Respiratory acidosis mechanisms and compensation US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Respiratory acidosis mechanisms and compensation. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Respiratory acidosis mechanisms and compensation US Medical PG Question 1: A 60-year-old woman is brought to the emergency department by her husband because of worsening shortness of breath over the past 2 days. Last week, she had a sore throat and a low-grade fever. She has coughed up white sputum each morning for the past 2 years. She has hypertension and type 2 diabetes mellitus. She has smoked 2 packs of cigarettes daily for 35 years. Current medications include metformin and lisinopril. On examination, she occasionally has to catch her breath between sentences. Her temperature is 38.1°C (100.6°F), pulse is 85/min, respirations are 16/min, and blood pressure is 140/70 mm Hg. Expiratory wheezes with a prolonged expiratory phase are heard over both lung fields. Arterial blood gas analysis on room air shows:
pH 7.33
PCO2 53 mm Hg
PO2 68 mm Hg
An x-ray of the chest shows hyperinflation of bilateral lung fields and flattening of the diaphragm. Which of the following additional findings is most likely in this patient?
- A. Decreased urinary bicarbonate excretion (Correct Answer)
- B. Decreased urinary chloride concentration
- C. Increased serum anion gap
- D. Increased urine osmolar gap
- E. Increased urinary pH
Respiratory acidosis mechanisms and compensation Explanation: ***Decreased urinary bicarbonate excretion***
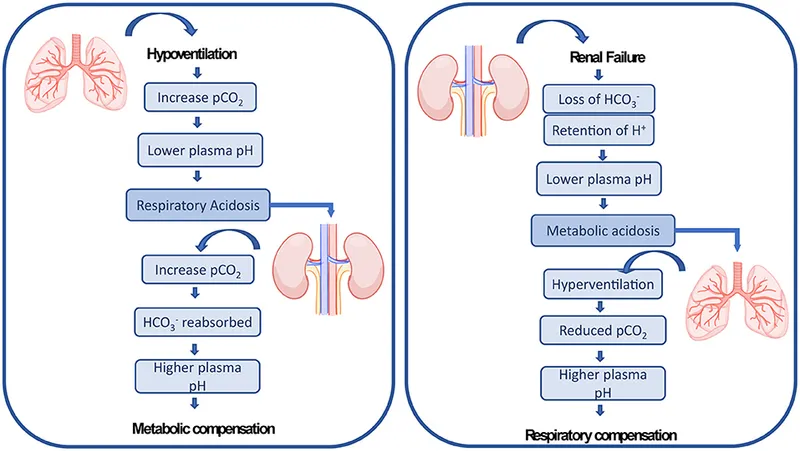
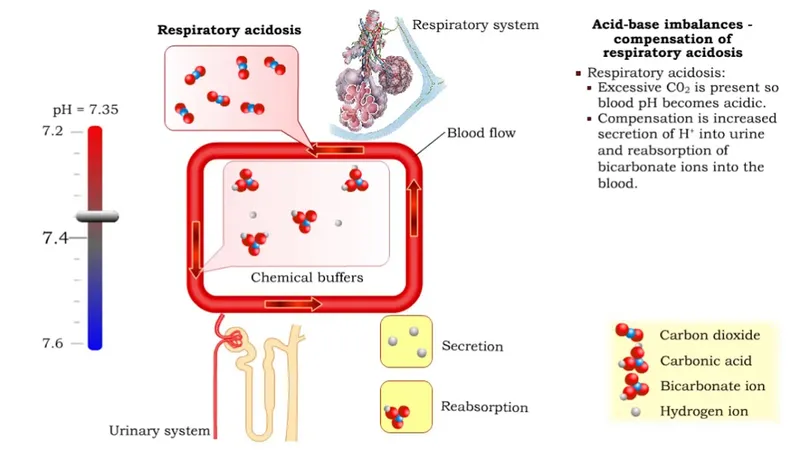
- The patient's ABG results (pH 7.33, PCO2 53 mmHg) indicate **chronic respiratory acidosis**, consistent with a **COPD exacerbation** on a background of chronic disease.
- In chronic respiratory acidosis, the kidneys compensate by **retaining bicarbonate** (increasing reabsorption) and **excreting hydrogen ions** to normalize pH.
- Therefore, urinary bicarbonate excretion is **decreased** as the kidneys conserve bicarbonate to buffer the chronic acidosis.
