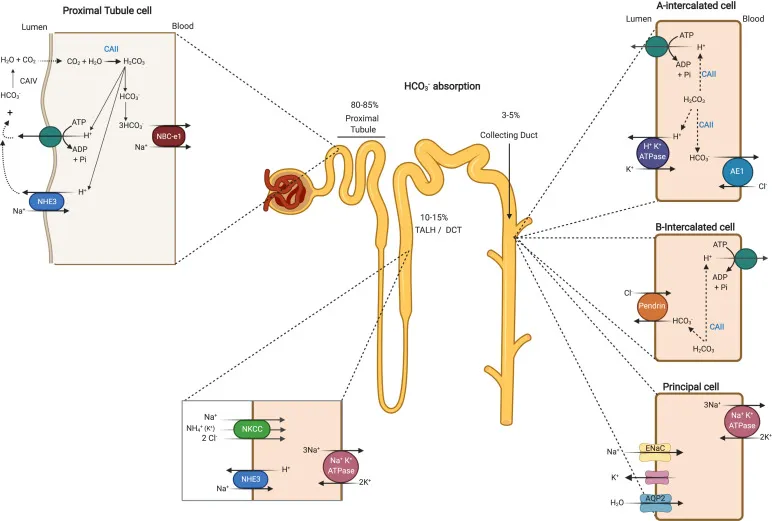
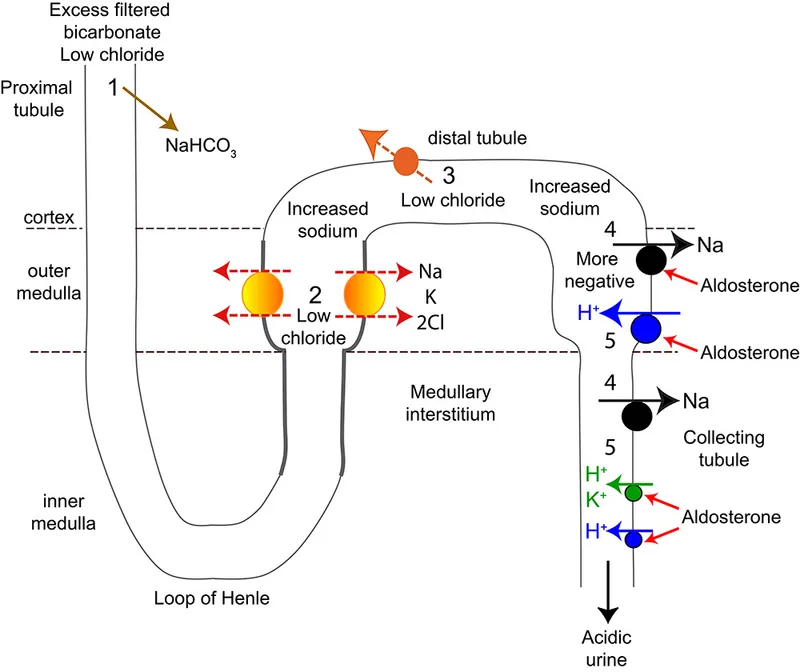
Metabolic alkalosis mechanisms and compensation US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Metabolic alkalosis mechanisms and compensation. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 1: A 52-year-old woman is brought to the emergency department by her husband because of weakness, abdominal pain, and a productive cough for 4 days. She also reports increased urination for the past 2 days. This morning, she had nausea and five episodes of vomiting. She has type 1 diabetes mellitus and hypertension. Current medications include insulin and lisinopril. She admits to have forgotten to take her medication in the last few days. Her temperature is 38.4°C (101.1°F), pulse is 134/min, respirations 31/min, and blood pressure is 95/61 mm Hg. Examination shows dry mucous membranes and decreased skin turgor. Abdominal examination shows diffuse tenderness with no guarding or rebound. Bowel sounds are normal. Laboratory studies show:
Serum
Na+ 139 mEq/L
K+ 5.3 mEq/L
Cl- 106 mEq/L
Glucose 420 mg/dL
Creatinine 1.0 mg/dL
Urine
Blood negative
Glucose 4+
Ketones 3+
Arterial blood gas analysis on room air shows:
pH 7.12
pCO2 17 mm Hg
pO2 86 mm Hg
HCO3- 12 mEq/L
Which of the following is the most likely underlying cause of this patient's increased potassium?
