Acid-base balance

On this page
🧪 The pH Command Center: Mastering Acid-Base Homeostasis
Your body defends a razor-thin pH range every second-deviate by just 0.3 units and consciousness fades, enzymes fail, and cardiac rhythms collapse. You'll master how lungs and kidneys orchestrate this defense through CO₂ elimination and bicarbonate reclamation, then decode arterial blood gases to distinguish respiratory from metabolic derangements. By integrating pattern recognition with evidence-based interventions, you'll transform complex acid-base disorders into systematic clinical decisions that save lives.
The body maintains acid-base homeostasis through three interconnected systems working in precise coordination:
-
Buffer Systems (immediate response: seconds)
- Bicarbonate system: 75% of total buffering capacity
- Phosphate system: 10% intracellular buffering
- Protein system: 15% hemoglobin and plasma proteins
- Hemoglobin: 6x more buffering capacity than plasma proteins
- Intracellular proteins: handle 60% of metabolic acid load
-
Respiratory System (rapid response: 1-3 minutes)
- CO₂ elimination adjusts within 10-15 breaths
- Maximum compensation: 12-24 hours
- Ventilation changes: 4-fold increase possible
-
Renal System (complete response: 3-5 days)
- Bicarbonate reabsorption: 99.9% efficiency
- Acid excretion: 50-100 mEq/day
- New bicarbonate generation: 1 mEq/kg/day
📌 Remember: BAR - Bicarbonate (immediate), Alveolar (rapid), Renal (complete) - The three-tier defense system with escalating timeframes
| System | Response Time | Mechanism | Capacity | Duration |
|---|---|---|---|---|
| Bicarbonate Buffer | Seconds | H⁺ + HCO₃⁻ ↔ H₂CO₃ | 75% total | Continuous |
| Respiratory | 1-3 minutes | CO₂ elimination | 4-fold ↑ ventilation | 12-24 hours |
| Renal | Hours-days | HCO₃⁻ reabsorption/generation | Unlimited | Permanent |
| Phosphate | Immediate | HPO₄²⁻ + H⁺ ↔ H₂PO₄⁻ | 10% total | Continuous |
| Protein | Immediate | Histidine residues | 15% total | Continuous |
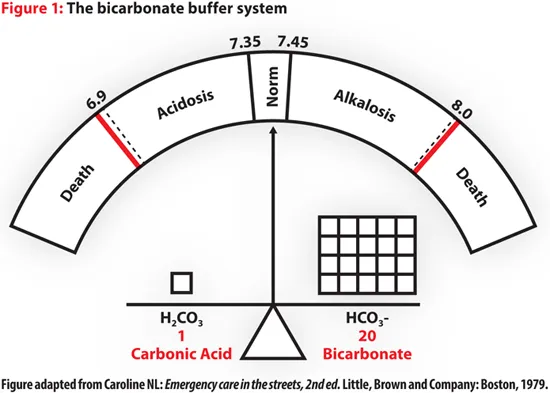
The Henderson-Hasselbalch equation provides the mathematical foundation for acid-base analysis:
$$pH = 6.1 + \log\frac{[HCO_3^-]}{0.03 \times PCO_2}$$
This equation reveals the 20:1 ratio of bicarbonate to dissolved CO₂ that maintains normal pH. When this ratio shifts, compensatory mechanisms activate to restore balance.
💡 Master This: The 20:1 rule - Normal bicarbonate (24 mEq/L) to dissolved CO₂ (1.2 mEq/L) ratio maintains pH 7.40. Any deviation triggers immediate buffer activation followed by respiratory and renal compensation.
📌 Remember: ROME - Respiratory Opposite, Metabolic Equal - pH and primary disorder move in opposite directions for respiratory disorders, same direction for metabolic disorders.
Connect these buffer fundamentals through respiratory control mechanisms to understand how ventilation provides the first line of active compensation.
