroke to occur.
*Troponin C*
- **Troponin C** is a regulatory protein that binds calcium, which then causes a conformational change in the troponin-tropomyosin complex, revealing the **actin binding sites** for myosin.
- It does not directly cause myosin detachment; instead, it facilitates the binding of myosin to actin.
*Tropomyosin*
- **Tropomyosin** is a long, fibrous protein that covers the **myosin-binding sites** on actin in a relaxed muscle, preventing cross-bridge formation.
- Its movement, regulated by troponin, allows myosin to bind, but it does not directly cause detachment.
*ADP*
- **ADP** is released from the myosin head during the power stroke, but its binding does not cause detachment; rather, it is present during the strongly bound state before **ATP** binds.
- The presence of **ADP** and inorganic phosphate (Pi) often promotes the strong binding of myosin to actin.
*cGMP*
- **cGMP** (cyclic guanosine monophosphate) is a second messenger involved in various cellular processes, including smooth muscle relaxation, but it is not directly involved in the cross-bridge cycle and detachment of **myosin from actin** in skeletal muscle.
- Its primary role in muscle physiology is often linked to nitric oxide signaling and vasodilation.
Electrical-mechanical coupling US Medical PG Question 4: An investigator is studying muscle contraction in tissue obtained from the thigh muscle of an experimental animal. After injection of radiolabeled ATP, the tissue is stimulated with electrical impulses. Radioassay of these muscle cells is most likely to show greatest activity in which of the following structures?
- A. H zone
- B. M line
- C. A band (Correct Answer)
- D. Z line
- E. I band
Electrical-mechanical coupling Explanation: ***A band***
- The **A band** contains the entire length of the **thick myosin filaments** along with the **overlap zone** where myosin and actin interact. Myosin has **ATPase activity**, meaning it binds and hydrolyzes **ATP** to power muscle contraction through cross-bridge cycling.
- Therefore, the greatest accumulation of **radiolabeled ATP** and its breakdown products would be found where **myosin heads** are located throughout the A band.
- The A band represents the most complete answer as it encompasses all regions containing myosin ATPase activity.
*H zone*
- The **H zone** is the central part of the **A band** where only **thick myosin filaments** are present, with no overlap with thin actin filaments.
- While myosin heads with ATPase activity are present here and would show radiolabeled ATP, the **H zone** is only a **subset** of the A band. The **A band** is the more comprehensive answer as it includes both the H zone and the overlap regions where most cross-bridge cycling occurs.
*M line*
- The **M line** is the very center of the **H zone** and anchors the **thick filaments**.
- It consists of structural proteins like **myomesin** and **creatine kinase**. While creatine kinase can phosphorylate ADP to regenerate ATP, it does not directly hydrolyze ATP for muscle contraction the way myosin ATPase does.
*Z line*
- The **Z line** (or Z disc) marks the boundaries of a **sarcomere** and anchors the **thin actin filaments**.
- It contains proteins like **alpha-actinin** and **desmin** but does not directly consume ATP for muscle contraction.
*I band*
- The **I band** contains only **thin actin filaments** and extends from the edge of the A band to the Z line.
- While actin is crucial for contraction, it does not possess **ATPase activity**; ATP hydrolysis primarily occurs at the **myosin heads** located in the A band.
Electrical-mechanical coupling US Medical PG Question 5: A healthy 22-year-old male participates in a research study you are leading to compare the properties of skeletal and cardiac muscle. You conduct a 3-phased experiment with the participant. In the first phase, you get him to lift up a 2.3 kg (5 lb) weight off a table with his left hand. In the second phase, you get him to do 20 burpees, taking his heart rate to 150/min. In the third phase, you electrically stimulate his gastrocnemius with a frequency of 50 Hz. You are interested in the tension and electrical activity of specific muscles as follows: Biceps in phase 1, cardiac muscle in phase 2, and gastrocnemius in phase 3. What would you expect to be happening in the phases and the respective muscles of interest?
