Cardiac cycle

On this page
💓 The Cardiac Engine: Orchestrating Life's Perfect Rhythm
Every heartbeat is a precisely choreographed mechanical and electrical event where timing, pressure, and valve coordination determine whether tissues receive life-sustaining blood or begin to fail. You'll discover how electrical impulses trigger coordinated contraction, how pressure gradients open and close valves in perfect sequence, what heart sounds reveal about underlying pathology, and how clinicians manipulate preload, afterload, and contractility to rescue failing hearts. Mastering the cardiac cycle transforms abstract physiology into the foundation for interpreting ECGs, diagnosing murmurs, and optimizing hemodynamics at the bedside.
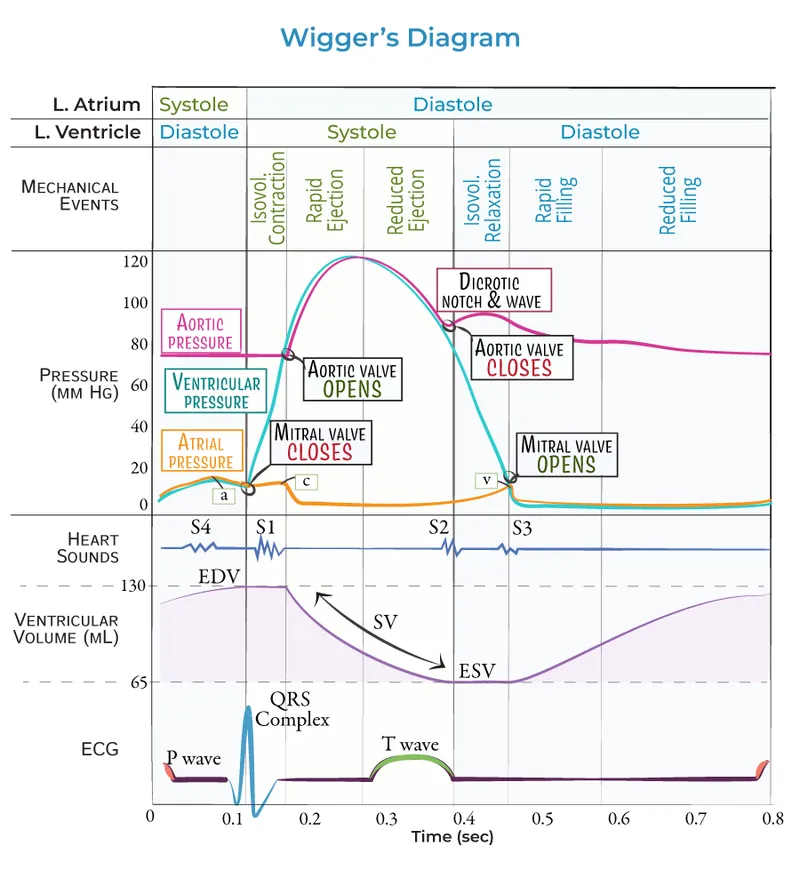
The cardiac cycle represents the complete sequence of mechanical and electrical events occurring during one heartbeat, typically lasting 0.8 seconds at rest. This intricate process involves precise coordination between atrial and ventricular chambers, four cardiac valves, and the electrical conduction system to generate the 5-6 liters per minute of cardiac output essential for tissue perfusion.
📌 Remember: PQRST-CYCLE - Pressure changes, QRS electrical activity, Relaxation (diastole), Systolic contraction, Timing coordination, Closure/opening of valves, Yielding stroke volume, Cardiac output generation, Lub-dub sounds, Ejection fraction optimization
The cardiac cycle fundamentally divides into two major phases: diastole (ventricular relaxation and filling, ~0.5 seconds) and systole (ventricular contraction and ejection, ~0.3 seconds). During diastole, ventricular pressure drops below atrial pressure, enabling passive filling of 70% of stroke volume, followed by active atrial contraction contributing the final 30%. Systole begins with isovolumetric contraction until ventricular pressure exceeds aortic pressure (80 mmHg), triggering ejection of 60-70% of end-diastolic volume.