- A. Muscle cell breakdown
- B. Extracellular potassium shift (Correct Answer)
- C. Repeated vomiting
- D. Increased renal potassium absorption
- E. Intracellular potassium shift
Metabolic alkalosis mechanisms and compensation Explanation: ***Extracellular potassium shift***
- The patient's **diabetic ketoacidosis (DKA)** leads to severe **acidosis (pH 7.12)**. In acidosis, hydrogen ions shift into cells, causing potassium to shift out of cells into the extracellular fluid to maintain electroneutrality, leading to **hyperkalemia** [2], [3].
- Additionally, the lack of insulin in DKA impairs the **Na+/K+-ATPase pump**, which normally moves potassium into cells, further contributing to extracellular potassium accumulation [2].
*Muscle cell breakdown*
- While significant muscle cell breakdown (e.g., in rhabdomyolysis) can release intracellular potassium into the circulation, there is no evidence of muscle injury in this patient.
- The primary driver of hyperkalemia in this context is metabolic acidosis and insulin deficiency, not muscle breakdown.
*Repeated vomiting*
- Repeated vomiting typically causes **hypokalemia** due to loss of gastric acid and subsequent renal potassium wasting [1].
- This patient's potassium is elevated, and while she did vomit, it is not the cause of hyperkalemia.
*Increased renal potassium absorption*
- Increased renal potassium absorption is not a typical physiological response to DKA and would usually be seen in conditions causing **hypovolemia with increased aldosterone** or certain **renal tubular acidosis** types, which are not the primary issue here.
- In DKA, the body tries to excrete excess potassium through the kidneys, although this can be impaired by reduced renal perfusion due to dehydration [3].
*Intracellular potassium shift*
- An intracellular potassium shift would lead to **hypokalemia**, not the hyperkalemia observed in this patient.
- Conditions like **alkalosis** or **insulin administration** cause potassium to move into cells.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 2: A 47-year-old man with a history of alcoholism undergoes an upper endoscopy, which reveals a superficial mucosal tear in the distal esophagus. Laboratory results show a metabolic alkalosis. What is the most likely mechanism of the acid/base disturbance in this patient?
- A. Hepatic cirrhosis
- B. Hypokalemia
- C. B12 deficiency
- D. Anemia
- E. Vomiting (Correct Answer)
Metabolic alkalosis mechanisms and compensation Explanation: ***Vomiting***
- The superficial mucosal tear in the distal esophagus ("Mallory-Weiss tear") is strongly associated with **forceful vomiting**, which can lead to significant loss of gastric acid.
- Loss of gastric acid (HCl) through vomiting causes a **metabolic alkalosis** as hydrogen ions are excreted, and the kidneys compensate by retaining bicarbonate.
*Hepatic cirrhosis*
- While common in alcoholics, **hepatic cirrhosis** typically leads to **metabolic acidosis** due to impaired lactate metabolism and renal dysfunction, rather than alkalosis.
- It would not directly explain the acute esophageal tear or the direct cause of metabolic alkalosis seen here.
*Hypokalemia*
- **Hypokalemia** can result from vomiting and can perpetuate metabolic alkalosis, but it is a consequence or a contributing factor, not the primary mechanism of acid-base disturbance.
- The initial loss of acid through vomiting is the direct cause of the alkalosis, which then often leads to compensatory hypokalemia.
*B12 deficiency*
- **B12 deficiency** is common in alcoholics but primarily causes **macrocytic anemia** and neurological symptoms, not metabolic alkalosis or esophageal tears.
- It has no direct physiological link to acid-base balance in a way that would cause metabolic alkalosis.
*Anemia*
- **Anemia** can be caused by chronic alcoholism or blood loss from the esophageal tear, but it does not directly lead to **metabolic alkalosis**.