- A. Increase of tension in experiments 2 and 3, with the same underlying mechanism
- B. Increase of tension in all phases (Correct Answer)
- C. Recruitment of large motor units followed by small motor units in experiment 1
- D. Fused tetanic contraction at the end of all three experiments
- E. Recruitment of small motor units at the start of experiments 1 and 2
Electrical-mechanical coupling Explanation: ***Increase of tension in all phases***
- In **phase 1**, lifting a 2.3 kg weight requires the **biceps** to contract, generating sufficient force (**tension**) to overcome gravity.
- In **phase 2**, the **cardiac muscle** increases its contractile force (**tension**) to meet the metabolic demands of **exercise**, leading to a heart rate of 150/min.
- In **phase 3**, electrical stimulation of the **gastrocnemius** at 50 Hz triggers muscle contraction, leading to an increase in **tension**.
*Increase of tension in experiments 2 and 3, with the same underlying mechanism*
- While tension increases in phases 2 and 3, the **underlying mechanisms differ**: cardiac muscle tension increases due to increased sympathetic stimulation and preload, while skeletal muscle tension increases due to unfused or fused tetanus from electrical stimulation.
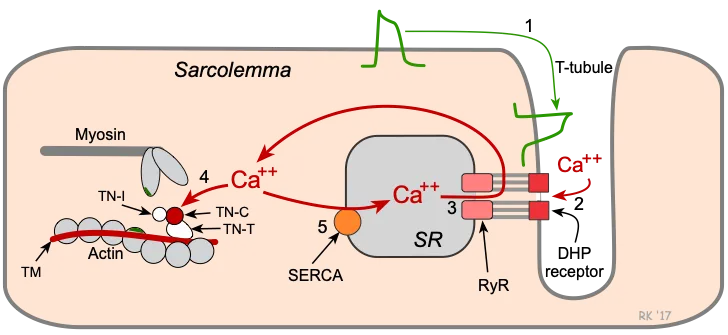
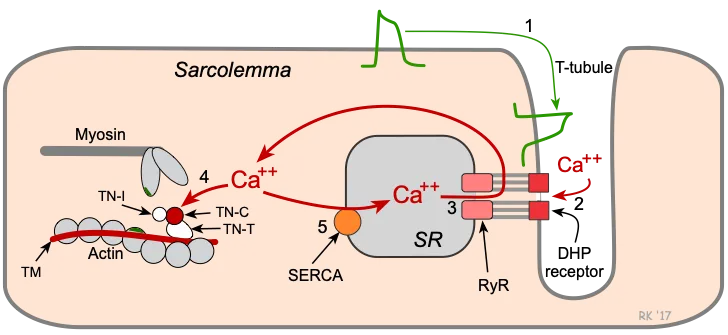
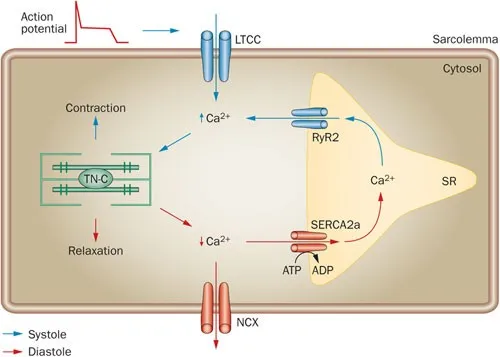
- Cardiac muscle contraction is regulated by **calcium-induced calcium release**, while skeletal muscle involves direct coupling of DHP receptor and ryanodine receptor.
*Recruitment of large motor units followed by small motor units in experiment 1*
- **Motor unit recruitment** follows the **size principle**, meaning smaller, more easily excitable motor units are activated first, followed by larger ones as more force is needed.
- Therefore, in phase 1, **small motor units** would be recruited first, not large ones.