🧪 The pH Command Center: Mastering Acid-Base Homeostasis
⚡ The Respiratory Engine: CO₂ Control and Ventilatory Response
Central chemoreceptors in the medulla oblongata provide 80% of ventilatory drive under normal conditions:
- Location: Ventrolateral medulla, 200-400 μm below surface
- Primary stimulus: CSF pH (reflects brain tissue pH)
- Response time: 1-3 minutes for full activation
- Sensitivity: 10-fold greater than peripheral chemoreceptors for CO₂
- CSF buffering: Limited (1/6 of plasma buffering capacity)
Peripheral chemoreceptors in the carotid and aortic bodies respond to multiple stimuli:
- Hypoxemia: PO₂ < 60 mmHg (steep response curve)
- Acidemia: pH < 7.35 (linear response)
- Hypercapnia: PCO₂ > 45 mmHg (synergistic with central)
- Response time: 10-15 seconds (faster than central)
⭐ Clinical Pearl: Chronic hypercapnia (PCO₂ > 50 mmHg for >24 hours) blunts central chemoreceptor sensitivity by 60-80%, shifting ventilatory drive to peripheral hypoxic stimulus. This explains why high-flow oxygen can suppress breathing in COPD patients.
| Stimulus | Receptor | Response Time | Threshold | Maximum Response |
|---|---|---|---|---|
| ↑ PCO₂ | Central | 1-3 minutes | 2-3 mmHg ↑ | 10-fold ↑ ventilation |
| ↓ pH | Peripheral | 10-15 seconds | pH < 7.35 | 5-fold ↑ ventilation |
| ↓ PO₂ | Peripheral | 10-15 seconds | PO₂ < 60 mmHg | 3-fold ↑ ventilation |
| ↑ K⁺ | Peripheral | 30 seconds | K⁺ > 5.5 mEq/L | 2-fold ↑ ventilation |
| Hypotension | Peripheral | 5-10 seconds | MAP < 80 mmHg | Variable |
-
Metabolic Acidosis: Expected PCO₂ = 1.5 × [HCO₃⁻] + 8 ± 2
- Winter's formula provides ±2 mmHg accuracy in 90% of cases
- Maximum compensation: PCO₂ 10-15 mmHg (respiratory muscle fatigue limit)
- Time to maximum: 12-24 hours
-
Metabolic Alkalosis: Expected PCO₂ = 0.7 × [HCO₃⁻] + 21 ± 2
- Limited compensation due to hypoxic drive
- Maximum PCO₂: 55-60 mmHg (hypoxemia threshold)
- Compensation degree: 40-60% of expected
💡 Master This: Respiratory compensation is never complete - pH never fully normalizes through ventilatory changes alone. If pH is normal with abnormal HCO₃⁻ and PCO₂, suspect mixed disorder or laboratory error.
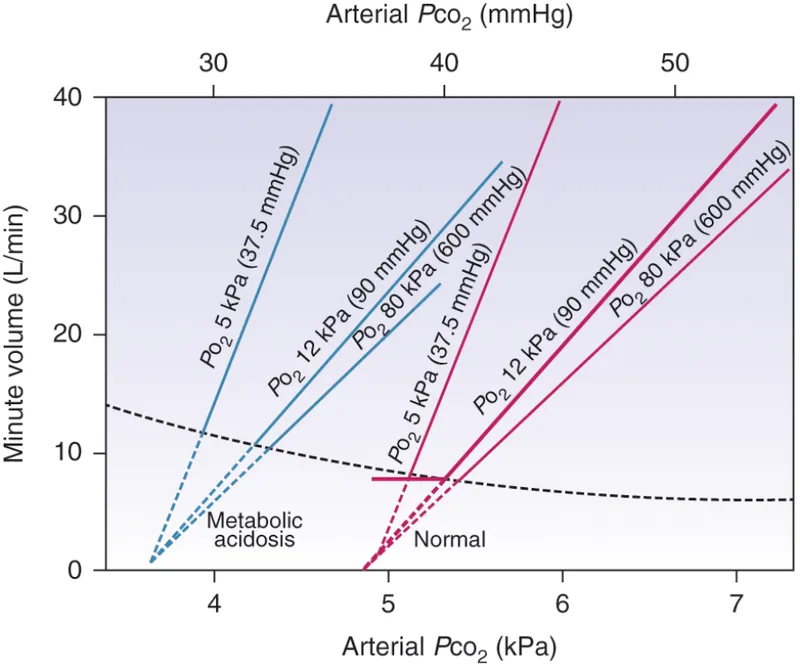
📌 Remember: CHAMPS - Central (CO₂), Hypoxic (peripheral), Acidosis (peripheral), Metabolic (both), Potassium (peripheral), Shock (peripheral) - The six major ventilatory stimuli and their receptor locations.