*Decreased urinary chloride concentration*
- This is typically seen in states of **metabolic alkalosis** (with volume contraction) or profound **volume depletion**, neither of which is the primary condition here.
- The patient has respiratory acidosis, not metabolic alkalosis.
*Increased serum anion gap*
- An increased anion gap indicates **metabolic acidosis** due to accumulation of unmeasured anions (e.g., lactate, ketones, toxins).
- The patient has **respiratory acidosis**, not metabolic acidosis with an anion gap.
- The anion gap is not directly affected by primary respiratory disorders.
*Increased urine osmolar gap*
- An increased urine osmolar gap suggests the presence of **unmeasured osmolytes** in the urine (e.g., from methanol or ethylene glycol ingestion).
- There is nothing in the patient's presentation to suggest toxic ingestion.
*Increased urinary pH*
- Increased urinary pH would occur if the kidneys were **excreting bicarbonate**, which happens in metabolic alkalosis or renal tubular acidosis.
- In chronic respiratory acidosis, the kidneys compensate by **excreting acid** (lowering urinary pH) and **retaining bicarbonate**.
- Therefore, urinary pH would be **decreased**, not increased.
Respiratory acidosis mechanisms and compensation US Medical PG Question 2: A 58-year-old man presents to the emergency department with a chief complaint of ringing in his ears that started several hours previously that has progressed to confusion. The patient denies any history of medical problems except for bilateral knee arthritis. He was recently seen by an orthopedic surgeon to evaluate his bilateral knee arthritis but has opted to not undergo knee replacement and prefers medical management. His wife noted that prior to them going on a hike today, he seemed confused and not himself. They decided to stay home, and roughly 14 hours later, he was no longer making any sense. Physical exam is notable for a confused man. The patient's vitals are being performed and his labs are being drawn. Which of the following is most likely to be seen on blood gas analysis?
- A. pH: 7.30, PaCO2: 15 mmHg, HCO3-: 16 mEq/L (Correct Answer)
- B. pH: 7.37, PaCO2: 41 mmHg, HCO3-: 12 mEq/L
- C. pH: 7.41, PaCO2: 65 mmHg, HCO3-: 34 mEq/L
- D. pH: 7.47, PaCO2: 11 mmHg, HCO3-: 24 mEq/L
- E. pH: 7.31, PaCO2: 31 mmHg, HCO3-: 15 mEq/L
Respiratory acidosis mechanisms and compensation Explanation: ***pH: 7.30, PaCO2: 15 mmHg, HCO3-: 16 mEq/L***
- This blood gas analysis shows a **low pH** (acidemia), **low PaCO2** (hypocapnia), and **low HCO3-** (bicarbonate). This pattern is consistent with a **primary metabolic acidosis** with a **compensatory respiratory alkalosis**.
- In this clinical scenario, the patient likely has **salicylate toxicity** (aspirin poisoning). Salicylate toxicity initially causes respiratory alkalosis due to direct stimulation of the respiratory center, followed by a high anion gap metabolic acidosis as salicylates interfere with cellular metabolism. This specific ABG reflects a mixed disorder where metabolic acidosis is predominant and respiratory compensation is attempting to raise the pH. The **tinnitus** and **confusion** are classic symptoms of salicylate toxicity.
*pH: 7.37, PaCO2: 41 mmHg, HCO3-: 12 mEq/L*
- This blood gas shows a **normal pH**, **normal PaCO2**, and **low HCO3-**. This suggests a **compensated metabolic acidosis**, where the body has fully compensated to bring the pH back to normal.
- While the patient likely has metabolic acidosis from salicylate toxicity, full compensation to a normal pH is less characteristic of an acute, severe presentation with significant neurological symptoms.
*pH: 7.41, PaCO2: 65 mmHg, HCO3-: 34 mEq/L*
- This blood gas shows a **normal pH**, **high PaCO2**, and **high HCO3-**. This indicates a **compensated respiratory acidosis**, where the kidneys have compensated for chronic CO2 retention.
- This pattern is not consistent with salicylate toxicity, which typically causes **respiratory alkalosis** early on, and later **metabolic acidosis**.
*pH: 7.47, PaCO2: 11 mmHg, HCO3-: 24 mEq/L*
- This blood gas analysis shows a **high pH** (alkalemia), **very low PaCO2** (severe hypocapnia), and a **normal HCO3-**. This indicates a **primary respiratory alkalosis** with no significant metabolic compensation.
