Drug distribution and protein binding US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Drug distribution and protein binding. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
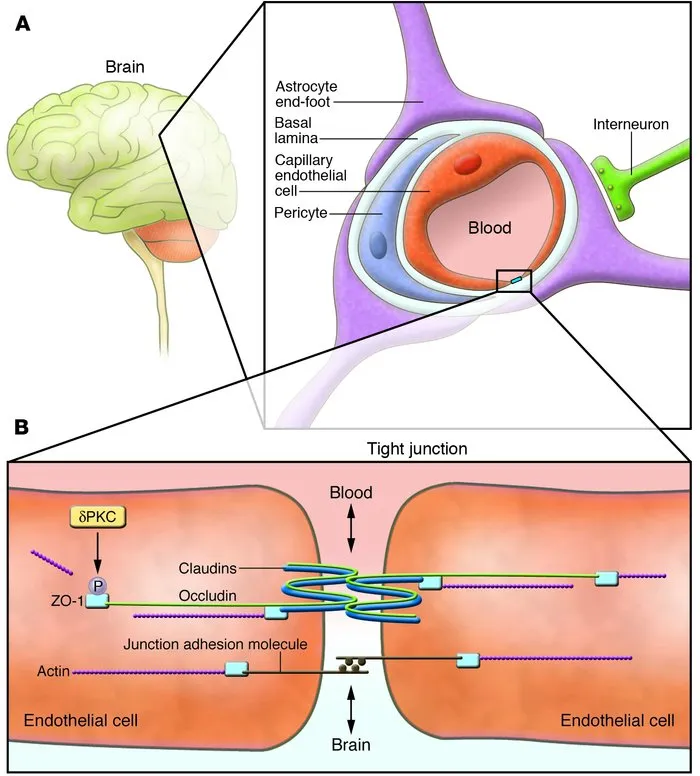
Drug distribution and protein binding US Medical PG Question 1: A 60-year-old woman is brought to the emergency department by ambulance after suffering a generalized tonic-clonic seizure. The seizure lasted 2 minutes, followed by a short period of unresponsiveness and loud breathing. Her blood pressure is 130/80 mm Hg, the heart rate is 76/min, and the respiratory rate is 15/min and regular. On physical examination, the patient is confused but follows commands and cannot recall recent events. The patient does not present with any other neurological deficits. T1/T2 MRI of the brain demonstrates a hypointense, contrast-enhancing mass within the right frontal lobe, surrounded by significant cerebral edema. Which of the following would you expect in the tissue surrounding the described lesion?
- A. Loss of endothelial tight junctions (Correct Answer)
- B. Replacement of interstitial fluid with cerebrospinal fluid (CSF)
- C. Increased intracellular concentrations of osmolytes
- D. Upregulation of aquaporin-4
- E. Increased interstitial fluid low in protein
Drug distribution and protein binding Explanation: ***Loss of endothelial tight junctions***
- The presence of a **contrast-enhancing mass** with surrounding edema suggests **vasogenic edema**, which is caused by the disruption of the **blood-brain barrier (BBB)**.
- This disruption primarily involves the **loss of tight junctions** between endothelial cells, allowing plasma proteins and fluid to leak into the interstitial space.
*Replacement of interstitial fluid with cerebrospinal fluid (CSF)*
- **CSF** is produced by the choroid plexus and flows through the ventricular system and subarachnoid space; it does not replace interstitial fluid within the brain parenchyma.
- While disruptions can occur, the primary mechanism of edema in this context is leakage from blood vessels, not direct replacement by CSF.
*Increased intracellular concentrations of osmolytes*
- This describes the mechanism of **cytotoxic edema**, where intracellular swelling occurs due to **cellular dysfunction** (e.g., ischemia) and the accumulation of osmolytes within cells.
- However, the patient's MRI findings of a **contrast-enhancing mass** and significant surrounding edema are more consistent with **vasogenic edema**, which is extracellular.
*Upregulation of aquaporin-4*
- **Aquaporin-4** channels are involved in water transport and are primarily associated with the development of **cytotoxic edema** or hydrocephalic edema by facilitating water movement across cell membranes.
- In **vasogenic edema**, the primary issue is the **breakdown of the BBB** and leakage of fluid and proteins, rather than altered aquaporin expression as the initial cause.
*Increased interstitial fluid low in protein*
- While there is **increased interstitial fluid**, the fluid in **vasogenic edema** is typically **rich in protein** (plasma proteins) because the **blood-brain barrier** is compromised.