- While blood loss can have various systemic effects, it does not involve the loss of gastric acid that defines a vomiting-induced alkalosis.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 3: A 24-year-old male is brought in by ambulance to the emergency department after he was found unresponsive at home for an unknown length of time. Upon arrival, he is found to be severely altered and unable to answer questions about his medical history. Based on clinical suspicion, a panel of basic blood tests are obtained including an arterial blood gas, which shows a pH of 7.32, a pCO2 of 70, and a bicarbonate level of 30 mEq/L. Which of the following is most likely the primary disturbance leading to the values found in the ABG?
- A. Respiratory acidosis (Correct Answer)
- B. Metabolic alkalosis
- C. Respiratory alkalosis
- D. Metabolic acidosis
- E. Mixed alkalosis
Metabolic alkalosis mechanisms and compensation Explanation: ***Respiratory acidosis***
- The **pH (7.32)** is acidic (normal 7.35-7.45), and the **pCO2 (70 mmHg)** is significantly elevated (normal 35-45 mmHg), indicating **primary respiratory acidosis** due to hypoventilation.
- The **bicarbonate (30 mEq/L)** is elevated above normal (22-26 mEq/L), indicating **partial metabolic compensation** by the kidneys retaining bicarbonate to buffer the acidosis.
- This pattern suggests **chronic respiratory acidosis** (e.g., from COPD, CNS depression, neuromuscular disease) with renal compensation.
*Metabolic alkalosis*
- This would present with **elevated pH** (>7.45) and **elevated bicarbonate** as the primary disturbance, often with compensatory elevation in pCO2.
- The patient's **pH is acidic (7.32)**, not alkalotic, ruling out metabolic alkalosis as the primary process.
*Respiratory alkalosis*
- This would present with **elevated pH** (>7.45) and **decreased pCO2** (<35 mmHg) due to hyperventilation.
- The patient has the opposite: **acidic pH and elevated pCO2**, ruling out respiratory alkalosis.
*Metabolic acidosis*
- This would present with **decreased pH** and **decreased bicarbonate** (<22 mEq/L) as the primary disturbance.
- While the pH is low, the **bicarbonate is elevated (30 mEq/L)**, not decreased, ruling out metabolic acidosis as the primary disorder.
*Mixed alkalosis*
- A mixed alkalosis would involve simultaneous respiratory and metabolic processes causing **elevated pH**.
- The patient's **pH is acidic (7.32)**, making any form of alkalosis impossible as the primary disturbance.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 4: A 52-year-old man with a history of Type 1 diabetes mellitus presents to the emergency room with increasing fatigue. Two days ago, he ran out of insulin and has not had time to obtain a new prescription. He denies fevers or chills. His temperature is 37.2 degrees Celsius, blood pressure 84/56 mmHg, heart rate 100/min, respiratory rate 20/min, and SpO2 97% on room air. His physical exam is otherwise within normal limits. An arterial blood gas analysis shows the following:
pH 7.25, PCO2 29, PO2 95, HCO3- 15.
Which of the following acid-base disorders is present?
- A. Respiratory alkalosis with appropriate metabolic compensation
- B. Respiratory acidosis with appropriate metabolic compensation
- C. Mixed metabolic and respiratory acidosis
- D. Metabolic acidosis with appropriate respiratory compensation (Correct Answer)
- E. Metabolic alkalosis with appropriate respiratory compensation
Metabolic alkalosis mechanisms and compensation Explanation: ***Metabolic acidosis with appropriate respiratory compensation***
- The patient's pH of 7.25 and HCO3- of 15 indicate **metabolic acidosis**, while the PCO2 of 29 indicates **respiratory compensation**.
- The compensation is **appropriate** as suggested by Winter's formula [Expected PCO2 = (1.5 x HCO3-) + 8 +/- 2; (1.5 x 15) + 8 = 30.5, which is close to 29].
*Respiratory alkalosis with appropriate metabolic compensation*
- This would involve a **pH > 7.45** and **low PCO2** with a secondary drop in HCO3-, which is not seen here.
- The patient's primary problem is a metabolic disturbance due to insulin deficiency.
*Respiratory acidosis with appropriate metabolic compensation*
- This disorder is characterized by a **low pH** and a **high PCO2**, with a secondary rise in HCO3-.
- The patient's PCO2 is low, indicating a compensatory response rather than a primary respiratory acidosis.