Connect respiratory control through renal mechanisms to understand how the kidneys provide definitive acid-base correction over days to weeks.
⚡ The Respiratory Engine: CO₂ Control and Ventilatory Response
🏭 The Renal Factory: Bicarbonate Processing and Acid Excretion
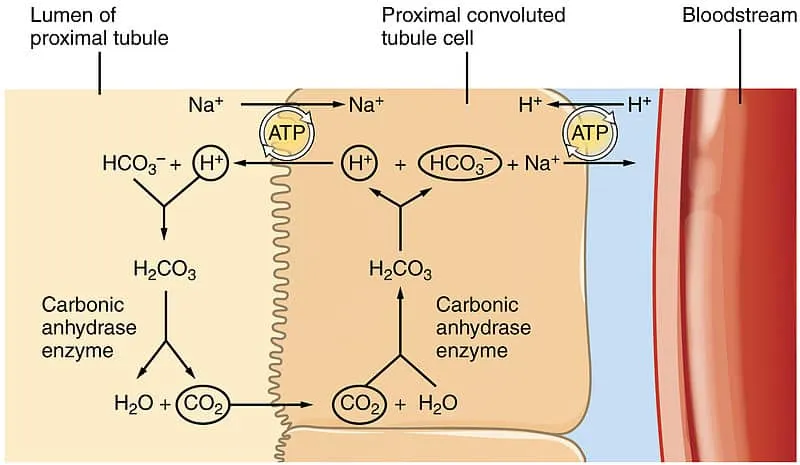
The kidneys handle acid-base balance through three integrated processes:
-
Bicarbonate Reabsorption (85% proximal tubule, 10% thick ascending limb, 5% collecting duct)
- Normal filtered load: 4,320 mEq/day (180 L × 24 mEq/L)
- Reabsorption efficiency: 99.9% (only 1-2 mEq lost daily)
- Rate-limiting step: H⁺-ATPase pump capacity
- Carbonic anhydrase dependency: 95% of reabsorption
-
Titratable Acid Excretion (20-40 mEq/day)
- Primary buffer: HPO₄²⁻ (pKa = 6.8)
- Urine pH range: 4.5-8.0 (normal 5.5-6.5)
- Maximum capacity: 100-150 mEq/day
- Phosphate dependency: limited by filtered phosphate load
-
Ammonia Production and Excretion (30-60 mEq/day, expandable to 300+ mEq/day)
- Glutamine metabolism in proximal tubule
- NH₃ diffusion and NH₄⁺ trapping in collecting duct
- pH-sensitive regulation: 10-fold increase possible
- Primary mechanism for new bicarbonate generation
⭐ Clinical Pearl: Net acid excretion = (NH₄⁺ + Titratable Acid) - HCO₃⁻ in urine. Normal value is 50-100 mEq/day, matching endogenous acid production. Values <20 mEq/day suggest renal tubular acidosis.
| Tubular Segment | HCO₃⁻ Reabsorption | Mechanism | Regulation | Capacity |
|---|---|---|---|---|
| Proximal (S1-S3) | 85% (3,600 mEq/day) | Na⁺/H⁺ exchanger + CA | Volume, K⁺, Ang II | High |
| Thick Ascending | 10% (430 mEq/day) | Na⁺-K⁺-2Cl⁻ + CA | ADH, prostaglandins | Moderate |
| Collecting Duct | 5% (220 mEq/day) | H⁺-ATPase, H⁺-K⁺-ATPase | Aldosterone, pH | Variable |
| Intercalated Cells | Variable | Type A (acid) vs Type B (base) | Acid-base status | Adaptive |
-
Metabolic Acidosis Response:
- Immediate: ↑ H⁺ secretion, ↑ NH₄⁺ production
- 6-12 hours: ↑ glutaminase activity (2-3 fold)
- 2-3 days: ↑ ammonia excretion (5-10 fold)
- 5-7 days: maximum adaptation (300+ mEq/day acid excretion)
-
Metabolic Alkalosis Response:
- ↓ H⁺ secretion, ↓ NH₄⁺ production
- Type B intercalated cell activation
- HCO₃⁻ wasting (if volume replete)
- Limited by chloride and potassium depletion
💡 Master This: Renal compensation requires adequate GFR (>30 mL/min), normal tubular function, and appropriate electrolyte balance. Chronic kidney disease limits compensation when GFR falls below 30 mL/min/1.73m².