- Fluid that is **low in protein** is characteristic of **hydrocephalic edema**, where CSF transudates into the periventricular white matter due to increased ventricular pressure.
Drug distribution and protein binding US Medical PG Question 2: An experimental infusable drug, X729, is currently being studied to determine its pharmacokinetics. The drug was found to have a half life of 1.5 hours and is eliminated by first order kinetics. What is the minimum number of hours required to reach a steady state concentration of >90%?
- A. 6 (Correct Answer)
- B. 3
- C. 7.5
- D. 1.5
- E. 4.5
Drug distribution and protein binding Explanation: ***6***
- For a drug eliminated by **first-order kinetics**, approximately **4 to 5 half-lives** are required to reach **steady-state concentration**.
- To reach >90% of steady-state, at least **4 half-lives** are needed, where **93.75%** of the steady state is achieved.
- The time taken would be **4 half-lives × 1.5 hours/half-life = 6 hours**, making this the **minimum time** to exceed 90%.
*3*
- This represents only **2 half-lives** (2 × 1.5 hours = 3 hours), which would achieve roughly **75%** of the steady-state concentration.
- This is insufficient to reach >90% of the steady-state concentration.
*7.5*
- This time point represents **5 half-lives** (5 × 1.5 hours = 7.5 hours), which would achieve approximately **97%** of the steady-state concentration.
- While this does exceed 90%, the question asks for the **minimum** number of hours required, and 90% is already exceeded at 6 hours (4 half-lives).
*1.5*
- This is only **1 half-life**, which would achieve approximately **50%** of the steady-state concentration.
- This is far too early to reach a >90% steady-state concentration.
*4.5*
- This represents **3 half-lives** (3 × 1.5 hours = 4.5 hours), achieving approximately **87.5%** of the steady-state concentration.
- While close to 90%, it does not quite reach "greater than 90%".
Drug distribution and protein binding US Medical PG Question 3: An investigator is developing a drug that selectively inhibits the retrograde axonal transport of rabies virus towards the central nervous system. To achieve this effect, this drug must target which of the following?
- A. Dynein (Correct Answer)
- B. Tubulin
- C. Nidogen
- D. Kinesin
- E. Acetylcholine
Drug distribution and protein binding Explanation: ***Dynein***
- **Dynein** is a microtubule-dependent motor protein responsible for **retrograde axonal transport**, moving cargo (like rabies virus) away from the axon terminals towards the cell body and ultimately the central nervous system.
- Inhibiting dynein would therefore prevent the **rabies virus** from traveling from the site of infection (e.g., muscle cell) to the central nervous system.
*Tubulin*
- **Tubulin** is the primary protein subunit that polymerizes to form **microtubules**, which serve as the tracks for axonal transport.
- Inhibiting tubulin polymerization would disrupt both **anterograde** and **retrograde transport** nonspecifically, leading to severe neurotoxicity rather than selective inhibition of rabies virus transport.
*Nidogen*
- **Nidogen** (also known as entactin) is a glycoprotein component of the **basal lamina**, an extracellular matrix structure.
- It plays a role in cell adhesion and tissue organization but is not directly involved in the intracellular motor processes of axonal transport.
*Kinesin*
- **Kinesin** is a microtubule-dependent motor protein primarily responsible for **anterograde axonal transport**, moving cargo from the cell body towards the axon terminals.
- Inhibiting kinesin would disrupt the outward movement of vesicles and organelles, but would not prevent the **inward retrograde transport** of the rabies virus.
*Acetylcholine*
- **Acetylcholine** is a neurotransmitter that plays a role in synaptic transmission in both the peripheral and central nervous systems.
- While rabies virus can affect neuronal function, acetylcholine itself is not a motor protein or a structural component directly involved in the physical process of **axonal transport**.
Drug distribution and protein binding US Medical PG Question 4: Which factor most strongly influences protein filtration at the glomerulus?
- A. Electrical charge
- B. Molecular size (Correct Answer)
- C. Shape
- D. Temperature
Drug distribution and protein binding Explanation: ***Molecular size***
- The glomerular filtration barrier, particularly the **slit diaphragms** between podocytes, acts as a size-selective filter, restricting the passage of larger molecules.
- Proteins like **albumin** (molecular radius ~36 Å, molecular weight ~69 kDa) are significantly large, making them difficult to pass through the filtration barrier.