*Mixed metabolic and respiratory acidosis*
- A mixed disorder would show a **low pH** due to both **low HCO3-** and **high PCO2**.
- The patient's PCO2 is low, indicating a compensatory response to metabolic acidosis, not an additional respiratory acidosis.
*Metabolic alkalosis with appropriate respiratory compensation*
- This would present with a **high pH (>7.45)** and **high HCO3-**, with compensatory **elevated PCO2**.
- The patient's pH and HCO3- are low, indicating acidosis, not alkalosis.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 5: A 17-year-old boy is brought to the physician by his father because of a 7-month history of fatigue, recurrent leg cramps, and increased urinary frequency. His pulse is 94/min and blood pressure is 118/85 mm Hg. Physical examination shows dry mucous membranes. Laboratory studies show:
Serum
Na+ 130 mEq/L
K+ 2.8 mEq/L
Cl- 92 mEq/L
Mg2+ 1.1 mEq/L
Ca2+ 10.6 mg/dL
Albumin 5.2 g/dL
Urine
Ca2+ 70 mg/24 h
Cl- 375 mEq/24h (N = 110–250)
Arterial blood gas analysis on room air shows a pH of 7.55 and an HCO3- concentration of 45 mEq/L. Impaired function of which of the following structures is the most likely cause of this patient's condition?
- A. Ascending loop of Henle
- B. Collecting duct
- C. Distal convoluted tubule (Correct Answer)
- D. Descending loop of Henle
- E. Proximal convoluted tubule
Metabolic alkalosis mechanisms and compensation Explanation: ***Distal convoluted tubule***
- The patient presents with **hypokalemia**, **metabolic alkalosis**, **hypomagnesemia**, and **hypocalciuria** (24-hour urine Ca2+ 70 mg, normal up to 250 mg), which are characteristic findings of **Gitelman syndrome**.
- **Gitelman syndrome** is caused by a loss-of-function mutation in the **thiazide-sensitive Na-Cl cotransporter (NCC)**, located in the **distal convoluted tubule**, leading to impaired reabsorption of Na+ and Cl- at this segment.
*Ascending loop of Henle*
- Impaired function of the **Na-K-2Cl cotransporter (NKCC2)** in the **thick ascending limb of the loop of Henle** causes **Bartter syndrome**.
- Bartter syndrome typically presents with **hypercalciuria**, in contrast to the hypocalciuria seen in this patient.
*Collecting duct*
- Dysfunction of the **collecting duct** can lead to various conditions, such as **renal tubular acidosis** or **diabetes insipidus**, depending on which channels or receptors are affected.
- However, the specific combination of **hypokalemia**, **metabolic alkalosis**, **hypomagnesemia**, and **hypocalciuria** points away from primary collecting duct dysfunction.
*Descending loop of Henle*
- The **descending loop of Henle** is primarily permeable to **water** and has a limited role in electrolyte reabsorption.
- Impairment here would primarily affect **urine concentration** and dilution but would not account for the specific electrolyte imbalances observed.
*Proximal convoluted tubule*
- The **proximal convoluted tubule** is responsible for reabsorbing a large fraction of filtered electrolytes, glucose, and amino acids.
- Dysfunction here (e.g., **Fanconi syndrome**) would typically present with **generalized aminoaciduria**, **glycosuria**, **phosphaturia**, and **proximal renal tubular acidosis**, which are not seen in this patient.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 6: A 54-year-old man presents with 3 days of non-bloody and non-bilious emesis every time he eats or drinks. He has become progressively weaker and the emesis has not improved. He denies diarrhea, fever, or chills and thinks his symptoms may be related to a recent event that involved sampling many different foods. His temperature is 97.5°F (36.4°C), blood pressure is 133/82 mmHg, pulse is 105/min, respirations are 15/min, and oxygen saturation is 98% on room air. Physical exam is notable for a weak appearing man with dry mucous membranes. His abdomen is nontender. Which of the following laboratory changes would most likely be seen in this patient?