*Fused tetanic contraction at the end of all three experiments*
- **Fused tetanic contraction** occurs in **skeletal muscle** when stimulation frequency is high enough that individual twitches summate completely, leading to sustained contraction.
- This phenomenon is **not possible in cardiac muscle** due to its long **refractory period**, which prevents sustained contraction and allows for adequate filling time.
*Recruitment of small motor units at the start of experiments 1 and 2*
- **Motor unit recruitment** applies to **skeletal muscle** (phase 1) and involves recruiting small motor units first for fine or gentle movements.
- **Cardiac muscle** (phase 2) does not have motor units; instead, it relies on the **Frank-Starling mechanism** and hormonal/nervous regulation to adjust its contractile force as a syncytium.
Electrical-mechanical coupling US Medical PG Question 6: Cardiac muscle serves many necessary functions, leading to a specific structure that serves these functions. The structure highlighted is an important histology component of cardiac muscle. What would be the outcome if this structure diffusely failed to function?
- A. Failure of potassium channels to appropriately open to repolarize the cell
- B. Failure of propagation of the action potential from the conduction system (Correct Answer)
- C. Ineffective excitation-contraction coupling due to insufficient calcium ions
- D. Inappropriate formation of cardiac valve leaflets
- E. Outflow tract obstruction
Electrical-mechanical coupling Explanation: ***Failure of propagation of the action potential from the conduction system***
- The highlighted structure, the **intercalated disc**, contains **gap junctions** which are crucial for the rapid, synchronized spread of **action potentials** between cardiac muscle cells.
- A diffuse failure of these structures would prevent the coordinated electrical activation of the myocardium, leading to a failure of impulse propagation and **compromised cardiac contraction**.
*Failure of potassium channels to appropriately open to repolarize the cell*
- This scenario describes a problem with **ion channel function** within individual cardiomyocytes, affecting their repolarization phase.
- While critical for a single cell's electrical activity, it does not directly relate to the primary function of **intercalated discs** in *propagating* action potentials across multiple cells.
*Ineffective excitation-contraction coupling due to insufficient calcium ions*
- This outcome would result from issues with **calcium handling** mechanisms, such as problems with the **sarcoplasmic reticulum** or **calcium channels**, which are internal to the cardiomyocyte.
- It is distinct from the role of **intercalated discs** in facilitating intercellular communication and electrical spread.
*Inappropriate formation of cardiac valve leaflets*
- The formation of cardiac valve leaflets is an intricate process during **embryological development** involving specific signaling pathways and cell migration.
- This structural defect is not directly related to the function of **intercalated discs** in mature cardiac muscle, which are involved in electrical and mechanical coupling.
*Outflow tract obstruction*
- **Outflow tract obstruction** is a congenital or acquired structural defect affecting the major arteries leaving the heart (e.g., aortic or pulmonary stenosis).
- This is a macroscopic structural anomaly that is not caused by a primary failure of **intercalated disc** function.
Electrical-mechanical coupling US Medical PG Question 7: A researcher is studying how electrical activity propagates across the heart. In order to do this, he decides to measure the rate at which an action potential moves within various groups of cardiac muscle tissue. In particular, he isolates fibers from areas of the heart with the following characteristics:
A) Dysfunction leads to fixed PR intervals prior to a dropped beat
B) Dysfunction leads to increasing PR intervals prior to a dropped beat
C) Dysfunction leads to tachycardia with a dramatically widened QRS complex
D) Dysfunction leads to tachycardia with a sawtooth pattern on electrocardiogram
Which of the following is the proper order of these tissues from fastest action potential propagation to slowest action potential propagation.
- A. B > D > C > A
- B. D > C > A > B
- C. B > C > D > A
- D. A > D > C > B (Correct Answer)
- E. A > C > D > B
Electrical-mechanical coupling Explanation: ***A > D > C > B***
* **Purkinje fibers (A)** have the fastest conduction velocity in the heart to ensure rapid and synchronous ventricular depolarization. The description of "fixed PR intervals prior to a dropped beat" in **Mobitz type II second-degree AV block** indicates an issue with conduction distal to the AV node, often in the His-Purkinje system, while still maintaining typical conduction through the atria and AV node for conducted beats.
