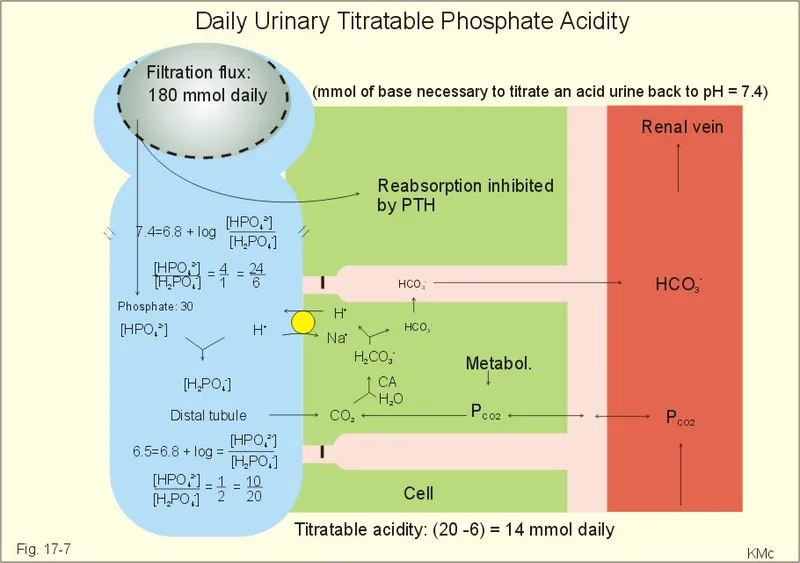
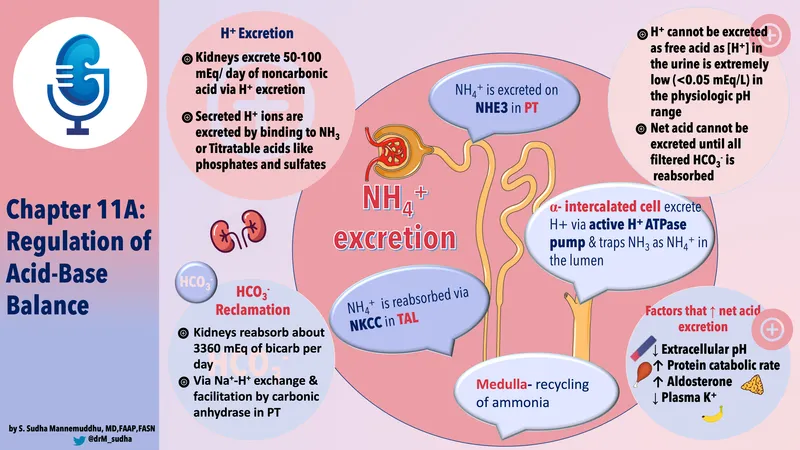
Titratable acid excretion US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Titratable acid excretion. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Titratable acid excretion US Medical PG Question 1: On cardiology service rounds, your team sees a patient admitted with an acute congestive heart failure exacerbation. In congestive heart failure, decreased cardiac function leads to decreased renal perfusion, which eventually leads to excess volume retention. To test your knowledge of physiology, your attending asks you which segment of the nephron is responsible for the majority of water absorption. Which of the following is a correct pairing of the segment of the nephron that reabsorbs the majority of all filtered water with the means by which that segment absorbs water?
- A. Distal convoluted tubule via passive diffusion following ion reabsorption
- B. Distal convoluted tubule via aquaporin channels
- C. Thick ascending loop of Henle via passive diffusion following ion reabsorption
- D. Proximal convoluted tubule via passive diffusion following ion reabsorption (Correct Answer)
- E. Collecting duct via aquaporin channels
Titratable acid excretion Explanation: ***Proximal convoluted tubule via passive diffusion following ion reabsorption***
- The **proximal convoluted tubule (PCT)** is responsible for reabsorbing approximately **65-70% of filtered water**, making it the primary site of water reabsorption in the nephron.
- This water reabsorption primarily occurs **passively**, following the active reabsorption of solutes (especially **sodium ions**), which creates an osmotic gradient.