- A. Metabolic alkalosis and hyperkalemia
- B. Non-anion gap metabolic acidosis and hypokalemia
- C. Respiratory acidosis and hyperkalemia
- D. Metabolic alkalosis and hypokalemia (Correct Answer)
- E. Anion gap metabolic acidosis and hypokalemia
Metabolic alkalosis mechanisms and compensation Explanation: ***Metabolic alkalosis and hypokalemia***
- Persistent **vomiting** leads to the loss of **gastric acid** (HCl) and **potassium**, resulting in **metabolic alkalosis** and **hypokalemia**. The loss of HCl directly removes acid from the body, and the subsequent renal compensation to conserve volume often exacerbates potassium loss.
- The patient's presentation with **dry mucous membranes**, increased heart rate (pulse 105/min), and persistent non-bloody, non-bilious emesis suggests significant volume depletion and electrolyte imbalances consistent with prolonged vomiting.
*Metabolic alkalosis and hyperkalemia*
- While metabolic alkalosis is expected due to gastric acid loss from vomiting, **hyperkalemia** is unlikely. Vomiting typically causes **hypokalemia** due to direct potassium loss and renal compensation mechanisms.
- The body attempts to compensate for volume depletion, leading to increased activity of the **renin-angiotensin-aldosterone system**, which promotes potassium excretion in the urine.
*Non-anion gap metabolic acidosis and hypokalemia*
- **Metabolic acidosis** is characterized by a decrease in blood pH and bicarbonate; however, profuse vomiting of gastric contents primarily leads to **alkalosis** due to the loss of hydrogen ions.
- **Non-anion gap metabolic acidosis** is usually seen in conditions involving bicarbonate loss from the kidneys or gut (e.g., diarrhea, renal tubular acidosis), not vomiting.
*Respiratory acidosis and hyperkalemia*
- **Respiratory acidosis** results from hypoventilation, leading to an increase in blood CO2, which is not suggested by the patient's normal respiratory rate and oxygen saturation.
- Profuse vomiting causes a loss of gastric acid and can lead to compensatory **hypoventilation** to retain CO2 (acid), but this is a secondary response to metabolic alkalosis, and primary respiratory acidosis is not the underlying issue.
*Anion gap metabolic acidosis and hypokalemia*
- **Anion gap metabolic acidosis** typically occurs with the accumulation of unmeasured acids (e.g., lactic acidosis, ketoacidosis, renal failure, poisoning), which is not indicated by the patient's symptoms.
- While **hypokalemia** is consistent with vomiting, the primary acid-base disturbance from prolonged emesis is metabolic alkalosis, not acidosis.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 7: A 57-year-old woman comes to the emergency department because of dizziness, nausea, and vomiting for 4 days. Her temperature is 37.3°C (99.1°F), pulse is 100/min, respirations are 20/min, and blood pressure is 110/70 mm Hg. Physical examination shows no abnormalities. Arterial blood gas analysis on room air shows:
pH 7.58
PCO2 43 mm Hg
PO2 96 mm Hg
HCO3- 32 mEq/L
The most appropriate next step in diagnosis is measurement of which of the following?
- A. Serum anion gap
- B. Urine albumin to creatinine ratio
- C. Serum osmolal gap
- D. Urine anion gap
- E. Urine chloride (Correct Answer)
Metabolic alkalosis mechanisms and compensation Explanation: ***Urine chloride***
- The patient presents with **metabolic alkalosis** (pH 7.58, HCO3- 32 mEq/L with minimal respiratory compensation).
- **Urine chloride** is the key diagnostic test to differentiate between **saline-responsive** (urine Cl <20 mEq/L) and **saline-unresponsive** (urine Cl >20 mEq/L) metabolic alkalosis.
- Given the patient's **4-day history of vomiting**, this is likely saline-responsive alkalosis from gastric HCl loss, which would be confirmed by low urine chloride and guide appropriate treatment with saline repletion.
*Serum anion gap*
- The **serum anion gap** is primarily used to evaluate causes of **metabolic acidosis** (differentiating high AG from normal AG acidosis).
- It would not provide useful information for determining the etiology of metabolic alkalosis.
*Urine albumin to creatinine ratio*
- The **urine albumin to creatinine ratio** screens for **kidney damage** or **proteinuria**.
- There is no clinical indication (e.g., elevated creatinine, edema, hypertension) to suggest kidney disease as the cause of her acid-base imbalance.