- While salicylate toxicity can cause respiratory alkalosis, severe confusion and the progression of symptoms suggest a more advanced stage, usually involving a metabolic acidosis component, making a pure, uncompensated respiratory alkalosis less likely.
*pH: 7.31, PaCO2: 31 mmHg, HCO3-: 15 mEq/L*
- This blood gas shows a **low pH**, **low PaCO2**, and **low HCO3-**. This also indicates a **metabolic acidosis** with **respiratory compensation**.
- However, compared to pH 7.30, PaCO2 15 mmHg, and HCO3- 16 mEq/L, this option shows slightly **less severe respiratory compensation** (PaCO2 is higher), which is less typical for the profound respiratory stimulation seen in severe salicylate poisoning. The chosen correct option demonstrates a more characteristic and maximal respiratory compensation for the degree of metabolic acidosis.
Respiratory acidosis mechanisms and compensation US Medical PG Question 3: A 72-year-old female is brought to the emergency department after being found unresponsive in her garage with an open bottle of unmarked fluid. She is confused and is unable to answer questions on arrival. Her medical history is significant for Alzheimer disease, but her family says she has no medical comorbidities. Serum analysis of this patient's blood shows a pH of 7.28 with a high anion gap. The electrolyte that is most likely significantly decreased in this patient follows which of the following concentration curves across the proximal tubule of the kidney?
- A. Curve C
- B. Curve E
- C. Curve B
- D. Curve A
- E. Curve D (Correct Answer)
Respiratory acidosis mechanisms and compensation Explanation: ***Curve D***
- The patient presents with **high anion gap metabolic acidosis**, which, in the context of an unknown fluid ingestion, is highly suggestive of **methanol** or **ethylene glycol poisoning**. These toxins are metabolized into toxic acids (**formic acid** from methanol; **glycolic acid, oxalic acid** from ethylene glycol).
- These toxic acid anions displace **bicarbonate** (HCO3-) in the blood to maintain electroneutrality, leading to a **decreased bicarbonate level**. Curve D represents bicarbonate, which is largely reabsorbed in the proximal tubule but significantly reduced in this scenario.
*Curve C*
- This curve likely represents a substance like **phosphate** or **urea**, which is partially reabsorbed and partially excreted.
- While phosphate levels can be affected in various metabolic derangements, it's not the primary electrolyte significantly decreased in **high anion gap metabolic acidosis** from toxic alcohol ingestion.
*Curve E*
- This curve typically represents a substance that is **filtered and then minimally reabsorbed** or even secreted, such as **creatinine** or **potassium** when excess is being excreted.
- **Potassium** levels can be variable in acidosis but are not typically the most significantly decreased electrolyte in this poisoning scenario.
*Curve B*
- This curve would normally represent an electrolyte that is **highly reabsorbed** in the proximal tubule, with very little remaining.
- This might represent substances like **glucose** (under normal conditions) or **amino acids**, which are not the primary electrolyte affected in this case.
*Curve A*
- This curve represents a substance that is **freely filtered** and then **neither reabsorbed nor secreted** significantly in the proximal tubule, such as **inulin**.
- This pattern does not correspond to an electrolyte whose level would be significantly decreased due to high anion gap metabolic acidosis.
Respiratory acidosis mechanisms and compensation US Medical PG Question 4: A 39-year-old woman is brought to the emergency room by her husband because of severe dyspnea and dizziness. Her symptoms started suddenly 30 minutes ago. She appears distressed. Arterial blood gas shows a pH of 7.51, pO2 of 100 mm Hg, and a pCO2 of 30 mm Hg. Which of the following is the most likely cause?
- A. Myasthenia gravis
- B. Opioid toxicity
- C. Panic attack (Correct Answer)
- D. Epiglottitis
- E. Pulmonary fibrosis
Respiratory acidosis mechanisms and compensation Explanation: ***Panic attack***
- The sudden onset of **severe dyspnea** and **dizziness** in a distressed patient, along with ABG results indicating **respiratory alkalosis** (pH 7.51, pCO2 30 mm Hg), is highly characteristic of a panic attack with hyperventilation.
- **Hyperventilation** leads to excessive CO2 exhalation, causing the pCO2 to drop and the pH to rise, resulting in symptoms like lightheadedness and dyspnea.