| Phase | Duration (sec) | Ventricular Pressure (mmHg) | Volume Change (mL) | Valve Status | Key Events |
|---|---|---|---|---|---|
| Early Diastole | 0.1 | 0-5 | +40-50 | AV open, SL closed | Rapid filling |
| Late Diastole | 0.4 | 5-8 | +20-30 | AV open, SL closed | Atrial kick |
| Isovolumetric Contraction | 0.05 | 8-80 | 0 | All closed | Pressure buildup |
| Ejection | 0.25 | 80-120 | -70 | AV closed, SL open | Stroke volume |
💡 Master This: Ventricular pressure must exceed aortic pressure (80 mmHg systolic) to open the aortic valve, while closure occurs when ventricular pressure drops below 60 mmHg, creating the dicrotic notch and S2 heart sound.
Understanding cardiac cycle timing becomes crucial when heart rate increases beyond 100 bpm, as diastolic time shortens disproportionately, reducing ventricular filling time and potentially compromising stroke volume despite increased contractility from sympathetic stimulation.
💓 The Cardiac Engine: Orchestrating Life's Perfect Rhythm
⚡ Electrical Command Center: The Spark That Drives the Squeeze
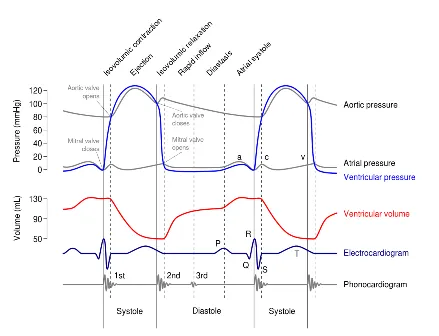
Excitation-contraction coupling transforms electrical depolarization into mechanical force through calcium-mediated cross-bridge formation. The sinoatrial node initiates depolarization at 60-100 bpm, spreading through atrial myocardium in 100 milliseconds, pausing at the AV node for 120 milliseconds to allow complete ventricular filling, then rapidly depolarizing ventricles via the His-Purkinje system in 80 milliseconds.
📌 Remember: DEPOLARIZE-CONTRACT - Depolarization triggers, Excitation spreads, Plateau phase sustains, Opening calcium channels, Liberating intracellular Ca2+, Activating troponin, Releasing tropomyosin, Initiating cross-bridges, Zone contraction, Ejecting blood, Closing valves, Opening for filling, New cycle, Timing reset, Repolarization, ATP restoration, Calcium reuptake, Troponin inhibition
The action potential plateau phase lasting 200-300 milliseconds prevents tetanic contraction by maintaining calcium channel opening and ensuring adequate ejection time. L-type calcium channels open during depolarization, triggering calcium-induced calcium release from the sarcoplasmic reticulum, increasing intracellular calcium concentration 10-fold from 0.1 μM to 1-2 μM.
- Electrical Timing Sequence:
- P wave: Atrial depolarization (80-100 ms)
- PR interval: AV conduction delay (120-200 ms)
- QRS complex: Ventricular depolarization (80-120 ms)
- Phase 0: Rapid sodium influx (+30 mV)
- Phase 1: Early repolarization (0 mV)
- Phase 2: Calcium plateau (200-300 ms)
- Phase 3: Potassium efflux repolarization (-90 mV)
⭐ Clinical Pearl: The absolute refractory period lasting 250 milliseconds prevents re-excitation during contraction, explaining why cardiac muscle cannot tetanize and maintains its pumping function.
💡 Master This: Calcium channel blockers reduce contractility by decreasing calcium influx during the plateau phase, while beta-blockers reduce heart rate by slowing SA node depolarization and prolonging AV node conduction time.
The electrical-mechanical coupling efficiency determines stroke volume, with optimal calcium handling producing ejection fractions of 55-70% in healthy hearts, while impaired calcium cycling in heart failure reduces ejection fraction below 40%.