* **Atrial muscle (D)** has a faster conduction velocity than the AV node but slower than Purkinje fibers. The "sawtooth pattern on electrocardiogram" unequivocally points to **atrial flutter**, which is characterized by rapid, regular depolarization of the atria.
* **Ventricular muscle (C)** has a conduction velocity slower than Purkinje fibers but faster than the AV node. "Tachycardia with a dramatically widened QRS complex" is characteristic of **ventricular tachycardia (VT)**, which arises from abnormal electrical activity within the ventricles.
* **AV node (B)** has the slowest conduction velocity in the heart, which allows for proper ventricular filling. "Increasing PR intervals prior to a dropped beat" describes **Mobitz type I second-degree AV block (Wenckebach phenomenon)**, which is due to progressive prolongation of conduction delay within the AV node itself.
*B > D > C > A*
* This order incorrectly places the **AV node (B)** as the fastest and **Purkinje fibers (A)** as the slowest, which is contrary to the known conduction velocities in the heart.
* The AV node is critical for delaying the impulse, making it the slowest, while Purkinje fibers are designed for rapid spread, making them the fastest.
*D > C > A > B*
* This option incorrectly places **atrial muscle (D)** as faster than **Purkinje fibers (A)**. Purkinje fibers have the fastest conduction velocity in the heart, considerably faster than atrial muscle.
*B > C > D > A*
* This arrangement incorrectly lists the **AV node (B)** as the fastest and **Purkinje fibers (A)** as the slowest. The AV node is the slowest for its physiological role of delaying ventricular contraction, while Purkinje fibers are optimized for rapid conduction.
*A > C > D > B*
* While placing **Purkinje fibers (A)** as the fastest and the **AV node (B)** as the slowest is correct, this order incorrectly places **ventricular muscle (C)** as faster than **atrial muscle (D)**. Atrial muscle generally conducts faster than ventricular muscle in normal physiology.
Electrical-mechanical coupling US Medical PG Question 8: A 72-year-old man with severe aortic regurgitation and compensated heart failure is being evaluated for surgical intervention. His echocardiogram shows LV end-diastolic dimension of 7.5 cm, ejection fraction of 45%, and severe aortic regurgitation with a regurgitant fraction of 60%. Pressure-volume loop analysis shows a markedly widened loop with increased stroke work. Evaluate the compensatory mechanisms maintaining his cardiac output and predict the timing for surgical intervention based on cardiac cycle mechanics.
- A. Surgery should be delayed until ejection fraction falls below 35% because current compensatory mechanisms are adequate as evidenced by maintained cardiac output
- B. Surgery is indicated now because the increased stroke work indicates the ventricle is operating at near-maximal preload reserve with impending decompensation despite preserved ejection fraction (Correct Answer)
- C. Surgery is contraindicated due to excessive left ventricular dimensions indicating irreversible remodeling with poor surgical outcomes
- D. Medical management with vasodilators should continue indefinitely because reduced afterload optimizes the pressure-volume relationship
- E. Surgery should wait until symptoms develop because pressure-volume loop changes alone do not predict outcomes in valvular disease
Electrical-mechanical coupling Explanation: ***Surgery is indicated now because the increased stroke work indicates the ventricle is operating at near-maximal preload reserve with impending decompensation despite preserved ejection fraction***
- In chronic **aortic regurgitation**, the ventricle undergoes **eccentric hypertrophy** to accommodate large volumes, but this patient has reached critical **LV end-diastolic dimensions** (>7.0 cm), signaling the limits of compensation.