*Distal convoluted tubule via passive diffusion following ion reabsorption*
- The **distal convoluted tubule (DCT)** reabsorbs a much smaller percentage of filtered water (around 5-10%) and its water reabsorption is largely **regulated by ADH**, not primarily simple passive diffusion following bulk ion reabsorption.
- While some passive water movement occurs, it is not the main mechanism or location for the majority of water reabsorption.
*Distal convoluted tubule via aquaporin channels*
- While aquaporin channels do play a role in water reabsorption in the DCT, particularly under the influence of **ADH**, the DCT is not the segment responsible for the **majority of all filtered water absorption**.
- The bulk of water reabsorption occurs earlier in the nephron, independently of ADH for the most part.
*Thick ascending loop of Henle via passive diffusion following ion reabsorption*
- The **thick ascending loop of Henle** is primarily involved in reabsorbing ions like Na+, K+, and Cl- but is largely **impermeable to water**.
- Its impermeability to water is crucial for creating the **osmotic gradient** in the renal medulla, which is necessary for later water reabsorption.
*Collecting duct via aquaporin channels*
- The **collecting duct** is critically important for **regulated water reabsorption** via **aquaporin-2 channels** under the influence of **ADH**, allowing for fine-tuning of urine concentration.
- However, it reabsorbs only a variable portion (typically 5-19%) of the remaining filtered water, not the **majority of all filtered water**.
Titratable acid excretion US Medical PG Question 2: In a healthy patient with no renal abnormalities, several mechanisms are responsible for moving various filtered substances into and out of the tubules. Para-aminohippurate (PAH) is frequently used to estimate renal blood flow when maintained at low plasma concentrations. The following table illustrates the effect of changing plasma PAH concentrations on PAH excretion:
Plasma PAH concentration (mg/dL) | Urinary PAH concentration (mg/dL)
0 | 0
10 | 60
20 | 120
30 | 150
40 | 180
Which of the following mechanisms best explains the decreased rate of increase in PAH excretion observed when plasma PAH concentration exceeds 20 mg/dL?
- A. Decreased glomerular filtration of PAH
- B. Increased rate of PAH reabsorption
- C. Increased flow rate of tubular contents
- D. Saturation of PAH transport carriers (Correct Answer)
- E. Increased diffusion rate of PAH
Titratable acid excretion Explanation: ***Saturation of PAH transport carriers***
- PAH is primarily cleared by **tubular secretion** via organic anion transporters (OATs) in the proximal tubule, which have a **finite transport maximum (Tm)**.
- When plasma PAH concentration exceeds the capacity of these carriers (as seen above 20 mg/dL), the transporters become saturated, leading to a **decreased incremental excretion** despite rising plasma levels.
*Decreased glomerular filtration of PAH*
- **Glomerular filtration rate (GFR)** for PAH is proportional to its plasma concentration and is typically constant in a healthy kidney, so it would not decrease with increasing plasma PAH.
- A decrease in GFR would lead to a *reduced* overall excretion, but not specifically explain the *decreased rate of increase* at higher plasma concentrations.
*Increased rate of PAH reabsorption*
- PAH is **minimally reabsorbed** in the renal tubules; its primary mechanism of removal from the blood is active secretion.
- An increase in reabsorption would lead to *less* PAH in the urine, but there's no physiological basis for increased reabsorption as plasma concentration rises.
*Increased flow rate of tubular contents*
- While an increased flow rate can sometimes affect solute reabsorption or secretion, it would generally lead to a more, not less, efficient clearance of secreted substances.
- This mechanism does not explain the **saturation kinetics** observed with PAH at higher plasma concentrations.
*Increased diffusion rate of PAH*
- PAH is a charged organic anion, and its movement across tubular membranes is primarily mediated by **active transport** rather than simple diffusion.
- Even if diffusion played a minor role, an increased diffusion rate would generally lead to *more* excretion, not the observed plateau in the rate of increase.