⚡ Electrical Command Center: The Spark That Drives the Squeeze
🔄 Pressure Dynamics: The Hydraulic Powerhouse
📌 Remember: PRESSURE-VOLUME-WORK - Preload determines filling, Resistance affects ejection, Elastance defines stiffness, Stroke volume equals difference, Systolic pressure peaks, Unloading during ejection, Relaxation enables filling, End-diastolic pressure, Ventricular compliance, Output depends on preload, Loop area equals work, Unloading afterload, Myocardial oxygen consumption, Ejection fraction calculation, Wall stress relationships, Optimal performance, Reserve mechanisms, Kinetic energy transfer
The Frank-Starling mechanism optimizes stroke volume through length-tension relationships, where increased preload (end-diastolic volume) enhances contractility up to optimal sarcomere length of 2.2 μm. Beyond this point, further stretching decreases contractility, explaining why excessive preload in heart failure reduces cardiac output despite increased filling pressures.
| Parameter | Normal Range | Heart Failure | Hypertrophy | Clinical Significance |
|---|---|---|---|---|
| End-Diastolic Pressure | 8-12 mmHg | 15-25 mmHg | 12-18 mmHg | Filling pressure |
| End-Diastolic Volume | 120-140 mL | 180-250 mL | 100-120 mL | Preload assessment |
| Ejection Fraction | 55-70% | <40% | 60-80% | Systolic function |
| Stroke Volume | 70-80 mL | 40-60 mL | 60-70 mL | Pump effectiveness |
| Stroke Work | 1.0-1.2 J | 0.6-0.8 J | 1.2-1.5 J | Energy efficiency |
- Pressure-Volume Determinants:
- Preload: End-diastolic volume (120-140 mL)
- Venous return determines filling
- Frank-Starling mechanism optimizes output
- Excessive preload reduces efficiency
- Afterload: Aortic pressure (80-120 mmHg)
- Systemic vascular resistance
- Arterial compliance effects
- Wall stress calculations (LaPlace law)
- Contractility: Intrinsic myocardial performance
- Calcium handling efficiency
- Cross-bridge cycling rate
- Sympathetic stimulation effects
- Preload: End-diastolic volume (120-140 mL)
💡 Master This: Afterload reduction with ACE inhibitors shifts the PV loop leftward, increasing stroke volume and reducing myocardial oxygen consumption, while positive inotropes shift the loop upward, increasing contractility but also energy demands.
Understanding PV relationships enables optimization of cardiac performance through targeted interventions: preload reduction for volume overload, afterload reduction for pressure overload, and inotropic support for contractile dysfunction.
🔄 Pressure Dynamics: The Hydraulic Powerhouse
🎵 Acoustic Signatures: Decoding the Heart's Symphony
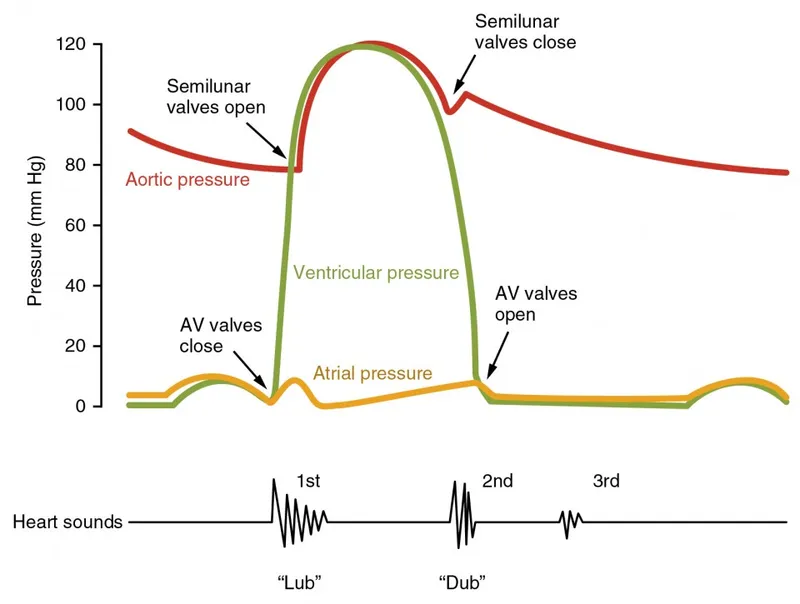
Heart sounds result from valve closure vibrations and turbulent blood flow, providing acoustic windows into cardiac mechanical function. S1 ("lub") occurs with mitral and tricuspid valve closure at the onset of systole, while S2 ("dub") results from aortic and pulmonary valve closure at end-systole. The 30-50 millisecond delay between aortic and pulmonary closure during inspiration creates physiological S2 splitting.