- An **ejection fraction (EF) of 45%** in the setting of severe AR is actually indicative of **systolic dysfunction**, as guidelines generally recommend intervention when EF falls below 50-55% due to the increased total stroke volume.
*Surgery should be delayed until ejection fraction falls below 35% because current compensatory mechanisms are adequate as evidenced by maintained cardiac output*
- Waiting for the **ejection fraction** to drop to 35% is dangerous; by this stage, the **myocardial damage** is often irreversible and postoperative outcomes are significantly poorer.
- A "maintained" cardiac output is deceptive here because the **total stroke work** is massive compared to the actual **forward flow**, leading to progressive heart failure.
*Surgery is should wait until symptoms develop because pressure-volume loop changes alone do not predict outcomes in valvular disease*
- **Asymptomatic patients** with severe AR require surgery if they meet specific **echocardiographic triggers** (like LV dimensions or EF) to prevent sudden death and permanent LV dysfunction.
- **Pressure-volume loop** analysis and chamber dimensions are highly predictive of the transition from a **compensated** to a **decompensated** state.
*Surgery is contraindicated due to excessive left ventricular dimensions indicating irreversible remodeling with poor surgical outcomes*
- While severe enlargement carries higher risk, an **LVEDD of 7.5 cm** is not a contraindication but rather an **urgent indication** for valve replacement to halt further decline.
- **Irreversible remodeling** is usually associated with even lower ejection fractions and severe **congestive heart failure** symptoms that do not respond to medical therapy.
*Medical management with vasodilators should continue indefinitely because reduced afterload optimizes the pressure-volume relationship*
- **Vasodilators** (like ACE inhibitors or CCBs) can reduce afterload and improve **forward flow**, but they do not stop the mechanical progression of **valvular regurgitation** or remodeling.
- **Surgical intervention** (AVR) is the only definitive treatment for severe chronic AR once the heart shows signs of **exhausted preload reserve** and declining contractility.
Electrical-mechanical coupling US Medical PG Question 9: A 35-year-old woman with constrictive pericarditis undergoes right heart catheterization showing equalization of diastolic pressures across all cardiac chambers (RA, RV, PA, PCWP all approximately 20 mmHg). Ventricular pressure tracings show a distinctive 'square root sign' during diastole. Evaluate the mechanism by which pericardial constriction alters the normal pressure dynamics during the cardiac cycle and predict the effect on cardiac output during exercise.
- A. Fixed total cardiac volume limits diastolic filling; cardiac output cannot increase normally with exercise due to inability to augment stroke volume through increased preload (Correct Answer)
- B. Systolic dysfunction prevents adequate ejection; cardiac output fails to increase due to reduced contractility independent of filling
- C. Valvular regurgitation worsens with exercise; cardiac output decreases due to increased regurgitant fraction with tachycardia
- D. Coronary perfusion is compromised during diastole; cardiac output cannot increase due to exercise-induced ischemia
- E. Pulmonary hypertension limits right ventricular output; cardiac output is restricted by inability to increase pulmonary blood flow
Electrical-mechanical coupling Explanation: ***Fixed total cardiac volume limits diastolic filling; cardiac output cannot increase normally with exercise due to inability to augment stroke volume through increased preload***
- In **constrictive pericarditis**, the rigid pericardium imposes a **fixed cardiac volume**, leading to the characteristic **equalization of diastolic pressures** across all four chambers.
- During exercise, the heart cannot utilize the **Frank-Starling mechanism** to increase **stroke volume** because the non-compliant pericardium prevents any further increase in **end-diastolic volume**.
*Systolic dysfunction prevents adequate ejection; cardiac output fails to increase due to reduced contractility independent of filling*
- Constrictive pericarditis is primarily a disorder of **diastolic filling**, not a primary myocardial failure of **systolic contractility**.
- While chronic constriction can cause secondary atrophy, the hallmark pathophysiology is the restriction of **ventricular expansion** during diastole.