Titratable acid excretion US Medical PG Question 3: A 72-year-old man being treated for benign prostatic hyperplasia (BPH) is admitted to the emergency department for 1 week of dysuria, nocturia, urge incontinence, and difficulty initiating micturition. His medical history is relevant for hypertension, active tobacco use, chronic obstructive pulmonary disease, and BPH with multiple urinary tract infections. Upon admission, he is found with a heart rate of 130/min, respiratory rate of 19/min, body temperature of 39.0°C (102.2°F), and blood pressure of 80/50 mm Hg. Additional findings during the physical examination include decreased breath sounds, wheezes, crackles at the lung bases, and intense right flank pain. A complete blood count shows leukocytosis and neutrophilia with a left shift. A sample for arterial blood gas analysis (ABG) was taken, which is shown below.
Laboratory test
Serum Na+ 140 mEq/L
Serum Cl- 102 mEq/L
Serum K+ 4.8 mEq/L
Serum creatinine (SCr) 2.3 mg/dL
Arterial blood gas
pH 7.12
Po2 82 mm Hg
Pco2 60 mm Hg
SO2% 92%
HCO3- 12.0 mEq/L
Which of the following best explains the patient’s condition?
- A. Metabolic acidosis complicated by respiratory alkalosis
- B. Non-anion gap metabolic acidosis
- C. Respiratory alkalosis complicated by metabolic acidosis
- D. Respiratory acidosis complicated by metabolic alkalosis
- E. Metabolic acidosis complicated by respiratory acidosis (Correct Answer)
Titratable acid excretion Explanation: ***Metabolic acidosis complicated by respiratory acidosis***
- The patient's pH is significantly low (7.12), indicating **acidemia**. The **HCO3- is markedly low (12 mEq/L)**, and PCO2 is elevated (60 mm Hg), suggesting both a metabolic and a respiratory component to the acidosis.
- The severe infection (fever, elevated heart rate, hypotension, flank pain, leukocytosis, elevated creatinine) and the signs of hypoperfusion contribute to **lactic acidosis (metabolic acidosis)**, while his history of COPD and lung findings (decreased breath sounds, wheezes, crackles) explain the impaired ventilation leading to **respiratory acidosis**.
*Metabolic acidosis complicated by respiratory alkalosis*
- While a **metabolic acidosis** is clearly present due to the low pH and HCO3-, the PCO2 is elevated, indicating **respiratory acidosis**, not alkalosis.
- Respiratory alkalosis would be characterized by a **low PCO2** due to hyperventilation.
*Non-anion gap metabolic acidosis*
- To determine the anion gap, we use the formula: **Na+ - (Cl- + HCO3-)**. In this case, 140 - (102 + 12) = 140 - 114 = **26 mEq/L**.
- An anion gap of 26 mEq/L, which is significantly elevated (normal range is typically 8-12 mEq/L), indicates an **anion gap metabolic acidosis**, not a non-anion gap one.
*Respiratory alkalosis complicated by metabolic acidosis*
- The low pH and HCO3- confirm **metabolic acidosis**, but the elevated PCO2 (60 mm Hg) indicates **respiratory acidosis**, not alkalosis, as the respiratory component is also acidotic.
- Respiratory alkalosis would result from **hyperventilation and a low PCO2**.
*Respiratory acidosis complicated by metabolic alkalosis*
- While the elevated PCO2 indicates **respiratory acidosis**, the HCO3- is significantly low (12 mEq/L), which points to a **metabolic acidosis**, not metabolic alkalosis.
- **Metabolic alkalosis** would be characterized by an **elevated HCO3-**.
Titratable acid excretion US Medical PG Question 4: A 25-year-old woman with an extensive psychiatric history is suspected of having metabolic acidosis after ingesting a large amount of aspirin in a suicide attempt. Labs are drawn and the values from the ABG are found to be: PCO2: 25, and HCO3: 15, but the pH value is smeared on the print-out and illegible. The medical student is given the task of calculating the pH using the pCO2 and HCO3 concentrations. He recalls from his first-year physiology course that the pKa of relevance for the bicarbonate buffering system is approximately 6.1. Which of the following is the correct formula the student should use, using the given values from the incomplete ABG?
