Renal regulation of acid-base balance US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Renal regulation of acid-base balance. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Renal regulation of acid-base balance US Medical PG Question 1: A 61-year-old male is given acetazolamide to treat open-angle glaucoma. Upon diuresis, his urine is found to be highly alkaline. Which of the following accounts for the alkaline nature of this patient’s urine?
- A. Inhibition of bicarbonate reabsorption in the proximal tubule (Correct Answer)
- B. Inhibition of bicarbonate reabsorption in beta-intercalated cells
- C. Inhibition of acid secretion in alpha-intercalated cells
- D. Inhibition of chloride reabsorption in the distal convoluted tubule
- E. Inhibition of chloride reabsorption in the thick ascending loop of Henle
Renal regulation of acid-base balance Explanation: ***Inhibition of bicarbonate reabsorption in the proximal tubule***
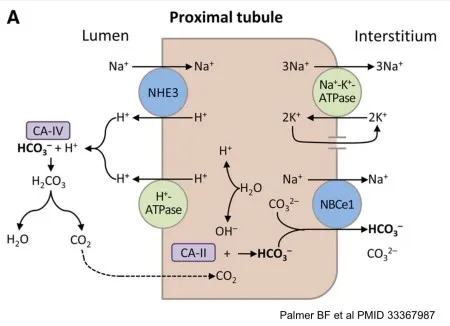
- **Acetazolamide** is a **carbonic anhydrase inhibitor** that primarily acts on the **proximal tubule** of the kidney.
- Its action here prevents the reabsorption of **bicarbonate (HCO3-)**, leading to its increased excretion in the urine and thus making the urine alkaline.
*Inhibition of chloride reabsorption in the distal convoluted tubule*
- This effect is typically associated with **thiazide diuretics**, which inhibit the **Na-Cl cotransporter** in the distal convoluted tubule.
- While it affects electrolyte balance, it does not directly lead to the observed **alkaline urine** in the manner described.
*Inhibition of bicarbonate reabsorption in beta-intercalated cells*
- **Beta-intercalated cells** in the collecting duct secrete bicarbonate, and their inhibition would lead to **acidic urine**, not alkaline.
- They play a role in **bicarbonate secretion**, not reabsorption as seen with acetazolamide's primary action.
*Inhibition of acid secretion in alpha-intercalated cells*
- **Alpha-intercalated cells** secrete acid (H+) into the urine. Inhibiting their function would reduce acid excretion, making the urine less acidic or even alkaline.
- However, the primary mechanism of acetazolamide's effect on urine pH is through **bicarbonate wasting** in the proximal tubule, not direct inhibition of acid secretion in the collecting duct.
*Inhibition of chloride reabsorption in the thick ascending loop of Henle*
- This is the mechanism of action for **loop diuretics** like furosemide, which inhibit the **Na-K-2Cl cotransporter**.
- While loop diuretics cause significant diuresis, they do not directly lead to the pronounced **urinary alkalinization** seen with acetazolamide.
Renal regulation of acid-base balance US Medical PG Question 2: A 67-year-old man presents to his primary care physician because of weak urine stream, and increasing difficulty in initiating and stopping urination. He also reports of mild generalized body aches and weakness during the day. The past medical history includes diabetes mellitus type 2 for 35 years and essential hypertension for 19 years. The medication list includes metformin, vildagliptin, and enalapril. The vital signs include: temperature 36.7°C (98.1°F), blood pressure 151/82 mm Hg, and pulse 88/min. The physical examination is remarkable for markedly enlarged, firm prostate without nodules. The laboratory test results are as follows:
Serum sodium 142 mEq/L
Serum potassium 5.7 mEq/L
Serum chloride 115 mEq/L
Serum bicarbonate 17 mEq/L
Serum creatinine 0.9 mg/dL
Arterial pH 7.31
Urine pH 5.3
Urine sodium 59 mEq/L
Urine potassium 6.2 mEq/L
Urine chloride 65 mEq/L
Which of the following most likely explains the patient’s findings?