*Myasthenia gravis*
- This is a **neuromuscular disorder** causing muscle weakness, which can lead to respiratory compromise over time, but typically does not present with such acute, sudden dyspnea and dizziness without prior symptoms.
- The ABG findings of respiratory alkalosis are not typical for a primary myasthenic crisis, which would likely show respiratory acidosis if respiratory failure were imminent.
*Opioid toxicity*
- Opioid overdose causes **respiratory depression**, leading to reduced respiratory rate and shallow breathing, which would result in **respiratory acidosis** (increased pCO2 and decreased pH), not alkalosis.
- The patient's pO2 of 100 mm Hg also argues against significant respiratory depression.
*Epiglottitis*
- Epiglottitis presents with a **rapidly worsening sore throat**, difficulty swallowing, drooling, and stridor, indicating upper airway obstruction.
- While it causes severe dyspnea, the ABG would likely show signs of hypoxemia and potentially acidosis due to airway compromise, not hyperventilation-induced alkalosis.
*Pulmonary fibrosis*
- This is a **chronic interstitial lung disease** that causes progressive dyspnea, often with a dry cough.
- The onset of symptoms is typically gradual, over months to years, not sudden within 30 minutes, and ABG would likely show hypoxemia with compensated respiratory alkalosis or acidosis depending on the stage, but not acutely severe hyperventilation-induced alkalosis.
Respiratory acidosis mechanisms and compensation US Medical PG Question 5: A 24-year-old woman presents to the emergency department after she was found agitated and screaming for help in the middle of the street. She says she also has dizziness and tingling in the lips and hands. Her past medical history is relevant for general anxiety disorder, managed medically with paroxetine. At admission, her pulse is 125/min, respiratory rate is 25/min, and body temperature is 36.5°C (97.7°F). Physical examination is unremarkable. An arterial blood gas sample is taken. Which of the following results would you most likely expect to see in this patient?
- A. pH: increased, HCO3-: increased, Pco2: increased
- B. pH: decreased, HCO3-: decreased, Pco2: decreased
- C. pH: decreased, HCO3-: increased, Pco2: increased
- D. pH: increased, HCO3-: decreased, Pco2: decreased (Correct Answer)
- E. pH: normal, HCO3-: increased, Pco2: increased
Respiratory acidosis mechanisms and compensation Explanation: ***pH: increased, HCO3-: decreased, Pco2: decreased***
- The patient's presentation with **agitation**, **dizziness**, **paresthesias** (tingling in lips and hands), and **tachypnea** (respiratory rate 25/min) is highly suggestive of **hyperventilation** due to an anxiety attack.
- **Hyperventilation** leads to excessive **CO2 expulsion**, causing a decrease in Pco2, which results in respiratory alkalosis (increased pH) and a compensatory decrease in HCO3-.
*pH: increased, HCO3-: increased, Pco2: increased*
- An **increased pH** coupled with **increased HCO3-** and **increased Pco2** would suggest a **metabolic alkalosis with respiratory compensation**, which is not consistent with the patient's acute hyperventilation.
- While pH is increased, the other values contradict the primary respiratory cause suggested by the symptoms.
*pH: decreased, HCO3-: decreased, Pco2: decreased*
- This profile describes **metabolic acidosis with respiratory compensation**, which would typically present with **Kussmaul breathing** and other signs of acidosis, not acute hyperventilation and agitation.
- Symptoms such as dizziness and tingling are associated with alkalosis, not acidosis.
*pH: decreased, HCO3-: increased, Pco2: increased*
- This pattern is characteristic of **respiratory acidosis with metabolic compensation**, often seen in conditions like **COPD exacerbation** or **opioid overdose** with hypoventilation.
- The patient's rapid breathing and clinical picture are not consistent with respiratory acidosis.
*pH: normal, HCO3-: increased, Pco2: increased*
- A **normal pH** with **increased HCO3-** and **increased Pco2** would indicate a **compensated metabolic alkalosis**.
- Her acute symptoms point to an uncompensated or acutely compensated respiratory disorder, not a compensated metabolic issue.
Respiratory acidosis mechanisms and compensation US Medical PG Question 6: A 66-year-old man is brought to the emergency department by his daughter because of 3 days of fever, chills, cough, and shortness of breath. The cough is productive of yellow sputum. His symptoms have not improved with rest and guaifenesin. His past medical history is significant for hypertension, for which he takes hydrochlorothiazide. He has a 30-pack-year history of smoking. His temperature is 38.9 C (102.0 F), blood pressure 88/56 mm Hg, and heart rate 105/min. Following resuscitation with normal saline, his blood pressure improves to 110/70 mm Hg. His arterial blood gas is as follows:
Blood pH 7.52, PaO2 74 mm Hg, PaCO2 28 mm Hg, and HCO3- 21 mEq/L.