📌 Remember: SOUND-VALVE-TIMING - S1 systole starts, Onset of contraction, Unified AV closure, Normal lub sound, Diastole follows S2, Valves slam shut, Aortic closes first, Lung valve follows, Vibrations create sound, Echo through chest, Timing reveals function, Inspiration splits S2, Mitral closes S1, Interval between sounds, Normal cycle, Gallops when abnormal
The intensity and timing of heart sounds correlate with hemodynamic parameters and valve function. S1 intensity increases with enhanced contractility and short PR intervals (<160 ms), while S2 intensity reflects aortic and pulmonary pressures. Abnormal sounds including S3 gallop (ventricular dysfunction) and S4 gallop (reduced compliance) indicate pathological states requiring intervention.
- Heart Sound Characteristics:
- S1 Components:
- Mitral closure: M1 component (10-15 ms before T1)
- Tricuspid closure: T1 component
- Frequency: 25-45 Hz (low-pitched)
- Duration: 100-150 ms
- S2 Components:
- Aortic closure: A2 component (higher pressure)
- Pulmonary closure: P2 component (30-50 ms after A2)
- Frequency: 50-70 Hz (higher-pitched)
- Inspiratory splitting: physiological
- S1 Components:
| Sound | Timing | Mechanism | Normal Intensity | Pathological Changes |
|---|---|---|---|---|
| S1 | Systole onset | AV valve closure | Apex loudest | Varies with PR interval |
| S2 | Systole end | SL valve closure | Base loudest | Splits with inspiration |
| S3 | Early diastole | Ventricular gallop | Absent in normal | CHF, volume overload |
| S4 | Late diastole | Atrial gallop | Absent in normal | Hypertrophy, ischemia |
💡 Master This: S3 gallop indicates elevated filling pressures (>18 mmHg) and reduced ventricular compliance, while S4 gallop suggests impaired ventricular relaxation with preserved systolic function.
Auscultation timing with palpated carotid pulse distinguishes systolic from diastolic events, with S1 preceding the carotid upstroke and S2 following peak pulse pressure by 80-100 milliseconds.
🎵 Acoustic Signatures: Decoding the Heart's Symphony
⚖️ Therapeutic Optimization: Mastering Cardiac Performance
Therapeutic manipulation of cardiac cycle parameters requires understanding the underlying pathophysiology and hemodynamic goals. Heart failure with reduced ejection fraction (HFrEF <40%) benefits from preload reduction (diuretics), afterload reduction (ACE inhibitors), and beta-blockade to improve diastolic filling time. Conversely, heart failure with preserved ejection fraction (HFpEF >50%) requires rate control and volume management without excessive preload reduction.
📌 Remember: OPTIMIZE-HEMODYNAMICS - Optimal preload balance, Pressure afterload reduction, Timing rate control, Inotropic support selective, Monitoring parameters, Intervention evidence-based, Zero tolerance complications, Efficiency maximization, Heart rate optimization, Ejection fraction improvement, Myocardial protection, Output augmentation, Diastolic enhancement, Yield stroke volume, Neurohormonal blockade, Arrhythmia prevention, Mortality reduction, Improved quality, Cardiac remodeling, Symptomatic relief
Evidence-based cardiac cycle optimization follows specific hemodynamic targets and monitoring parameters. ACE inhibitors reduce afterload by 15-20%, improving stroke volume and reducing myocardial oxygen consumption. Beta-blockers reduce heart rate by 20-25%, prolonging diastolic filling time and improving coronary perfusion, while reducing mortality by 35% in HFrEF patients.