- A. Fanconi syndrome
- B. Type 1 renal tubular acidosis
- C. Type 2 renal tubular acidosis
- D. Type 4 renal tubular acidosis (Correct Answer)
- E. End-stage renal disease
Renal regulation of acid-base balance Explanation: ***Type 4 renal tubular acidosis***
- The patient presents with **hyperkalemia** (serum potassium 5.7 mEq/L) and **non-anion gap metabolic acidosis** (serum bicarbonate 17 mEq/L, pH 7.31) with a relatively low urine pH (5.3). This combination is characteristic of **Type 4 RTA**, which often results from **hypoaldosteronism** or renal **aldosterone resistance**.
- His long-standing **diabetes mellitus** can cause damage to the **juxtaglomerular apparatus** or autonomic neuropathy, impairing renin and ultimately aldosterone secretion. Additionally, **enalapril** (an ACE inhibitor) contributes to reduced aldosterone levels, further exacerbating the condition.
*Fanconi syndrome*
- This syndrome is characterized by a generalized defect in the **proximal tubule**, leading to the loss of **glucose**, **amino acids**, **phosphate**, and **bicarbonate** in the urine.
- While it causes RTA, it typically presents with **hypokalemia** due to increased potassium excretion, which is contrary to this patient's **hyperkalemia**.
*Type 1 renal tubular acidosis*
- This type involves a defect in **distal tubule** hydrogen ion secretion, resulting in **metabolic acidosis** with a **high urine pH** (>5.5) despite systemic acidosis.
- Patients with Type 1 RTA typically present with **hypokalemia** due to increased potassium excretion, unlike the hyperkalemia seen in this patient.
*Type 2 renal tubular acidosis*
- This condition involves impaired **bicarbonate reabsorption** in the **proximal tubule**. Initially, it leads to metabolic acidosis with a high urine pH.
- However, once the filtered bicarbonate load falls below the compromised reabsorptive capacity, the urine pH can become acidic. Like Type 1 RTA, it is typically associated with **hypokalemia**.
*End-stage renal disease*
- While ESRD can cause metabolic acidosis and hyperkalemia due to severe reduction in GFR, the patient's **creatinine** (0.9 mg/dL) is within the normal range, indicating preserved renal function.
- Also, ESRD typically involves much broader complications of uremia beyond just electrolyte imbalances, which are not described here.
Renal regulation of acid-base balance US Medical PG Question 3: A 72-year-old female is brought to the emergency department after being found unresponsive in her garage with an open bottle of unmarked fluid. She is confused and is unable to answer questions on arrival. Her medical history is significant for Alzheimer disease, but her family says she has no medical comorbidities. Serum analysis of this patient's blood shows a pH of 7.28 with a high anion gap. The electrolyte that is most likely significantly decreased in this patient follows which of the following concentration curves across the proximal tubule of the kidney?
- A. Curve C
- B. Curve E
- C. Curve B
- D. Curve A
- E. Curve D (Correct Answer)
Renal regulation of acid-base balance Explanation: ***Curve D***
- The patient presents with **high anion gap metabolic acidosis**, which, in the context of an unknown fluid ingestion, is highly suggestive of **methanol** or **ethylene glycol poisoning**. These toxins are metabolized into toxic acids (**formic acid** from methanol; **glycolic acid, oxalic acid** from ethylene glycol).
- These toxic acid anions displace **bicarbonate** (HCO3-) in the blood to maintain electroneutrality, leading to a **decreased bicarbonate level**. Curve D represents bicarbonate, which is largely reabsorbed in the proximal tubule but significantly reduced in this scenario.
*Curve C*
- This curve likely represents a substance like **phosphate** or **urea**, which is partially reabsorbed and partially excreted.
- While phosphate levels can be affected in various metabolic derangements, it's not the primary electrolyte significantly decreased in **high anion gap metabolic acidosis** from toxic alcohol ingestion.
*Curve E*
- This curve typically represents a substance that is **filtered and then minimally reabsorbed** or even secreted, such as **creatinine** or **potassium** when excess is being excreted.
- **Potassium** levels can be variable in acidosis but are not typically the most significantly decreased electrolyte in this poisoning scenario.
*Curve B*
- This curve would normally represent an electrolyte that is **highly reabsorbed** in the proximal tubule, with very little remaining.
- This might represent substances like **glucose** (under normal conditions) or **amino acids**, which are not the primary electrolyte affected in this case.