Which of the following acid-base disturbances best characterizes this patient's condition?
- A. Metabolic acidosis
- B. Respiratory acidosis
- C. Respiratory alkalosis (Correct Answer)
- D. Normal acid-base status
- E. Metabolic alkalosis
Respiratory acidosis mechanisms and compensation Explanation: ***Respiratory alkalosis***
- The patient's pH of **7.52** indicates alkalemia. A **PaCO2 of 28 mm Hg** (normal range 35-45 mm Hg) is low, indicating a respiratory component.
- The **primary disturbance is respiratory alkalosis** due to hyperventilation from pneumonia/sepsis causing tachypnea and increased CO2 elimination.
- The HCO3- of 21 mEq/L (normal range 22-26 mEq/L) is at the lower limit of normal. In acute respiratory alkalosis, bicarbonate remains near normal since **metabolic compensation takes 2-3 days** to develop significantly.
- With a 3-day history, minimal renal compensation is expected, consistent with the near-normal bicarbonate.
*Metabolic acidosis*
- Metabolic acidosis would present with a **low pH** and a **low HCO3-**, which is not seen here.
- The patient's pH is **alkaline (7.52)**, not acidic, ruling out this diagnosis.
*Respiratory acidosis*
- Respiratory acidosis would be characterized by a **low pH** and a **high PaCO2**, indicating hypoventilation.
- The patient's **PaCO2 is low (28 mm Hg)** and the **pH is high**, directly contradicting respiratory acidosis.
*Normal acid-base status*
- A normal acid-base status would have a **pH between 7.35 and 7.45** and PaCO2 between 35-45 mm Hg.
- The patient's **pH of 7.52** and **PaCO2 of 28 mm Hg** are both abnormal, specifically indicating alkalemia and hypocapnia.
*Metabolic alkalosis*
- Metabolic alkalosis would feature a **high pH** and a **high HCO3-** (typically >26 mEq/L), often resulting from conditions like vomiting or diuretic use.
- While the patient is on hydrochlorothiazide, his **HCO3- is 21 mEq/L** (low-normal, not elevated), indicating this is not a primary metabolic alkalosis.
Respiratory acidosis mechanisms and compensation US Medical PG Question 7: A 52-year-old man with a history of Type 1 diabetes mellitus presents to the emergency room with increasing fatigue. Two days ago, he ran out of insulin and has not had time to obtain a new prescription. He denies fevers or chills. His temperature is 37.2 degrees Celsius, blood pressure 84/56 mmHg, heart rate 100/min, respiratory rate 20/min, and SpO2 97% on room air. His physical exam is otherwise within normal limits. An arterial blood gas analysis shows the following:
pH 7.25, PCO2 29, PO2 95, HCO3- 15.
Which of the following acid-base disorders is present?
- A. Respiratory alkalosis with appropriate metabolic compensation
- B. Respiratory acidosis with appropriate metabolic compensation
- C. Mixed metabolic and respiratory acidosis
- D. Metabolic acidosis with appropriate respiratory compensation (Correct Answer)
- E. Metabolic alkalosis with appropriate respiratory compensation
Respiratory acidosis mechanisms and compensation Explanation: ***Metabolic acidosis with appropriate respiratory compensation***
- The patient's pH of 7.25 and HCO3- of 15 indicate **metabolic acidosis**, while the PCO2 of 29 indicates **respiratory compensation**.
- The compensation is **appropriate** as suggested by Winter's formula [Expected PCO2 = (1.5 x HCO3-) + 8 +/- 2; (1.5 x 15) + 8 = 30.5, which is close to 29].
*Respiratory alkalosis with appropriate metabolic compensation*
- This would involve a **pH > 7.45** and **low PCO2** with a secondary drop in HCO3-, which is not seen here.
- The patient's primary problem is a metabolic disturbance due to insulin deficiency.
*Respiratory acidosis with appropriate metabolic compensation*
- This disorder is characterized by a **low pH** and a **high PCO2**, with a secondary rise in HCO3-.