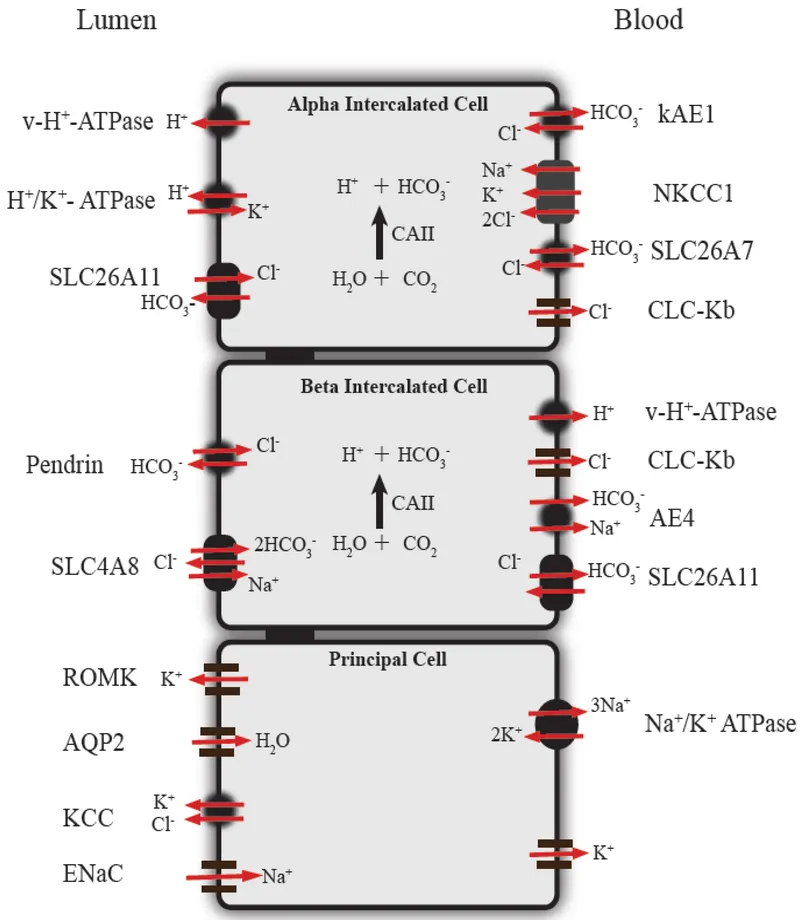
📌 Remember: HARDEST - H⁺ secretion, Ammonia production, Reabsorption of HCO₃⁻, Distal acidification, Electrolyte balance, Sodium retention, Titratable acid - The seven renal mechanisms for acid-base control.
Connect renal processing through pattern recognition frameworks to understand how clinical presentations reveal underlying acid-base disorders.
🏭 The Renal Factory: Bicarbonate Processing and Acid Excretion
🎯 The Diagnostic Matrix: ABG Pattern Recognition and Clinical Correlation
The 5-Step ABG Analysis provides systematic pattern recognition:
-
Step 1: pH Assessment (Normal 7.35-7.45)
- Acidemia: pH < 7.35
- Alkalemia: pH > 7.45
- Normal pH with abnormal HCO₃⁻/PCO₂ = mixed disorder
-
Step 2: Primary Disorder Identification
- Metabolic: HCO₃⁻ abnormal in same direction as pH
- Respiratory: PCO₂ abnormal in opposite direction to pH
- Mixed: Both HCO₃⁻ and PCO₂ abnormal
-
Step 3: Compensation Assessment
- Respiratory compensation: immediate to 24 hours
- Renal compensation: 2-5 days for completion
- Never complete (pH never fully normal)
-
Step 4: Anion Gap Calculation (if metabolic acidosis)
- Normal: 8-12 mEq/L (Na⁺ - [Cl⁻ + HCO₃⁻])
- High anion gap: >12 mEq/L
- Delta-delta ratio: Δ anion gap / Δ HCO₃⁻
-
Step 5: Clinical Correlation
- History and physical examination
- Electrolyte abnormalities
- Underlying disease processes
⭐ Clinical Pearl: Expected compensation formulas predict normal physiologic response. Deviations >±2 from predicted values suggest mixed disorders or inadequate compensation time.