- A. 15/6.1 + log[10/(0.03*25)]
- B. 6.1 + log[15/(0.03*25)] (Correct Answer)
- C. 10^6.1 + 15/0.03*25
- D. 6.1 + log[0.03/15*25]
- E. 6.1 + log[25/(15*0.03)]
Titratable acid excretion Explanation: ***6.1 + log[15/(0.03*25)]***
- This formula correctly represents the Henderson-Hasselbalch equation for the bicarbonate buffer system: **pH = pKa + log([HCO3-]/[0.03 * PCO2])**.
- Here, **pKa is 6.1**, **[HCO3-] is 15**, and **[0.03 * PCO2] is 0.03 * 25**, making this the appropriate calculation for pH.
*15/6.1 + log[10/(0.03*25)]*
- This formula incorrectly places the pKa in the denominator of the first term and introduces an arbitrary '10' in the numerator of the logarithmic term.
- The **Henderson-Hasselbalch equation** dictates that pKa is added, not divided into, another component, and the logarithmic term should reflect the ratio of bicarbonate to carbonic acid.
*10^6.1 + 15/0.03*25*
- This option incorrectly uses an exponentiation of pKa and adds it to an unrelated fractional term, which does not correspond to the Henderson-Hasselbalch equation structure.
- The formula for pH calculation is a sum of pKa and a logarithmic term, not an exponentiation and a simple fraction.
*6.1 + log[0.03/15*25]*
- This option incorrectly inverts the ratio within the logarithm, placing the carbonic acid component (0.03 * PCO2) in the numerator and bicarbonate in the denominator.
- The correct Henderson-Hasselbalch equation requires the **bicarbonate concentration in the numerator** and the carbonic acid concentration in the denominator.
*6.1 + log [25/(15*0.03)]*
- This option incorrectly places the PCO2 (25) in the numerator of the logarithmic term and the product of HCO3- and 0.03 in the denominator.
- The correct ratio for the Henderson-Hasselbalch equation is **[HCO3-] / [0.03 * PCO2]**.
Titratable acid excretion US Medical PG Question 5: A 70-year-old woman is brought to the emergency department due to worsening lethargy. She lives with her husband who says she has had severe diarrhea for the past few days. Examination shows a blood pressure of 85/60 mm Hg, pulse of 100/min, and temperature of 37.8°C (100.0°F). The patient is stuporous, while her skin appears dry and lacks turgor. Laboratory tests reveal:
Serum electrolytes
Sodium 144 mEq/L
Potassium 3.5 mEq/L
Chloride 115 mEq/L
Bicarbonate 19 mEq/L
Serum pH 7.3
PaO2 80 mm Hg
Pco2 38 mm Hg
This patient has which of the following acid-base disturbances?
- A. Chronic respiratory acidosis
- B. Anion gap metabolic acidosis with respiratory compensation
- C. Anion gap metabolic acidosis
- D. Non-anion gap metabolic acidosis with respiratory compensation (Correct Answer)
- E. Non-anion gap metabolic acidosis
Titratable acid excretion Explanation: ***Non-anion gap metabolic acidosis with respiratory compensation***
- This patient has significant **diarrhea**, which causes a loss of **bicarbonate** from the gastrointestinal tract, leading to a **non-anion gap metabolic acidosis**.
- The **serum pH of 7.3** confirms acidosis, and the **Pco2 of 38 mm Hg** (which is slightly below the normal range, considering the acidosis) indicates effective **respiratory compensation** for the metabolic disturbance. Calculating the **anion gap** = Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L** (normal range 8-12 mEq/L), which is within normal limits.
*Chronic respiratory acidosis*
- This would involve an elevated **Pco2** and a compensatory increase in **bicarbonate**, neither of which are observed in this patient.
- The patient's primary problem is loss of bicarbonate due to diarrhea, not impaired CO2 excretion.
*Anion gap metabolic acidosis with respiratory compensation*
- An **anion gap metabolic acidosis** would show an elevated anion gap (>12 mEq/L), which is not present here (anion gap is 10 mEq/L).
- While respiratory compensation is occurring, the underlying acidosis is **non-anion gap**.