*Curve A*
- This curve represents a substance that is **freely filtered** and then **neither reabsorbed nor secreted** significantly in the proximal tubule, such as **inulin**.
- This pattern does not correspond to an electrolyte whose level would be significantly decreased due to high anion gap metabolic acidosis.
Renal regulation of acid-base balance US Medical PG Question 4: A 45-year-old woman presents with severe, acute-onset colicky abdominal pain and nausea. She also describes bone pain, constipation, headache, decreased vision, and menstrual irregularity. Past medical history is significant for surgical removal of an insulinoma one year ago. Two months ago, she was prescribed fluoxetine for depression but hasn’t found it very helpful. Family history is significant for a rare genetic syndrome. Non-contrast CT, CBC, CMP, and urinalysis are ordered in the diagnostic work-up. Urine sediment is significant for the findings shown in the picture. Which of the following will also be a likely significant finding in the diagnostic workup?
- A. Decreased urine pH (Correct Answer)
- B. Hypokalemia and non-anion gap acidosis
- C. Diagnosis confirmed with cyanide-nitroprusside test
- D. Elevated hemoglobin on CBC with significantly low levels of EPO
- E. Imaging demonstrates staghorn calculi
Renal regulation of acid-base balance Explanation: ***Decreased urine pH***
- The urine sediment shows **uric acid crystals** (rhomboid or rosette-shaped), which are pathognomonic for acidic urine.
- Uric acid stones form when **urine pH < 5.5**, as uric acid is insoluble in acidic conditions.
- This patient's complex presentation with history of insulinoma and family history of rare genetic syndrome suggests **MEN1 (Multiple Endocrine Neoplasia Type 1)**, which includes parathyroid adenomas, pancreatic tumors, and pituitary tumors.
- The symptoms of bone pain, abdominal pain, constipation, and neurological changes ("stones, bones, abdominal groans, psychiatric moans") suggest **hypercalcemia from primary hyperparathyroidism**.
- While calcium stones (not uric acid) are more common in hypercalcemia, the image specifically shows uric acid crystals, making decreased urine pH the most relevant finding in this diagnostic workup.
*Hypokalemia and non-anion gap acidosis*
- This constellation is characteristic of **Type 1 (distal) renal tubular acidosis**.
- While RTA can predispose to kidney stones (typically calcium phosphate in alkaline urine), it does not match the uric acid crystals shown in the image.
- The patient's symptoms are more consistent with hypercalcemia from MEN1 rather than RTA.
*Diagnosis confirmed with cyanide-nitroprusside test*
- The **cyanide-nitroprusside test** detects elevated cystine levels and is used to diagnose **cystinuria**.
- However, the image shows **uric acid crystals**, not hexagonal cystine crystals.
- This test would not be relevant to the current clinical picture.
*Elevated hemoglobin on CBC with significantly low levels of EPO*
- This suggests **polycythemia vera** or primary polycythemia, a myeloproliferative disorder.
- While some MEN syndromes can have associated findings, this is unrelated to the uric acid crystals and the primary presentation.
- There is no indication of polycythemia in this patient's presentation.
*Imaging demonstrates staghorn calculi*
- **Staghorn calculi** are typically **struvite stones** caused by urease-producing bacteria (e.g., *Proteus*) in **alkaline urine** with urinary tract infections.
- The image shows **uric acid crystals**, which form in acidic urine and typically produce small stones, not staghorn calculi.
- Staghorn calculi are inconsistent with the presented urine sediment findings.
Renal regulation of acid-base balance US Medical PG Question 5: A 70-year-old woman is brought to the emergency department due to worsening lethargy. She lives with her husband who says she has had severe diarrhea for the past few days. Examination shows a blood pressure of 85/60 mm Hg, pulse of 100/min, and temperature of 37.8°C (100.0°F). The patient is stuporous, while her skin appears dry and lacks turgor. Laboratory tests reveal:
Serum electrolytes
Sodium 144 mEq/L
Potassium 3.5 mEq/L
Chloride 115 mEq/L
Bicarbonate 19 mEq/L
Serum pH 7.3
PaO2 80 mm Hg
Pco2 38 mm Hg
This patient has which of the following acid-base disturbances?