- The patient's PCO2 is low, indicating a compensatory response rather than a primary respiratory acidosis.
*Mixed metabolic and respiratory acidosis*
- A mixed disorder would show a **low pH** due to both **low HCO3-** and **high PCO2**.
- The patient's PCO2 is low, indicating a compensatory response to metabolic acidosis, not an additional respiratory acidosis.
*Metabolic alkalosis with appropriate respiratory compensation*
- This would present with a **high pH (>7.45)** and **high HCO3-**, with compensatory **elevated PCO2**.
- The patient's pH and HCO3- are low, indicating acidosis, not alkalosis.
Respiratory acidosis mechanisms and compensation US Medical PG Question 8: A 70-year-old woman is brought to the emergency department due to worsening lethargy. She lives with her husband who says she has had severe diarrhea for the past few days. Examination shows a blood pressure of 85/60 mm Hg, pulse of 100/min, and temperature of 37.8°C (100.0°F). The patient is stuporous, while her skin appears dry and lacks turgor. Laboratory tests reveal:
Serum electrolytes
Sodium 144 mEq/L
Potassium 3.5 mEq/L
Chloride 115 mEq/L
Bicarbonate 19 mEq/L
Serum pH 7.3
PaO2 80 mm Hg
Pco2 38 mm Hg
This patient has which of the following acid-base disturbances?
- A. Chronic respiratory acidosis
- B. Anion gap metabolic acidosis with respiratory compensation
- C. Anion gap metabolic acidosis
- D. Non-anion gap metabolic acidosis with respiratory compensation (Correct Answer)
- E. Non-anion gap metabolic acidosis
Respiratory acidosis mechanisms and compensation Explanation: ***Non-anion gap metabolic acidosis with respiratory compensation***
- This patient has significant **diarrhea**, which causes a loss of **bicarbonate** from the gastrointestinal tract, leading to a **non-anion gap metabolic acidosis**.
- The **serum pH of 7.3** confirms acidosis, and the **Pco2 of 38 mm Hg** (which is slightly below the normal range, considering the acidosis) indicates effective **respiratory compensation** for the metabolic disturbance. Calculating the **anion gap** = Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L** (normal range 8-12 mEq/L), which is within normal limits.
*Chronic respiratory acidosis*
- This would involve an elevated **Pco2** and a compensatory increase in **bicarbonate**, neither of which are observed in this patient.
- The patient's primary problem is loss of bicarbonate due to diarrhea, not impaired CO2 excretion.
*Anion gap metabolic acidosis with respiratory compensation*
- An **anion gap metabolic acidosis** would show an elevated anion gap (>12 mEq/L), which is not present here (anion gap is 10 mEq/L).
- While respiratory compensation is occurring, the underlying acidosis is **non-anion gap**.
*Anion gap metabolic acidosis*
- This diagnosis requires an **elevated anion gap**, which is calculated as Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L**.
- Since the anion gap is within the normal range, an anion gap metabolic acidosis is excluded.
*Non-anion gap metabolic acidosis*
- While the patient does have a **non-anion gap metabolic acidosis** due to bicarbonate loss from diarrhea, this option doesn't account for the **respiratory compensation** indicated by the Pco2.
- The slightly reduced Pco2 demonstrates the body's attempt to normalize pH, making "with respiratory compensation" a more complete description.
Respiratory acidosis mechanisms and compensation US Medical PG Question 9: A 24-year-old male is brought in by ambulance to the emergency department after he was found unresponsive at home for an unknown length of time. Upon arrival, he is found to be severely altered and unable to answer questions about his medical history. Based on clinical suspicion, a panel of basic blood tests are obtained including an arterial blood gas, which shows a pH of 7.32, a pCO2 of 70, and a bicarbonate level of 30 mEq/L. Which of the following is most likely the primary disturbance leading to the values found in the ABG?
- A. Respiratory acidosis (Correct Answer)
- B. Metabolic alkalosis
- C. Respiratory alkalosis
- D. Metabolic acidosis
- E. Mixed alkalosis
Respiratory acidosis mechanisms and compensation Explanation: ***Respiratory acidosis***
- The **pH (7.32)** is acidic (normal 7.35-7.45), and the **pCO2 (70 mmHg)** is significantly elevated (normal 35-45 mmHg), indicating **primary respiratory acidosis** due to hypoventilation.