*Anion gap metabolic acidosis*
- This diagnosis requires an **elevated anion gap**, which is calculated as Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L**.
- Since the anion gap is within the normal range, an anion gap metabolic acidosis is excluded.
*Non-anion gap metabolic acidosis*
- While the patient does have a **non-anion gap metabolic acidosis** due to bicarbonate loss from diarrhea, this option doesn't account for the **respiratory compensation** indicated by the Pco2.
- The slightly reduced Pco2 demonstrates the body's attempt to normalize pH, making "with respiratory compensation" a more complete description.
Titratable acid excretion US Medical PG Question 6: A group of researchers wish to develop a clinical trial assessing the efficacy of a specific medication on the urinary excretion of amphetamines in intoxicated patients. They recruit 50 patients for the treatment arm and 50 patients for the control arm of the study. Demographics are fairly balanced between the two groups. The primary end points include (1) time to recovery of mental status, (2) baseline heart rate, (3) urinary pH, and (4) specific gravity. Which medication should they use in order to achieve a statistically significant result positively favoring the intervention?
- A. Potassium citrate
- B. Ascorbic acid (Correct Answer)
- C. Tap water
- D. Sodium bicarbonate
- E. Aluminum hydroxide
Titratable acid excretion Explanation: ***Ascorbic acid***
- Urinary excretion of **weak bases** like amphetamines is enhanced in an **acidic urine environment**. Ascorbic acid, or vitamin C, is an acidic substance that, when administered, can significantly **lower urinary pH**.
- By acidifying the urine, ascorbic acid promotes the **ionization of amphetamines** in the renal tubules, making them less lipid-soluble and decreasing their reabsorption, thereby **increasing their urinary excretion**.
*Potassium citrate*
- Potassium citrate is a **urinary alkalinizer**, meaning it would increase the pH of the urine.
- Increasing urinary pH would **decrease the excretion of acidic drugs** and **increase the reabsorption of basic drugs** like amphetamines, which is the opposite of the desired effect.
*Tap water*
- Administering tap water would primarily lead to **diuresis** (increased urine production) but would have a **negligible effect on urinary pH**.
- While increased urine volume can dilute the concentration of amphetamines, it does not significantly alter the **renal clearance rate based on pH**, which is crucial for weak bases.
*Sodium bicarbonate*
- Sodium bicarbonate is a potent **urinary alkalinizer**, used to increase the pH of the urine.
- Just like potassium citrate, a higher urinary pH would **inhibit the excretion of amphetamines** by promoting their non-ionized, lipid-soluble form and increasing their reabsorption.
*Aluminum hydroxide*
- Aluminum hydroxide is primarily an **antacid** and phosphate binder, used for conditions like GERD or hyperphosphatemia; it has **no significant direct effect on urinary pH or amphetamine excretion**.
- Its action is largely confined to the gastrointestinal tract, and it does not get absorbed in a way that would acidify the urine.
Titratable acid excretion US Medical PG Question 7: A group of investigators is studying a drug to treat refractory angina pectoris. This drug works by selectively inhibiting the late influx of sodium ions into cardiac myocytes. At high doses, the drug also partially inhibits the degradation of fatty acids. Which of the following is the most likely effect of this drug?
- A. Increased prolactin release
- B. Decreased uric acid excretion
- C. Decreased serum pH
- D. Increased oxygen efficiency (Correct Answer)
- E. Decreased insulin release
Titratable acid excretion Explanation: ***Increased oxygen efficiency***
- Inhibiting the **late sodium current** reduces intracellular calcium overload, preventing diastolic dysfunction and improving myocardial relaxation.
- Partial inhibition of **fatty acid degradation** shifts myocardial metabolism towards glucose utilization, which is more oxygen-efficient.
*Increased prolactin release*
- This drug does not act on **dopamine receptors**, which are typically involved in regulating prolactin release.
- **Ranolazine**, the drug described, has no known effect on the endocrine system, specifically prolactin.
*Decreased uric acid excretion*
- **Uric acid excretion** is primarily affected by renal handling, often influenced by diuretics or drugs that compete for renal transporters, which is not a mechanism of this drug.