*Serum osmolal gap*
- The **serum osmolal gap** detects **exogenous osmotically active substances** like toxic alcohols (methanol, ethylene glycol) or mannitol.
- These typically cause **high anion gap metabolic acidosis**, not metabolic alkalosis, making this test inappropriate for this patient.
*Urine anion gap*
- The **urine anion gap** differentiates causes of **normal anion gap metabolic acidosis** by assessing urinary ammonium excretion (positive in RTA, negative in GI losses).
- It is not indicated for the evaluation of metabolic alkalosis.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 8: A 58-year-old man presents to the emergency department with a chief complaint of ringing in his ears that started several hours previously that has progressed to confusion. The patient denies any history of medical problems except for bilateral knee arthritis. He was recently seen by an orthopedic surgeon to evaluate his bilateral knee arthritis but has opted to not undergo knee replacement and prefers medical management. His wife noted that prior to them going on a hike today, he seemed confused and not himself. They decided to stay home, and roughly 14 hours later, he was no longer making any sense. Physical exam is notable for a confused man. The patient's vitals are being performed and his labs are being drawn. Which of the following is most likely to be seen on blood gas analysis?
- A. pH: 7.30, PaCO2: 15 mmHg, HCO3-: 16 mEq/L (Correct Answer)
- B. pH: 7.37, PaCO2: 41 mmHg, HCO3-: 12 mEq/L
- C. pH: 7.41, PaCO2: 65 mmHg, HCO3-: 34 mEq/L
- D. pH: 7.47, PaCO2: 11 mmHg, HCO3-: 24 mEq/L
- E. pH: 7.31, PaCO2: 31 mmHg, HCO3-: 15 mEq/L
Metabolic alkalosis mechanisms and compensation Explanation: ***pH: 7.30, PaCO2: 15 mmHg, HCO3-: 16 mEq/L***
- This blood gas analysis shows a **low pH** (acidemia), **low PaCO2** (hypocapnia), and **low HCO3-** (bicarbonate). This pattern is consistent with a **primary metabolic acidosis** with a **compensatory respiratory alkalosis**.
- In this clinical scenario, the patient likely has **salicylate toxicity** (aspirin poisoning). Salicylate toxicity initially causes respiratory alkalosis due to direct stimulation of the respiratory center, followed by a high anion gap metabolic acidosis as salicylates interfere with cellular metabolism. This specific ABG reflects a mixed disorder where metabolic acidosis is predominant and respiratory compensation is attempting to raise the pH. The **tinnitus** and **confusion** are classic symptoms of salicylate toxicity.
*pH: 7.37, PaCO2: 41 mmHg, HCO3-: 12 mEq/L*
- This blood gas shows a **normal pH**, **normal PaCO2**, and **low HCO3-**. This suggests a **compensated metabolic acidosis**, where the body has fully compensated to bring the pH back to normal.
- While the patient likely has metabolic acidosis from salicylate toxicity, full compensation to a normal pH is less characteristic of an acute, severe presentation with significant neurological symptoms.
*pH: 7.41, PaCO2: 65 mmHg, HCO3-: 34 mEq/L*
- This blood gas shows a **normal pH**, **high PaCO2**, and **high HCO3-**. This indicates a **compensated respiratory acidosis**, where the kidneys have compensated for chronic CO2 retention.
- This pattern is not consistent with salicylate toxicity, which typically causes **respiratory alkalosis** early on, and later **metabolic acidosis**.
*pH: 7.47, PaCO2: 11 mmHg, HCO3-: 24 mEq/L*
- This blood gas analysis shows a **high pH** (alkalemia), **very low PaCO2** (severe hypocapnia), and a **normal HCO3-**. This indicates a **primary respiratory alkalosis** with no significant metabolic compensation.
- While salicylate toxicity can cause respiratory alkalosis, severe confusion and the progression of symptoms suggest a more advanced stage, usually involving a metabolic acidosis component, making a pure, uncompensated respiratory alkalosis less likely.
*pH: 7.31, PaCO2: 31 mmHg, HCO3-: 15 mEq/L*
- This blood gas shows a **low pH**, **low PaCO2**, and **low HCO3-**. This also indicates a **metabolic acidosis** with **respiratory compensation**.