| Primary Disorder | Expected Compensation | Time Frame | Limits |
|---|---|---|---|
| Metabolic Acidosis | PCO₂ = 1.5 × [HCO₃⁻] + 8 ± 2 | 12-24 hours | PCO₂ ≥ 10 mmHg |
| Metabolic Alkalosis | PCO₂ = 0.7 × [HCO₃⁻] + 21 ± 2 | 12-24 hours | PCO₂ ≤ 55 mmHg |
| Acute Respiratory Acidosis | ↑ HCO₃⁻ by 1 per 10 ↑ PCO₂ | Minutes | Limited buffering |
| Chronic Respiratory Acidosis | ↑ HCO₃⁻ by 3.5 per 10 ↑ PCO₂ | 3-5 days | HCO₃⁻ ≤ 45 mEq/L |
| Acute Respiratory Alkalosis | ↓ HCO₃⁻ by 2 per 10 ↓ PCO₂ | Minutes | Limited buffering |
| Chronic Respiratory Alkalosis | ↓ HCO₃⁻ by 5 per 10 ↓ PCO₂ | 3-5 days | HCO₃⁻ ≥ 12 mEq/L |
- Methanol (formic acid accumulation)
- Uremia (organic acids, phosphates)
- Diabetic ketoacidosis (β-hydroxybutyrate, acetoacetate)
- Propylene glycol, Paracetamol
- Iron, Isoniazid
- Lactic acidosis (Type A: hypoxia, Type B: metformin)
- Ethylene glycol (oxalic acid)
- Salicylates (uncoupling oxidative phosphorylation)
💡 Master This: Delta-delta ratio = (Anion gap - 12) / (24 - HCO₃⁻). Normal ratio 1.0-2.0 suggests pure high anion gap acidosis. Ratio <1 suggests concurrent normal anion gap acidosis; ratio >2 suggests concurrent metabolic alkalosis.
Normal anion gap metabolic acidosis uses HARDUPS mnemonic:
- Hyperalimentation, Hyperchloremic
- Acetazolamide, Addison's disease
- Renal tubular acidosis, Recovery from ketoacidosis
- Diarrhea, Diuretics (carbonic anhydrase inhibitors)
- Ureteral diversions (ureterosigmoidostomy)
- Posthypocapnia, Paracetamol (late)
- Saline administration (dilutional)
📌 Remember: ROME WASN'T BUILT - Respiratory Opposite, Metabolic Equal, Winter's formula Acidosis, Saline Normal gap, Time for compensation Builds Up In Lung and kidney Tissues - Complete ABG interpretation framework.
Connect pattern recognition through treatment algorithms to understand evidence-based management approaches for each acid-base disorder.
🎯 The Diagnostic Matrix: ABG Pattern Recognition and Clinical Correlation
⚖️ The Treatment Command Center: Evidence-Based Management Algorithms
Metabolic Acidosis Management prioritizes cause-specific treatment:
-
Severe Acidosis (pH < 7.1, HCO₃⁻ < 8 mEq/L)
- Bicarbonate therapy: 1-2 mEq/kg IV over 1-2 hours
- Target pH: 7.2-7.25 (not normal)
- Monitor for overcorrection: check ABG every 30-60 minutes
- Complications: paradoxical CSF acidosis, hypokalemia, volume overload
-
Diabetic Ketoacidosis (DKA)
- Insulin therapy: 0.1 units/kg/hour IV
- Fluid resuscitation: 15-20 mL/kg first hour
- Avoid bicarbonate unless pH < 7.0
- Monitor glucose drop: 50-75 mg/dL/hour
- Resolution criteria: anion gap < 12 mEq/L, HCO₃⁻ > 15 mEq/L
-
Lactic Acidosis
- Address underlying hypoperfusion
- Avoid bicarbonate (may worsen intracellular acidosis)
- Lactate clearance: >10% per hour indicates improvement
- Consider dialysis if pH < 7.1 and refractory
⭐ Clinical Pearl: Bicarbonate therapy in DKA may paradoxically worsen cerebral acidosis and delay ketone clearance. Reserve for pH < 7.0 or severe hyperkalemia (K⁺ > 6.5 mEq/L).