- This drug does not interfere with the **organic anion transporters (OATs)** responsible for uric acid secretion.
*Decreased serum pH*
- Changes in **serum pH** are usually associated with severe metabolic or respiratory disturbances, which are not direct effects of a drug targeting cardiac ion channels and metabolism.
- The drug's mechanism of action does not directly produce **acidic byproducts** or inhibit acid-base regulatory systems.
*Decreased insulin release*
- Insulin release is primarily stimulated by **glucose** and modulated by various endocrine pathways, none of which are directly targeted by a drug that inhibits cardiac sodium channels and fatty acid oxidation.
- There is no evidence that this class of drugs affects **pancreatic beta-cell function**.
Titratable acid excretion US Medical PG Question 8: A 21-year-old man presents to the emergency department with acute back pain. The pain began a few hours prior to presentation and is located on the left lower back. The pain is described to be “shock-like,” 9/10 in pain severity, and radiates to the left groin. His temperature is 98.6°F (37°C), blood pressure is 120/75 mmHg, pulse is 101/min, and respirations are 18/min. The patient appears uncomfortable and is mildly diaphoretic. There is costovertebral angle tenderness and genitourinary exam is unremarkable. A non-contrast computerized tomography (CT) scan of the abdomen and pelvis demonstrates an opaque lesion affecting the left ureter with mild hydronephrosis. Straining of the urine with urine crystal analysis is demonstrated. Which of the following amino acids is most likely poorly reabsorbed by this patient’s kidney?
- A. Isoleucine
- B. Aspartic acid
- C. Phenylalanine
- D. Lysine (Correct Answer)
- E. Histidine
Titratable acid excretion Explanation:
***Lysine***
- The patient's symptoms (acute, severe, radiating back pain, CVA tenderness, hydronephrosis, and opaque lesion on CT) are highly characteristic of a **kidney stone**.
- Given the patient's young age and the nature of the amino acid question, thinking of **cystinuria** is appropriate, where the basic amino acids **COLA** (cystine, ornithine, lysine, arginine) are poorly reabsorbed.
*Isoleucine*
- **Isoleucine** is a branched-chain amino acid, not one of the basic amino acids impacted by cystinuria.
- Its malabsorption is not associated with the formation of kidney stones.
*Aspartic acid*
- **Aspartic acid** is an acidic amino acid and is not involved in the transport defects seen in cystinuria.
- There is no direct link between aspartic acid malabsorption and kidney stone formation.
*Phenylalanine*
- **Phenylalanine** is an aromatic amino acid and its metabolism is associated with disorders like phenylketonuria, not kidney stones.
- It is not one of the amino acids whose renal reabsorption is impaired in cystinuria.
*Histidine*
- **Histidine** is an essential amino acid, but it is not one of the basic amino acids (COLA) whose transport is affected in cystinuria.
- Poor reabsorption of histidine is not typically associated with kidney stone formation.
Titratable acid excretion US Medical PG Question 9: Renal clearance of substance Y is experimentally studied. At a constant glomerular filtration rate, it is found that the amount of substance Y excreted is greater than the amount filtered. This holds true across all physiologic values on the titration curve. Substance Y is most similar to which of the following?
- A. Para-amino hippuric acid (Correct Answer)
- B. Albumin
- C. Bicarbonate
- D. Magnesium
- E. Glucose
Titratable acid excretion Explanation: ***Para-amino hippuric acid***
- If the amount of a substance excreted is **greater than the amount filtered**, it indicates that the substance undergoes both **glomerular filtration** and **tubular secretion**.
- **Para-amino hippuric acid (PAH)** is a classic example of a substance that is extensively filtered and actively secreted by the renal tubules, making its clearance rate very high and a good estimate of **renal plasma flow**.
*Albumin*
- **Albumin** is a large protein that is normally **not filtered** by the glomerulus due to its size and negative charge.
- Its presence in the urine, indicating a greater amount excreted than filtered (which is normally zero), would suggest **glomerular damage**, but it does not undergo active tubular secretion.