- A. Chronic respiratory acidosis
- B. Anion gap metabolic acidosis with respiratory compensation
- C. Anion gap metabolic acidosis
- D. Non-anion gap metabolic acidosis with respiratory compensation (Correct Answer)
- E. Non-anion gap metabolic acidosis
Renal regulation of acid-base balance Explanation: ***Non-anion gap metabolic acidosis with respiratory compensation***
- This patient has significant **diarrhea**, which causes a loss of **bicarbonate** from the gastrointestinal tract, leading to a **non-anion gap metabolic acidosis**.
- The **serum pH of 7.3** confirms acidosis, and the **Pco2 of 38 mm Hg** (which is slightly below the normal range, considering the acidosis) indicates effective **respiratory compensation** for the metabolic disturbance. Calculating the **anion gap** = Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L** (normal range 8-12 mEq/L), which is within normal limits.
*Chronic respiratory acidosis*
- This would involve an elevated **Pco2** and a compensatory increase in **bicarbonate**, neither of which are observed in this patient.
- The patient's primary problem is loss of bicarbonate due to diarrhea, not impaired CO2 excretion.
*Anion gap metabolic acidosis with respiratory compensation*
- An **anion gap metabolic acidosis** would show an elevated anion gap (>12 mEq/L), which is not present here (anion gap is 10 mEq/L).
- While respiratory compensation is occurring, the underlying acidosis is **non-anion gap**.
*Anion gap metabolic acidosis*
- This diagnosis requires an **elevated anion gap**, which is calculated as Na - (Cl + HCO3) = 144 - (115 + 19) = **10 mEq/L**.
- Since the anion gap is within the normal range, an anion gap metabolic acidosis is excluded.
*Non-anion gap metabolic acidosis*
- While the patient does have a **non-anion gap metabolic acidosis** due to bicarbonate loss from diarrhea, this option doesn't account for the **respiratory compensation** indicated by the Pco2.
- The slightly reduced Pco2 demonstrates the body's attempt to normalize pH, making "with respiratory compensation" a more complete description.
Renal regulation of acid-base balance US Medical PG Question 6: Which mechanism primarily regulates sodium reabsorption in the collecting duct?
- A. Glomerulotubular balance
- B. Atrial natriuretic peptide
- C. Antidiuretic hormone
- D. Aldosterone (Correct Answer)
Renal regulation of acid-base balance Explanation: ***Aldosterone***
- **Aldosterone** is the primary hormone that stimulates **sodium reabsorption** and **potassium secretion** in the principal cells of the collecting duct.
- It acts by increasing the synthesis and activity of **ENaC channels** on the apical membrane and **Na+/K+-ATPase pumps** on the basolateral membrane.
*Glomerulotubular balance*
- **Glomerulotubular balance** refers to the mechanism by which the **proximal tubule** reabsorbs a constant fraction of the filtered load, regardless of changes in glomerular filtration rate (GFR).
- This mechanism maintains a relatively constant delivery of fluid and solutes to downstream segments but does not primarily regulate sodium in the collecting duct.
*Atrial natriuretic peptide*
- **Atrial natriuretic peptide (ANP)** primarily **inhibits sodium reabsorption** in the collecting duct, leading to **natriuresis** and **diuresis**, which is the opposite of sodium reabsorption.
- ANP is released in response to atrial stretch, indicating increased blood volume.
*Antidiuretic hormone*
- **Antidiuretic hormone (ADH)** primarily regulates **water reabsorption** in the collecting duct by increasing the insertion of **aquaporin-2 channels** into the apical membrane, making the collecting duct permeable to water.
- While ADH can indirectly affect sodium concentration by influencing water movement, it does not directly regulate sodium transport to the same extent as aldosterone.
Renal regulation of acid-base balance US Medical PG Question 7: A 17-year-old boy is brought to the physician by his father because of a 7-month history of fatigue, recurrent leg cramps, and increased urinary frequency. His pulse is 94/min and blood pressure is 118/85 mm Hg. Physical examination shows dry mucous membranes. Laboratory studies show:
Serum
Na+ 130 mEq/L
K+ 2.8 mEq/L
Cl- 92 mEq/L
Mg2+ 1.1 mEq/L
Ca2+ 10.6 mg/dL
Albumin 5.2 g/dL
Urine
Ca2+ 70 mg/24 h
Cl- 375 mEq/24h (N = 110–250)
Arterial blood gas analysis on room air shows a pH of 7.55 and an HCO3- concentration of 45 mEq/L. Impaired function of which of the following structures is the most likely cause of this patient's condition?