| Condition | First-Line Treatment | Target Parameters | Monitoring | Success Rate |
|---|---|---|---|---|
| DKA | Insulin + fluids | Glucose 150-250 mg/dL | AG, pH q2-4h | 95-98% |
| Lactic Acidosis | Treat underlying cause | Lactate clearance >10%/h | Lactate q1-2h | 60-80% |
| Renal Failure | Dialysis | HCO₃⁻ 18-22 mEq/L | Pre/post ABG | 90-95% |
| Diarrhea | Fluid/electrolyte replacement | Normal AG, HCO₃⁻ >15 | Electrolytes q6h | 85-90% |
| Salicylate | Alkaline diuresis/dialysis | Salicylate <30 mg/dL | Level q4h | 90-95% |
-
Saline-Responsive (Urine Cl⁻ < 20 mEq/L)
- Normal saline: 1-2 L over 4-6 hours
- Potassium replacement: 40-80 mEq/day
- Target: HCO₃⁻ 24-28 mEq/L
- Success rate: 90-95% with adequate replacement
-
Saline-Resistant (Urine Cl⁻ > 40 mEq/L)
- Treat underlying mineralocorticoid excess
- Potassium-sparing diuretics
- Consider acetazolamide: 250-500 mg BID
- Monitor for hypokalemia and volume depletion
Respiratory Disorder Management emphasizes ventilatory optimization:
-
Respiratory Acidosis
- Improve ventilation: ↑ tidal volume or respiratory rate
- Bronchodilators for airway obstruction
- Non-invasive ventilation: CPAP/BiPAP
- Mechanical ventilation if pH < 7.25 with respiratory failure
-
Respiratory Alkalosis
- Treat underlying cause (anxiety, pain, hypoxia)
- Rebreathing techniques for anxiety-induced hyperventilation
- Adjust ventilator settings: ↓ minute ventilation
- Sedation if mechanically ventilated
💡 Master This: Never fully correct pH rapidly - aim for 50-75% correction over 24 hours. Rapid correction can cause rebound alkalosis, cerebral edema, or arrhythmias. The body's compensation mechanisms need time to readjust.
📌 Remember: TREAT CAUSE - Target underlying pathology, Replace deficits gradually, Evaluate compensation, Avoid overcorrection, Time allows adaptation, Check electrolytes, Assess response, Understand limits, Support natural mechanisms, Expect gradual improvement - The systematic treatment approach.
Connect treatment principles through multi-system integration to understand how complex medical conditions create mixed acid-base disorders.
⚖️ The Treatment Command Center: Evidence-Based Management Algorithms
🌐 The Integration Network: Multi-System Interactions and Complex Scenarios
Cardiopulmonary Integration demonstrates how heart and lung dysfunction create complex acid-base patterns:
-
Cardiogenic Shock with Pulmonary Edema
- Primary: Lactic acidosis (↓ tissue perfusion)
- Secondary: Respiratory acidosis (↓ gas exchange)
- Compensation: Limited respiratory response due to lung dysfunction
- Treatment challenge: Fluid restriction vs. bicarbonate space
-
COPD Exacerbation with Heart Failure
- Chronic respiratory acidosis (baseline PCO₂ 50-60 mmHg)
- Acute respiratory acidosis (PCO₂ 70-80 mmHg)
- Metabolic alkalosis (diuretic-induced)
- Net effect: Near-normal pH with severely abnormal components
-
Pulmonary Embolism with RV Failure
- Acute respiratory alkalosis (hyperventilation)
- Developing lactic acidosis (RV failure)
- Progression: Alkalosis → mixed → acidosis over 6-12 hours
⭐ Clinical Pearl: Triple acid-base disorders occur in 5-10% of critically ill patients. Classic example: Diabetic with pneumonia and vomiting = metabolic acidosis (DKA) + respiratory alkalosis (sepsis) + metabolic alkalosis (vomiting).