*Bicarbonate*
- **Bicarbonate** is freely filtered at the glomerulus and is primarily **reabsorbed** in the renal tubules, particularly in the proximal tubule.
- Therefore, the amount of bicarbonate excreted is typically **much less than** the amount filtered, not greater.
*Magnesium*
- **Magnesium** is filtered by the glomeruli and undergoes complex regulation involving both **reabsorption and secretion** in various parts of the renal tubule, though reabsorption predominates.
- While magnesium balance is maintained by the kidneys, its excretion does not typically exceed filtration to the extent described for substances primarily handled by secretion.
*Glucose*
- **Glucose** is freely filtered at the glomerulus and is almost **completely reabsorbed** in the proximal tubule under normal physiological conditions.
- The amount of glucose excreted is typically zero, and only exceeds filtration when the **tubular reabsorptive capacity is saturated**, as in uncontrolled diabetes, but it is reabsorbed, not secreted.
Titratable acid excretion US Medical PG Question 10: A person is exercising strenuously on a treadmill for 1 hour. An arterial blood gas measurement is then taken. Which of the following are the most likely values?
- A. pH 7.56, PaO2 100, PCO2 44, HCO3 38
- B. pH 7.32, PaO2 42, PCO2 50, HCO3 27
- C. pH 7.57 PaO2 100, PCO2 23, HCO3 21 (Correct Answer)
- D. pH 7.38, PaO2 100, PCO2 69 HCO3 42
- E. pH 7.36, PaO2 100, PCO2 40, HCO3 23
Titratable acid excretion Explanation: ***pH 7.57, PaO2 100, PCO2 23, HCO3 21***
- After 1 hour of strenuous exercise, this represents **respiratory alkalosis with mild metabolic compensation**, which is the expected finding in a healthy individual during sustained vigorous exercise.
- The **low PCO2 (23 mmHg)** reflects appropriate **hyperventilation** in response to increased metabolic demands and lactic acid production. During intense exercise, minute ventilation increases dramatically, often exceeding the rate of CO2 production.
- The **slightly elevated pH (7.57)** and **mildly decreased HCO3 (21 mEq/L)** indicate that respiratory compensation has slightly overshot, creating mild alkalosis, while the bicarbonate is consumed both in buffering lactate and through renal compensation.
- **Normal PaO2 (100 mmHg)** confirms adequate oxygenation maintained by increased ventilation.
*pH 7.36, PaO2 100, PCO2 40, HCO3 23*
- These are **completely normal arterial blood gas values** with no evidence of any physiological stress or compensation.
- After 1 hour of strenuous exercise, we would expect **hyperventilation with decreased PCO2**, not a normal PCO2 of 40 mmHg. This profile would be consistent with rest, not vigorous exercise.
- The absence of any respiratory or metabolic changes makes this inconsistent with the clinical scenario.
*pH 7.56, PaO2 100, PCO2 44, HCO3 38*
- This profile suggests **metabolic alkalosis** (high pH, high HCO3) with inadequate respiratory compensation (normal to slightly elevated PCO2).
- This is **not consistent with strenuous exercise**, which produces metabolic acid (lactate), not metabolic base. The elevated HCO3 suggests vomiting, diuretic use, or other causes of metabolic alkalosis.
*pH 7.32, PaO2 42, PCO2 50, HCO3 27*
- This indicates **respiratory acidosis** (low pH, high PCO2) with **severe hypoxemia** (PaO2 42 mmHg).
- During strenuous exercise, healthy individuals **increase ventilation** to enhance O2 delivery and remove CO2, so both hypoxemia and hypercapnia are unexpected and would suggest severe cardiopulmonary disease or hypoventilation.
*pH 7.38, PaO2 100, PCO2 69, HCO3 42*
- This demonstrates **compensated respiratory acidosis** (normal pH, markedly elevated PCO2 and HCO3).
- The **very high PCO2 (69 mmHg)** indicates severe **hypoventilation**, which is the opposite of what occurs during exercise. This profile suggests chronic respiratory failure with metabolic compensation, such as in severe COPD.
More Titratable acid excretion US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.