- A. Ascending loop of Henle
- B. Collecting duct
- C. Distal convoluted tubule (Correct Answer)
- D. Descending loop of Henle
- E. Proximal convoluted tubule
Renal regulation of acid-base balance Explanation: ***Distal convoluted tubule***
- The patient presents with **hypokalemia**, **metabolic alkalosis**, **hypomagnesemia**, and **hypocalciuria** (24-hour urine Ca2+ 70 mg, normal up to 250 mg), which are characteristic findings of **Gitelman syndrome**.
- **Gitelman syndrome** is caused by a loss-of-function mutation in the **thiazide-sensitive Na-Cl cotransporter (NCC)**, located in the **distal convoluted tubule**, leading to impaired reabsorption of Na+ and Cl- at this segment.
*Ascending loop of Henle*
- Impaired function of the **Na-K-2Cl cotransporter (NKCC2)** in the **thick ascending limb of the loop of Henle** causes **Bartter syndrome**.
- Bartter syndrome typically presents with **hypercalciuria**, in contrast to the hypocalciuria seen in this patient.
*Collecting duct*
- Dysfunction of the **collecting duct** can lead to various conditions, such as **renal tubular acidosis** or **diabetes insipidus**, depending on which channels or receptors are affected.
- However, the specific combination of **hypokalemia**, **metabolic alkalosis**, **hypomagnesemia**, and **hypocalciuria** points away from primary collecting duct dysfunction.
*Descending loop of Henle*
- The **descending loop of Henle** is primarily permeable to **water** and has a limited role in electrolyte reabsorption.
- Impairment here would primarily affect **urine concentration** and dilution but would not account for the specific electrolyte imbalances observed.
*Proximal convoluted tubule*
- The **proximal convoluted tubule** is responsible for reabsorbing a large fraction of filtered electrolytes, glucose, and amino acids.
- Dysfunction here (e.g., **Fanconi syndrome**) would typically present with **generalized aminoaciduria**, **glycosuria**, **phosphaturia**, and **proximal renal tubular acidosis**, which are not seen in this patient.
Renal regulation of acid-base balance US Medical PG Question 8: A 32-year-old woman is admitted to the emergency department for 36 hours of intense left-sided back pain that extends into her left groin. She reports that the pain started a day after a charitable 5 km (3.1 mi) marathon. The past medical history is relevant for multiple complaints of eye dryness and dry mouth. Physical examination is unremarkable, except for intense left-sided costovertebral pain. The results from laboratory tests are shown.
Laboratory test Result
Serum Na+ 137
Serum Cl- 110
Serum K+ 3.0
Serum creatinine (SCr) 0.82
Arterial blood gas Result
pH 7.28
pO2 98 mm Hg
pCO2 28.5 mm Hg
SaO2% 98%
HCO3- 15 mm Hg
Which of the following explains this patient’s condition?
- A. Carbonic acid accumulation
- B. Decreased bicarbonate renal absorption
- C. Decreased renal excretion of hydrogen ions (H+) (Correct Answer)
- D. Decreased synthesis of ammonia (NH3)
- E. Decreased excretion of nonvolatile acids
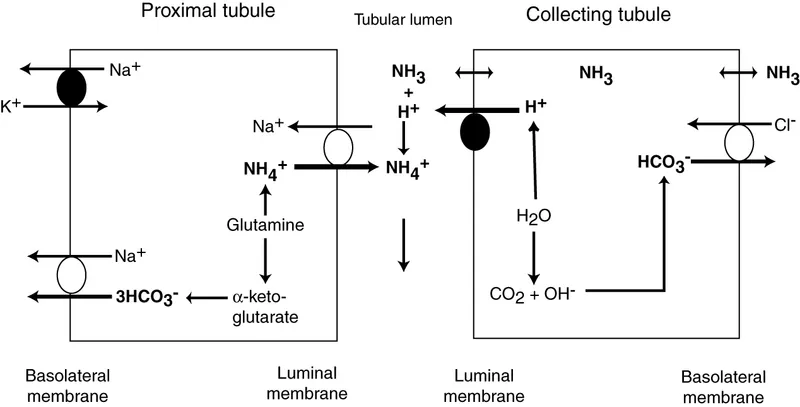
Renal regulation of acid-base balance Explanation: ***Decreased renal excretion of hydrogen ions (H+)***
- The patient presents with **metabolic acidosis** (pH 7.28, HCO3- 15 mEq/L) with **respiratory compensation** (pCO2 28.5 mm Hg). The anion gap is **normal** (Na+ - (Cl- + HCO3-) = 137 - (110 + 15) = **12 mEq/L**), indicating a **non-anion gap metabolic acidosis**.