| Clinical Scenario | Primary Disorder | Secondary Effect | Compensation | Net pH |
|---|---|---|---|---|
| Septic Shock | Lactic acidosis | Respiratory alkalosis | Limited renal | 7.25-7.35 |
| COPD + Diuretics | Respiratory acidosis | Metabolic alkalosis | Renal limited | 7.35-7.45 |
| DKA + Pneumonia | Metabolic acidosis | Respiratory acidosis | Competing | 7.10-7.25 |
| CHF + Hyperventilation | Metabolic alkalosis | Respiratory alkalosis | Additive | 7.50-7.60 |
| Renal Failure + COPD | Metabolic acidosis | Respiratory acidosis | None | 7.15-7.25 |
-
Diabetic Ketoacidosis with Renal Impairment
- Ketoacid production: 300-500 mEq/day
- Reduced renal acid excretion: <50 mEq/day
- Severe acidosis: pH 6.9-7.1
- Recovery time: 48-72 hours vs. 12-24 hours with normal kidneys
-
Primary Hyperaldosteronism
- Metabolic alkalosis (H⁺ loss)
- Hypokalemia (K⁺ wasting)
- Volume expansion (Na⁺ retention)
- Paradoxical aciduria (distal H⁺ secretion)
-
Addison's Disease
- Metabolic acidosis (↓ mineralocorticoid)
- Hyperkalemia (↓ K⁺ excretion)
- Volume depletion (Na⁺ loss)
- Normal anion gap pattern
Pharmacologic Interactions create predictable acid-base disturbances:
-
Salicylate Poisoning (classic mixed disorder)
- Early: Respiratory alkalosis (direct CNS stimulation)
- Late: Metabolic acidosis (uncoupled oxidative phosphorylation)
- Transition occurs at salicylate levels >40 mg/dL
- Treatment: Alkaline diuresis (target urine pH 7.5-8.0)
-
Metformin-Associated Lactic Acidosis (MALA)
- Incidence: 0.03-0.06 cases per 1,000 patient-years
- Risk factors: eGFR < 30 mL/min/1.73m², contrast exposure
- Mortality: 30-50% if lactate > 15 mmol/L
- Treatment: Hemodialysis (removes both metformin and lactate)
-
Carbonic Anhydrase Inhibitor Effects
- Acetazolamide: Normal anion gap metabolic acidosis
- Mechanism: ↓ proximal HCO₃⁻ reabsorption
- Onset: 2-4 hours, peak effect 6-12 hours
- Compensation: Respiratory alkalosis (↑ ventilation)
💡 Master This: Delta-delta analysis becomes crucial in mixed disorders. Calculate expected anion gap change vs. actual bicarbonate change to identify concurrent metabolic alkalosis or additional normal anion gap acidosis.
ICU Integration Patterns reveal common multi-system scenarios:
-
Post-Cardiac Arrest
- Lactic acidosis (global hypoperfusion)
- Respiratory acidosis (initial apnea)
- Iatrogenic alkalosis (bicarbonate administration)
- Timeline: Acidosis → mixed → gradual normalization over 24-48 hours
-
Liver Failure with Renal Dysfunction
- Respiratory alkalosis (hyperammonemia)
- Metabolic acidosis (lactate, renal failure)
- Altered drug metabolism affecting acid-base medications
- Prognosis: pH patterns predict 30-day mortality
📌 Remember: COMPLEX CASES - Cardiopulmonary interactions, Organ failure combinations, Medication effects, Physiologic compensation, Limited reserve, Endocrine influences, Xtra monitoring needed, Combined treatments, Anticipate changes, Systemic approach, Evaluate trends, Support all systems - Framework for multi-system acid-base management.
Connect multi-system understanding through rapid mastery tools to develop clinical expertise frameworks for immediate application.
🌐 The Integration Network: Multi-System Interactions and Complex Scenarios
🎯 The Clinical Mastery Arsenal: Rapid Assessment and Decision Tools
The 30-Second ABG Assessment Protocol:
- 5-Second Scan: pH (7.35-7.45), PCO₂ (35-45 mmHg), HCO₃⁻ (22-26 mEq/L)
- 10-Second Analysis: Primary disorder identification using ROME
- 15-Second Compensation: Apply prediction formulas (±2 tolerance)
- 20-Second Integration: Anion gap if metabolic acidosis
- 30-Second Decision: Treatment priority and monitoring plan
📌 Remember: RAPID ABG - Read values quickly, Assess pH first, Primary disorder next, Identify compensation, Determine anion gap, Apply clinical context, Build treatment plan, Go with systematic approach - The 30-second framework.