- Therapeutic Targets by Condition:
- Heart Failure (HFrEF):
- Target EF improvement: >40%
- Optimal heart rate: 60-70 bpm
- PCWP goal: <18 mmHg
- Cardiac index: >2.2 L/min/m²
- Hypertensive Heart Disease:
- Blood pressure: <130/80 mmHg
- LV mass regression: >10%
- Diastolic function preservation
- Prevent adverse remodeling
- Ischemic Cardiomyopathy:
- Heart rate: <70 bpm
- Optimal medical therapy
- Revascularization consideration
- Device therapy evaluation
- Heart Failure (HFrEF):
| Intervention | Mechanism | Hemodynamic Effect | Mortality Benefit | Monitoring Parameter |
|---|---|---|---|---|
| ACE Inhibitors | Afterload reduction | ↑SV, ↓PCWP | 20-25% reduction | Creatinine, K+ |
| Beta Blockers | Rate control, ↓contractility | ↑filling time | 35% reduction | Heart rate, BP |
| Diuretics | Preload reduction | ↓PCWP, ↓edema | Symptomatic only | Volume status, electrolytes |
| Aldosterone Antagonists | Neurohormonal blockade | ↓remodeling | 30% reduction | K+, creatinine |
💡 Master This: Cardiac resynchronization therapy (CRT) improves cardiac cycle efficiency by 15-20% in patients with EF <35%, QRS >150 ms, and optimal medical therapy, reducing hospitalizations by 40%.
Hemodynamic monitoring guides therapeutic optimization, with pulmonary capillary wedge pressure <18 mmHg, cardiac index >2.2 L/min/m², and mixed venous oxygen saturation >65% indicating adequate cardiac cycle performance.
⚖️ Therapeutic Optimization: Mastering Cardiac Performance
🔗 Integrative Mastery: The Cardiac Cycle Ecosystem
📌 Remember: INTEGRATE-SYSTEMS - Inotropic state varies, Neurohormonal control, Timing coordination, Endocrine influences, Genetic factors, Renin-angiotensin system, Autonomic modulation, Tissue perfusion, Energy metabolism, Systemic circulation, Yield optimization, Stress adaptation, Thermoregulation, Exercise response, Myocardial efficiency, Synchronization electrical
Advanced cardiac cycle integration involves understanding ventricular-arterial coupling, where optimal efficiency occurs when arterial elastance equals ventricular elastance (Ea/Ees = 1.0). This relationship determines stroke work efficiency and myocardial oxygen consumption, with uncoupling in disease states reducing cardiac performance despite preserved individual component function.
- Multi-System Integration Factors:
- Vascular System Coupling:
- Arterial compliance: 1.5-2.0 mL/mmHg
- Venous compliance: 30-50 mL/mmHg
- Systemic vascular resistance: 800-1200 dynes·s/cm⁵
- Pulmonary vascular resistance: 150-250 dynes·s/cm⁵
- Neurohormonal Regulation:
- Sympathetic stimulation: ↑HR, ↑contractility
- Parasympathetic tone: ↓HR, ↓AV conduction
- Renin-angiotensin activation: ↑afterload, ↑preload
- Natriuretic peptides: ↓preload, ↓afterload
- Metabolic Integration:
- Oxygen consumption: 8-12 mL O₂/100g/min
- Coronary flow reserve: 3-5x baseline
- Substrate utilization: 60% fatty acids, 40% glucose
- ATP production efficiency: 38 molecules/glucose
- Vascular System Coupling:
| Integration Level | Normal Function | Pathological State | Compensation Mechanism | Clinical Marker |
|---|---|---|---|---|
| Ventricular-Arterial | Ea/Ees = 1.0 | Ea/Ees >1.5 | ↑contractility | Stroke work efficiency |
| Neurohormonal | Balanced autonomic | SNS activation | RAAS suppression | BNP, norepinephrine |
| Metabolic | Efficient ATP use | ↑O₂ consumption | Substrate switching | Lactate, VO₂ |
| Cellular | Ca²⁺ homeostasis | Ca²⁺ overload | SERCA upregulation | Troponin, CK-MB |
💡 Master This: Cardiac cycle optimization requires simultaneous attention to mechanical efficiency (PV loop optimization), electrical stability (rhythm management), vascular coupling (impedance matching), and metabolic efficiency (substrate utilization), achieving integrated cardiovascular performance.