- The **bicarbonate (30 mEq/L)** is elevated above normal (22-26 mEq/L), indicating **partial metabolic compensation** by the kidneys retaining bicarbonate to buffer the acidosis.
- This pattern suggests **chronic respiratory acidosis** (e.g., from COPD, CNS depression, neuromuscular disease) with renal compensation.
*Metabolic alkalosis*
- This would present with **elevated pH** (>7.45) and **elevated bicarbonate** as the primary disturbance, often with compensatory elevation in pCO2.
- The patient's **pH is acidic (7.32)**, not alkalotic, ruling out metabolic alkalosis as the primary process.
*Respiratory alkalosis*
- This would present with **elevated pH** (>7.45) and **decreased pCO2** (<35 mmHg) due to hyperventilation.
- The patient has the opposite: **acidic pH and elevated pCO2**, ruling out respiratory alkalosis.
*Metabolic acidosis*
- This would present with **decreased pH** and **decreased bicarbonate** (<22 mEq/L) as the primary disturbance.
- While the pH is low, the **bicarbonate is elevated (30 mEq/L)**, not decreased, ruling out metabolic acidosis as the primary disorder.
*Mixed alkalosis*
- A mixed alkalosis would involve simultaneous respiratory and metabolic processes causing **elevated pH**.
- The patient's **pH is acidic (7.32)**, making any form of alkalosis impossible as the primary disturbance.
Respiratory acidosis mechanisms and compensation US Medical PG Question 10: A 30-year-old woman presents to the emergency department with breathlessness for the last hour. She is unable to provide any history due to her dyspnea. Her vitals include: respiratory rate 20/min, pulse 100/min, and blood pressure 144/84 mm Hg. On physical examination, she is visibly obese, and her breathing is labored. There are decreased breath sounds and hyperresonance to percussion across all lung fields bilaterally. An arterial blood gas is drawn, and the patient is placed on inhaled oxygen. Laboratory findings reveal:
pH 7.34
pO2 63 mm Hg
pCO2 50 mm Hg
HCO3 22 mEq/L
Her alveolar partial pressure of oxygen is 70 mm Hg. Which of the following is the most likely etiology of this patient’s symptoms?
- A. Right to left shunt
- B. Alveolar hypoventilation (Correct Answer)
- C. Ventricular septal defect
- D. Impaired gas diffusion
- E. Ventilation/perfusion mismatch
Respiratory acidosis mechanisms and compensation Explanation: ***Alveolar hypoventilation***
- The patient exhibits features of **obesity** and **labored breathing** with decreased breath sounds and hyperresonance, along with arterial blood gas results showing **respiratory acidosis** (pH 7.34, pCO2 50 mmHg) and **hypoxia** (pO2 63 mmHg).
- The calculated A-a gradient (Alveolar O2 - arterial O2) is low (70 mmHg - 63 mmHg = 7 mmHg), indicating that the problem is primarily with **overall ventilation** rather than a defect in gas exchange across the alveolar-capillary membrane.
*Right to left shunt*
- A right-to-left shunt would cause a **large A-a gradient**, as deoxygenated blood bypasses the lungs and mixes with oxygenated blood.
- While it causes **hypoxemia**, it would not typically be associated with hypercapnia unless very severe, and the A-a gradient calculation here does not support a significant shunt.
*Ventricular septal defect*
- A ventricular septal defect is a **structural heart abnormality** that can cause a left-to-right shunt initially, leading to pulmonary hypertension and eventually a right-to-left shunt (Eisenmenger syndrome).
- While it can cause hypoxemia due to shunting, it would not primarily manifest with increased pCO2 or the specific lung physical exam findings of decreased breath sounds and hyperresonance in the absence of other cardiac signs.
*Impaired gas diffusion*
- Impaired gas diffusion would lead to a **large A-a gradient** and **hypoxemia**, but typically not significant hypercapnia unless the impairment is extremely severe.
- Conditions like **pulmonary fibrosis** or **emphysema** cause impaired diffusion, but the patient's presentation and particularly the low A-a gradient do not support this.
*Ventilation/perfusion mismatch*
- A V/Q mismatch also causes a **large A-a gradient** and **hypoxemia**, as some areas of the lung are either poorly ventilated or poorly perfused.
- While it can cause hypercapnia in severe cases, the primary issue indicated by the low A-a gradient here is one of overall inadequate ventilation, not selective areas of ventilation-perfusion imbalance.
More Respiratory acidosis mechanisms and compensation US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.