| Time | Action | Key Points | Decision |
|---|---|---|---|
| 0-5 sec | Read Values | pH, PCO₂, HCO₃⁻ | Normal vs Abnormal |
| 5-10 sec | Primary Disorder | ROME rule | Metabolic vs Respiratory |
| 10-15 sec | Compensation | Prediction formulas | Adequate vs Mixed |
| 15-20 sec | Anion Gap | If metabolic acidosis | MUDPILES vs HARDUPS |
| 20-25 sec | Clinical Context | History, exam, labs | Underlying cause |
| 25-30 sec | Treatment Plan | Priorities, monitoring | Immediate vs Supportive |
-
Life-Threatening Values:
- pH < 7.1 or > 7.6 (immediate intervention required)
- HCO₃⁻ < 8 mEq/L (severe metabolic acidosis)
- PCO₂ > 80 mmHg with pH < 7.25 (respiratory failure)
- Anion gap > 25 mEq/L (severe organic acidosis)
-
Compensation Limits:
- Maximum respiratory compensation: PCO₂ 10-15 mmHg
- Maximum renal compensation: HCO₃⁻ 45 mEq/L (chronic respiratory acidosis)
- Respiratory alkalosis limit: PCO₂ 15-20 mmHg
- Metabolic alkalosis limit: HCO₃⁻ 45-50 mEq/L
Pattern Recognition Drill Framework:
-
Classic Presentations:
- DKA: pH 7.1, HCO₃⁻ 8, PCO₂ 20, AG 25
- COPD: pH 7.35, HCO₃⁻ 32, PCO₂ 60, AG 10
- Diarrhea: pH 7.25, HCO₃⁻ 12, PCO₂ 25, AG 10
- Anxiety: pH 7.50, HCO₃⁻ 18, PCO₂ 25, AG 12
-
Mixed Disorder Clues:
- Normal pH with abnormal HCO₃⁻ and PCO₂
- Compensation beyond predicted limits
- Delta-delta ratio <1 or >2
- Clinical scenario suggesting multiple processes
Treatment Priority Matrix for systematic management:
-
Immediate (pH < 7.1 or > 7.6)
- Bicarbonate for severe acidosis
- Ventilatory support for respiratory failure
- Dialysis for refractory acidosis
- Continuous monitoring
-
Urgent (pH 7.1-7.25 or 7.5-7.6)
- Cause-specific treatment
- Supportive care
- Frequent monitoring (q2-4h)
- Anticipate complications
-
Routine (pH 7.25-7.5)
- Address underlying condition
- Support natural compensation
- Standard monitoring
- Prevent progression
⭐ Clinical Pearl: The Rule of 15s - In metabolic acidosis, if the last two digits of pH equal 15 minus the last digit of PCO₂, compensation is appropriate. Example: pH 7.25 (25) and PCO₂ 30 (0): 25 = 15 - (-10), suggesting mixed disorder.
💡 Master This: Clinical context always trumps numbers. A pH of 7.35 may be life-threatening in a patient with chronic respiratory acidosis (baseline pH 7.32), while pH 7.25 may be well-tolerated in DKA with appropriate compensation.
Monitoring and Follow-up Protocols:
- Severe Disorders: ABG every 30-60 minutes until stable
- Moderate Disorders: ABG every 2-4 hours during treatment
- Mild Disorders: ABG every 6-12 hours or PRN
- Resolution Criteria: pH 7.35-7.45, appropriate compensation, clinical improvement
📌 Remember: MASTER TOOLS - Monitor trends not just values, Assess clinical response, Systematic approach always, Treat underlying causes, Expect gradual improvement, Recognize mixed disorders, Time allows compensation, Optimize all systems, Organize priorities, Limit overcorrection, Support natural mechanisms - The complete clinical mastery framework for acid-base excellence.
🎯 The Clinical Mastery Arsenal: Rapid Assessment and Decision Tools
Practice Questions: Acid-base balance
Test your understanding with these related questions
A 27-year-old man with a past medical history of type I diabetes mellitus presents to the emergency department with altered mental status. The patient was noted as becoming more lethargic and confused over the past day, prompting his roommates to bring him in. His temperature is 99.0°F (37.2°C), blood pressure is 107/68 mmHg, pulse is 120/min, respirations are 17/min, and oxygen saturation is 98% on room air. Laboratory values are ordered as seen below. Serum: Na+: 144 mEq/L Cl-: 100 mEq/L K+: 6.3 mEq/L HCO3-: 16 mEq/L BUN: 20 mg/dL Glucose: 599 mg/dL Creatinine: 1.4 mg/dL Ca2+: 10.2 mg/dL Which of the following is the appropriate endpoint of treatment for this patient?