The future of cardiac cycle management involves precision medicine approaches using genetic markers, advanced imaging, and artificial intelligence to optimize individual patient hemodynamics based on personalized physiological signatures rather than population-based guidelines.
🔗 Integrative Mastery: The Cardiac Cycle Ecosystem
🎯 Clinical Command Center: Rapid Cardiac Cycle Mastery
Rapid cardiac cycle mastery requires systematic assessment frameworks combining electrical, mechanical, and hemodynamic parameters. The CYCLE-CHECK approach provides comprehensive evaluation: Cardiac rhythm analysis, Yielding stroke volume assessment, Contractility evaluation, Loading conditions (preload/afterload), Ejection fraction measurement, Compliance assessment, Heart sound analysis, Electrical-mechanical timing, Coronary perfusion adequacy, Key hemodynamic parameters.
📌 Remember: MASTER-CARDIAC-CYCLE - Mechanical efficiency, Acoustic signatures, Systolic function, Timing relationships, Electrical coupling, Rhythm stability, Compliance assessment, Afterload evaluation, Rate optimization, Diastolic function, Inotropic state, Acoustic analysis, Coronary perfusion, Cycle efficiency, Yield maximization, Clinical correlation, Loading optimization, Ejection assessment
Essential cardiac cycle parameters for rapid clinical assessment include heart rate (60-100 bpm), blood pressure (120/80 mmHg), ejection fraction (55-70%), cardiac output (4-6 L/min), and filling pressures (PCWP <12 mmHg). Abnormal values trigger specific diagnostic and therapeutic pathways based on underlying pathophysiology and hemodynamic goals.
- Rapid Assessment Arsenal:
- Electrical Parameters:
- Heart rate: 60-100 bpm
- PR interval: 120-200 ms
- QRS duration: <120 ms
- QT interval: <440 ms
- Mechanical Parameters:
- Ejection fraction: 55-70%
- Stroke volume: 70-80 mL
- Cardiac output: 4-6 L/min
- Cardiac index: 2.5-3.5 L/min/m²
- Hemodynamic Thresholds:
- CVP: 2-8 mmHg
- PCWP: 6-12 mmHg
- SVR: 800-1200 dynes·s/cm⁵
- PVR: 150-250 dynes·s/cm⁵
- Electrical Parameters:
| Clinical Scenario | Key Assessment | Critical Values | Immediate Action | Monitoring Parameter |
|---|---|---|---|---|
| Acute MI | EF, wall motion | EF <40%, RWMA | Reperfusion, ACE-I | Troponin, echo |
| Heart Failure | BNP, EF, PCWP | BNP >400, EF <40% | Diuresis, vasodilators | Daily weights, BUN |
| Shock | CO, SVR, PCWP | CI <2.2, SVR varies | Volume vs pressors | Mixed venous O₂ |
| Arrhythmia | Rate, rhythm | HR >150 or <50 | Rate/rhythm control | Continuous monitoring |
💡 Master This: The cardiac cycle efficiency equation integrates all parameters: Efficiency = (Stroke Work / Myocardial O₂ Consumption) × 100, with normal values >15% and heart failure reducing efficiency to <10%.
Mastering cardiac cycle assessment enables rapid diagnosis, appropriate intervention, and optimal patient outcomes through systematic evaluation of the heart's mechanical and electrical performance in health and disease.
🎯 Clinical Command Center: Rapid Cardiac Cycle Mastery
Practice Questions: Cardiac cycle
Test your understanding with these related questions
A 75-year-old man presents to the emergency department after an episode of syncope while walking outside with his wife. His wife states that he suddenly appeared pale and collapsed to the ground. She says he remained unconscious for 1 minute. He says he noticed a fluttering in his chest and excessive sweating before the episode. He has type 2 diabetes mellitus, essential hypertension, and chronic stable angina. He has not started any new medications in the past few months. Vital signs reveal: temperature 37.0°C (98.6°F), blood pressure 135/72 mm Hg, and pulse 72/min. Physical examination is unremarkable. ECG shows an old bifascicular block. Echocardiogram and 24-hour Holter monitoring are normal. Which of the following is the best next step in the evaluation of this patient's condition?