*Valvular regurgitation worsens with exercise; cardiac output decreases due to increased regurgitant fraction with tachycardia*
- This condition is an **extracardiac restriction** of the ventricles rather than a primary **valvular pathology** such as mitral or tricuspid regurgitation.
- Tachycardia generally decreases **regurgitant fraction** in conditions like mitral regurgitation because there is less time for backflow during systole.
*Coronary perfusion is compromised during diastole; cardiac output cannot increase due to exercise-induced ischemia*
- While the **square root sign** and high diastolic pressures exist, they do not typically cause **microvascular ischemia** as the primary limiting factor for cardiac output.
- The limitation is **mechanical** (volumetric) rather than **ischemic**; the ventricles simply cannot expand to accommodate more blood volume.
*Pulmonary hypertension limits right ventricular output; cardiac output is restricted by inability to increase pulmonary blood flow*
- Although **pulmonary artery** pressures are elevated (equalizing with other chambers), this is due to **back-pressure** from left-sided filling restriction, not primary pulmonary vascular disease.
- The primary pathology is the **global restriction** of all chambers by the pericardium, rather than an isolated failure of the **pulmonary circulation**.
Electrical-mechanical coupling US Medical PG Question 10: A 58-year-old man with severe coronary artery disease develops a ventricular aneurysm following an anterior myocardial infarction. Pressure-volume loop analysis shows a distinctive notch during the ejection phase. He has reduced ejection fraction of 30% but normal filling pressures. Evaluate the pathophysiologic mechanism explaining the notch in the pressure-volume loop and its clinical significance.
- A. Mitral regurgitation causes retrograde flow during systole appearing as a loop notch
- B. Diastolic dysfunction creates abnormal pressure-volume relationships during filling
- C. Coronary steal phenomenon redirects blood flow creating pressure fluctuations
- D. Increased afterload from peripheral vasoconstriction causes interrupted ejection
- E. Paradoxical systolic bulging of the aneurysm redistributes stroke volume, creating biphasic ejection
Electrical-mechanical coupling Explanation: ***Paradoxical systolic bulging of the aneurysm redistributes stroke volume, creating biphasic ejection***
- In a **ventricular aneurysm**, the non-contractile scarred tissue bulges outward during systole, absorbing energy that should be used for **forward stroke volume**.
- This **dyskinetic movement** causes a temporary redistribution of volume within the ventricle, resulting in a characteristic **notch** or irregularity in the ejection limb of the pressure-volume loop.
*Mitral regurgitation causes retrograde flow during systole appearing as a loop notch*
- **Mitral regurgitation** typically eliminates the **isovolumetric contraction** phase and broadens the PV loop, rather than creating a specific notch during the ejection phase.
- While it involves abnormal flow, the clinical indicator here is a **ventricular aneurysm**, which has a distinct mechanical effect on wall motion.
*Diastolic dysfunction creates abnormal pressure-volume relationships during filling*
- **Diastolic dysfunction** primarily affects the lower portion of the loop by shifting the **end-diastolic pressure-volume relationship (EDPVR)** curve upwards.
- The patient has **normal filling pressures**, suggesting that the primary pathology is systolic-mechanical rather than related to impaired relaxation or compliance.
*Coronary steal phenomenon redirects blood flow creating pressure fluctuations*
- **Coronary steal** is a microvascular phenomenon involving the redistribution of blood flow within the **myocardium** itself, not the intraventricular volume.
- It leads to **ischemia**, but does not create a mechanical "notch" in the pressure-volume loop ejection phase during a single cardiac cycle.
*Increased afterload from peripheral vasoconstriction causes interrupted ejection*
- Increased **afterload** typically tallies the PV loop by increasing the **systolic peaks**, but it does not cause a dip or notch in the phase where the semi-lunar valves are open.
- **Interrupted ejection** is a result of structural wall abnormalities (like dyskinesis) rather than systemic **vascular resistance** variations.
More Electrical-mechanical coupling US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.