- The history of **dry eyes and dry mouth** strongly suggests **Sjögren syndrome**, which is commonly associated with **Type 1 (distal) renal tubular acidosis**.
- In **Type 1 RTA**, the distal tubule alpha-intercalated cells cannot adequately secrete H+ ions, leading to metabolic acidosis with **inability to acidify urine** (urine pH > 5.5). Associated findings include **hypokalemia** (K+ 3.0), **nephrolithiasis** (calcium phosphate stones due to alkaline urine), and hypercalciuria.
- The left-sided flank pain radiating to the groin is consistent with **nephrolithiasis**, a common complication of Type 1 RTA.
*Carbonic acid accumulation*
- **Carbonic acid accumulation** indicates **respiratory acidosis** with elevated pCO2, which is not present here.
- The patient has a **low pCO2 (28.5 mm Hg)**, representing appropriate **respiratory compensation** for the primary metabolic acidosis.
*Decreased bicarbonate renal absorption*
- **Decreased bicarbonate renal absorption** characterizes **Type 2 (proximal) RTA**.
- While Type 2 RTA also causes non-anion gap metabolic acidosis, it is **not typically associated with Sjögren syndrome** and would present with different features (glycosuria, aminoaciduria, phosphaturia as part of Fanconi syndrome).
- Type 2 RTA can acidify urine to pH < 5.5 when serum HCO3- is low, unlike Type 1 RTA.
*Decreased synthesis of ammonia (NH3)*
- **Decreased ammonia synthesis** is characteristic of **Type 4 RTA** or severe chronic kidney disease.
- Type 4 RTA presents with **hyperkalemia** (due to hypoaldosteronism), not the hypokalemia seen in this patient.
- The normal serum creatinine (0.82 mg/dL) rules out significant renal failure.
*Decreased excretion of nonvolatile acids*
- **Decreased excretion of nonvolatile acids** would cause **elevated anion gap metabolic acidosis** (e.g., lactic acidosis, ketoacidosis, or advanced renal failure with accumulation of organic acids).
- This patient has a **normal anion gap (12 mEq/L)** and **normal renal function** (creatinine 0.82 mg/dL), making this mechanism unlikely.
- The clinical context of Sjögren syndrome with dry eyes/mouth points specifically to distal RTA.
Renal regulation of acid-base balance US Medical PG Question 9: A 24-year-old male is brought in by ambulance to the emergency department after he was found unresponsive at home for an unknown length of time. Upon arrival, he is found to be severely altered and unable to answer questions about his medical history. Based on clinical suspicion, a panel of basic blood tests are obtained including an arterial blood gas, which shows a pH of 7.32, a pCO2 of 70, and a bicarbonate level of 30 mEq/L. Which of the following is most likely the primary disturbance leading to the values found in the ABG?
- A. Respiratory acidosis (Correct Answer)
- B. Metabolic alkalosis
- C. Respiratory alkalosis
- D. Metabolic acidosis
- E. Mixed alkalosis
Renal regulation of acid-base balance Explanation: ***Respiratory acidosis***
- The **pH (7.32)** is acidic (normal 7.35-7.45), and the **pCO2 (70 mmHg)** is significantly elevated (normal 35-45 mmHg), indicating **primary respiratory acidosis** due to hypoventilation.
- The **bicarbonate (30 mEq/L)** is elevated above normal (22-26 mEq/L), indicating **partial metabolic compensation** by the kidneys retaining bicarbonate to buffer the acidosis.
- This pattern suggests **chronic respiratory acidosis** (e.g., from COPD, CNS depression, neuromuscular disease) with renal compensation.