- However, compared to pH 7.30, PaCO2 15 mmHg, and HCO3- 16 mEq/L, this option shows slightly **less severe respiratory compensation** (PaCO2 is higher), which is less typical for the profound respiratory stimulation seen in severe salicylate poisoning. The chosen correct option demonstrates a more characteristic and maximal respiratory compensation for the degree of metabolic acidosis.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 9: Two hours after undergoing laparoscopic roux-en-Y gastric bypass surgery, a 44-year-old man complains of pain in the site of surgery and nausea. He has vomited twice in the past hour. He has hypertension, type 2 diabetes mellitus, and hypercholesterolemia. Current medications include insulin, atorvastatin, hydrochlorothiazide, acetaminophen, and prophylactic subcutaneous heparin. He drinks two to three beers daily and occasionally more on weekends. He is 177 cm (5 ft 10 in) tall and weighs 130 kg (286 lb); BMI is 41.5 kg/m2. His temperature is 37.3°C (99.1°F), pulse is 103/min, and blood pressure is 122/82 mm Hg. Examination shows five laparoscopic incisions with no erythema or discharge. The abdomen is soft and non-distended. There is slight diffuse tenderness to palpation. Bowel sounds are reduced. Laboratory studies show:
Hematocrit 45%
Serum
Na+ 136 mEq/L
K+ 3.5 mEq/L
Cl- 98 mEq/L
Urea nitrogen 31 mg/dL
Glucose 88 mg/dL
Creatinine 1.1 mg/dL
Arterial blood gas analysis on room air shows:
pH 7.28
pCO2 32 mm Hg
pO2 74 mm Hg
HCO3- 14.4 mEq/L
Which of the following is the most likely cause for the acid-base status of this patient?
- A. Hypoxia (Correct Answer)
- B. Uremia
- C. Late dumping syndrome
- D. Vomiting
- E. Early dumping syndrome
Metabolic alkalosis mechanisms and compensation Explanation: ***Hypoxia***
- The patient exhibits **metabolic acidosis** (pH 7.28, HCO3- 14.4 mEq/L) with **appropriate respiratory compensation** (pCO2 32 mm Hg using Winter's formula: expected pCO2 = 1.5 × 14.4 + 8 ± 2 = 29.6 ± 2).
- The **pO2 of 74 mm Hg is significantly low** (normal range on room air: 80-100 mm Hg), indicating **hypoxemia** that leads to **tissue hypoxia** and **anaerobic metabolism**.
- In the setting of **obesity (BMI 41.5)** and **immediate post-operative status** after laparoscopic surgery, multiple factors contribute to hypoxemia including **atelectasis, reduced functional residual capacity, pain limiting deep breathing, residual anesthetic effects, and pneumoperitoneum effects**.
- Tissue hypoxia results in **lactic acidosis** (a high anion gap metabolic acidosis), which explains the acid-base disturbance. The **elevated BUN (31 mg/dL) with relatively normal creatinine** suggests prerenal azotemia from hypoperfusion, further supporting inadequate tissue oxygenation.
- The **tachycardia (103/min)** represents a compensatory response to improve oxygen delivery to hypoxic tissues.
*Vomiting*
- Vomiting causes loss of **gastric HCl**, resulting in **hypochloremic metabolic ALKALOSIS** (elevated pH and HCO3-), not acidosis.
- While this patient is vomiting, the acid-base status shows **acidosis**, which is the opposite of what vomiting typically causes.
- The low **Cl- (98 mEq/L)** is consistent with some gastric acid loss, but the dominant acid-base disorder is metabolic acidosis from another cause.
*Uremia*
- **Uremia** causes high anion gap metabolic acidosis due to retention of organic acids and phosphates in renal failure.
- While the **BUN is elevated (31 mg/dL)**, the **creatinine (1.1 mg/dL) is essentially normal**, especially for a patient with high muscle mass (130 kg).
- The BUN elevation is more consistent with **prerenal azotemia** (dehydration/hypoperfusion) rather than intrinsic renal failure causing uremic acidosis.
*Late dumping syndrome*
- **Late dumping syndrome** occurs **1-3 hours after eating** and results from rapid carbohydrate absorption causing hyperinsulinemia and subsequent **reactive hypoglycemia**.