*Metabolic alkalosis*
- This would present with **elevated pH** (>7.45) and **elevated bicarbonate** as the primary disturbance, often with compensatory elevation in pCO2.
- The patient's **pH is acidic (7.32)**, not alkalotic, ruling out metabolic alkalosis as the primary process.
*Respiratory alkalosis*
- This would present with **elevated pH** (>7.45) and **decreased pCO2** (<35 mmHg) due to hyperventilation.
- The patient has the opposite: **acidic pH and elevated pCO2**, ruling out respiratory alkalosis.
*Metabolic acidosis*
- This would present with **decreased pH** and **decreased bicarbonate** (<22 mEq/L) as the primary disturbance.
- While the pH is low, the **bicarbonate is elevated (30 mEq/L)**, not decreased, ruling out metabolic acidosis as the primary disorder.
*Mixed alkalosis*
- A mixed alkalosis would involve simultaneous respiratory and metabolic processes causing **elevated pH**.
- The patient's **pH is acidic (7.32)**, making any form of alkalosis impossible as the primary disturbance.
Renal regulation of acid-base balance US Medical PG Question 10: A person is exercising strenuously on a treadmill for 1 hour. An arterial blood gas measurement is then taken. Which of the following are the most likely values?
- A. pH 7.56, PaO2 100, PCO2 44, HCO3 38
- B. pH 7.32, PaO2 42, PCO2 50, HCO3 27
- C. pH 7.57 PaO2 100, PCO2 23, HCO3 21 (Correct Answer)
- D. pH 7.38, PaO2 100, PCO2 69 HCO3 42
- E. pH 7.36, PaO2 100, PCO2 40, HCO3 23
Renal regulation of acid-base balance Explanation: ***pH 7.57, PaO2 100, PCO2 23, HCO3 21***
- After 1 hour of strenuous exercise, this represents **respiratory alkalosis with mild metabolic compensation**, which is the expected finding in a healthy individual during sustained vigorous exercise.
- The **low PCO2 (23 mmHg)** reflects appropriate **hyperventilation** in response to increased metabolic demands and lactic acid production. During intense exercise, minute ventilation increases dramatically, often exceeding the rate of CO2 production.
- The **slightly elevated pH (7.57)** and **mildly decreased HCO3 (21 mEq/L)** indicate that respiratory compensation has slightly overshot, creating mild alkalosis, while the bicarbonate is consumed both in buffering lactate and through renal compensation.
- **Normal PaO2 (100 mmHg)** confirms adequate oxygenation maintained by increased ventilation.
*pH 7.36, PaO2 100, PCO2 40, HCO3 23*
- These are **completely normal arterial blood gas values** with no evidence of any physiological stress or compensation.
- After 1 hour of strenuous exercise, we would expect **hyperventilation with decreased PCO2**, not a normal PCO2 of 40 mmHg. This profile would be consistent with rest, not vigorous exercise.
- The absence of any respiratory or metabolic changes makes this inconsistent with the clinical scenario.
*pH 7.56, PaO2 100, PCO2 44, HCO3 38*
- This profile suggests **metabolic alkalosis** (high pH, high HCO3) with inadequate respiratory compensation (normal to slightly elevated PCO2).
- This is **not consistent with strenuous exercise**, which produces metabolic acid (lactate), not metabolic base. The elevated HCO3 suggests vomiting, diuretic use, or other causes of metabolic alkalosis.
*pH 7.32, PaO2 42, PCO2 50, HCO3 27*
- This indicates **respiratory acidosis** (low pH, high PCO2) with **severe hypoxemia** (PaO2 42 mmHg).
- During strenuous exercise, healthy individuals **increase ventilation** to enhance O2 delivery and remove CO2, so both hypoxemia and hypercapnia are unexpected and would suggest severe cardiopulmonary disease or hypoventilation.
*pH 7.38, PaO2 100, PCO2 69, HCO3 42*
- This demonstrates **compensated respiratory acidosis** (normal pH, markedly elevated PCO2 and HCO3).
- The **very high PCO2 (69 mmHg)** indicates severe **hypoventilation**, which is the opposite of what occurs during exercise. This profile suggests chronic respiratory failure with metabolic compensation, such as in severe COPD.
More Renal regulation of acid-base balance US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.