- This patient's **glucose is normal (88 mg/dL)**, and symptoms began only **2 hours post-surgery** in the fasting state, not after a meal.
- Late dumping does not cause metabolic acidosis.
*Early dumping syndrome*
- **Early dumping syndrome** occurs **10-30 minutes after eating** due to rapid gastric emptying of hyperosmolar contents into the small intestine, causing fluid shifts.
- Symptoms include **cramping, diarrhea, vasomotor symptoms (flushing, palpitations, dizziness)**, not metabolic acidosis.
- This patient has not yet eaten post-operatively, making dumping syndrome impossible.
Metabolic alkalosis mechanisms and compensation US Medical PG Question 10: A 30-year-old man is brought to the emergency room by ambulance after being found unconscious in his car parked in his garage with the engine running. His wife arrives and reveals that his past medical history is significant for severe depression treated with fluoxetine. He is now disoriented to person, place, and time. His temperature is 37.8 deg C (100.0 deg F), blood pressure is 100/50 mmHg, heart rate is 100/min, respiratory rate is 10/min, and SaO2 is 100%. On physical exam, there is no evidence of burn wounds. He has moist mucous membranes and no abnormalities on cardiac and pulmonary auscultation. His respirations are slow but spontaneous. His capillary refill time is 4 seconds. He is started on 100% supplemental oxygen by non-rebreather mask. His preliminary laboratory results are as follows:
Arterial blood pH 7.20, PaO2 102 mm Hg, PaCO2 23 mm Hg, HCO3 10 mm Hg, WBC count 9.2/µL, Hb 14 mg/dL, platelets 200,000/µL, sodium 137 mEq/L, potassium 5.0 mEq/L, chloride 96 mEq/L, BUN 28 mg/dL, creatinine 1.0 mg/dL, and glucose 120 mg/dL. Which of the following is the cause of this patient's acid-base abnormality?
- A. Decreased oxygen delivery to tissues (Correct Answer)
- B. Decreased ability for the tissues to use oxygen
- C. Increased anions from toxic ingestion
- D. Increased metabolic rate
- E. Decreased minute ventilation
Metabolic alkalosis mechanisms and compensation Explanation: ***Decreased oxygen delivery to tissues***
- The patient's presentation in a running car in a garage suggests **carbon monoxide (CO) poisoning**. CO binds to hemoglobin with higher affinity than oxygen, forming **carboxyhemoglobin (COHb)**, which impairs oxygen delivery to tissues despite normal PaO2.
- The **metabolic acidosis (pH 7.20, HCO3 10)** with an elevated anion gap (Na - (Cl + HCO3) = 137 - (96 + 10) = 31) and altered mental status are consistent with widespread tissue hypoxia due to decreased oxygen delivery, leading to **lactic acid accumulation**.
*Decreased ability for the tissues to use oxygen*
- This scenario typically occurs in conditions like **cyanide poisoning**, where cellular metabolism is inhibited, preventing oxygen utilization despite adequate delivery.
- Cyanide poisoning often presents with a narrower or normal anion gap metabolic acidosis and a **"cherry red" skin color**, which are not specifically noted here.
*Increased anions from toxic ingestion*
- While there is an **elevated anion gap metabolic acidosis**, merely stating "increased anions from toxic ingestion" is less precise than identifying the underlying mechanism of oxygen deprivation.
- Many toxins can cause an elevated anion gap, but the specific context of **CO poisoning** points to tissue hypoxia as the primary driver of acidosis, not just the presence of other toxic anions.
*Increased metabolic rate*
- An increased metabolic rate, as seen in conditions like **sepsis** or hyperthyroidism, can lead to increased acid production and metabolic acidosis.
- However, in this case, the **depressed respiratory rate** and context of CO exposure point away from a primary state of hypermetabolism.
*Decreased minute ventilation*
- **Decreased minute ventilation** would primarily lead to **respiratory acidosis** (elevated PaCO2) due to CO2 retention.
- The patient's lab results show a **low PaCO2 (23 mmHg)**, indicating respiratory compensation for a metabolic acidosis, not a primary respiratory problem.
More Metabolic alkalosis mechanisms and compensation US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.