- Size selectivity is the **primary and most important** factor in protein filtration.
*Electrical charge*
- The glomerular basement membrane contains **negatively charged proteoglycans** (heparan sulfate), which repel negatively charged proteins like albumin, contributing to their retention.
- While important, the role of electrical charge is **secondary** to molecular size in preventing the bulk passage of most proteins.
*Shape*
- While abnormal protein shapes (e.g., **amyloid fibrils**) can impact filtration in specific disease states, the typical physiological filtration of most proteins is primarily governed by size and charge.
- The inherent shape of normal globular proteins plays a less direct role compared to their overall size.
*Temperature*
- **Physiological temperature** is relatively constant in the body and does not directly influence the molecular interactions and physical properties of the glomerular filtration barrier in a way that significantly alters protein filtration.
- Temperature changes would lead to denaturation or aggregation, which are not the primary determinants of normal protein filtration.
Drug distribution and protein binding US Medical PG Question 5: A 35-year-old woman is started on a new experimental intravenous drug X. In order to make sure that she is able to take this drug safely, the physician in charge of her care calculates the appropriate doses to give to this patient. Data on the properties of drug X from a subject with a similar body composition to the patient is provided below:
Weight: 100 kg
Dose provided: 1500 mg
Serum concentration 15 mg/dL
Bioavailability: 1
If the patient has a weight of 60 kg and the target serum concentration is 10 mg/dL, which of the following best represents the loading dose of drug X that should be given to this patient?
- A. 300 mg
- B. 450 mg
- C. 150 mg
- D. 1000 mg
- E. 600 mg (Correct Answer)
Drug distribution and protein binding Explanation: ***600 mg***
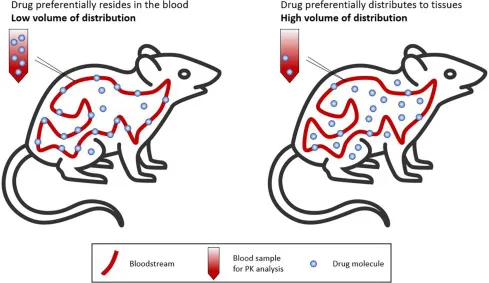
- First, calculate the **volume of distribution (Vd)** using the provided data: **Vd = Total Dose / Serum Concentration**. Converting units: 15 mg/dL = 150 mg/L. Therefore, Vd = 1500 mg / 150 mg/L = **10 L** (for the 100 kg subject).
- Since the Vd value is for a 100 kg person, Vd per kg = 10 L / 100 kg = **0.1 L/kg**. For the 60 kg patient, the Vd = 0.1 L/kg × 60 kg = **6 L**.
- The **loading dose = Target Serum Concentration × Vd / Bioavailability**. Converting target concentration: 10 mg/dL = 100 mg/L. Therefore: (100 mg/L × 6 L) / 1 = **600 mg**.
*300 mg*
- This value is obtained if an incorrect **Vd** or target concentration was used, potentially through miscalculation or incorrect unit conversion.
- For instance, if the **Vd** was inaccurately calculated at 3 L (instead of 6 L), this could lead to the incorrect answer.
*450 mg*
- This result might occur if the **Vd calculation** was flawed or if the target concentration was incorrectly interpreted.
- A potential error could involve using a Vd of 4.5 L which would result in 450 mg, or if the drug amount was simply prorated by weight without properly considering the Vd per kg.
*150 mg*
- This value suggests a significant error in the calculation of the **volume of distribution** or the target concentration.
- It might be obtained if the **Vd** was mistakenly taken as 1.5 L or if the dose was divided by the original serum concentration without accounting for the new patient's weight and desired concentration.
*1000 mg*
- This value is significantly higher than the correct answer, indicating an overestimation of the **Vd** or target concentration.
- It could result from using the original dose (1500 mg) and attempting to scale it incorrectly by weight alone (1500 mg × 60/100 = 900 mg, close to 1000), or if unit conversions were mishandled during the Vd determination.
Drug distribution and protein binding US Medical PG Question 6: Drug A is an experimental compound being investigated for potential use as a protectant against venous thrombosis. Binding assays reveal that the drug’s primary mechanism of action is to block carboxylation of glutamic acid residues in certain serum proteins. Drug A is most similar to which of the following:
- A. Streptokinase
- B. Bivalirudin
- C. Warfarin (Correct Answer)
- D. Heparin
- E. Rivaroxaban
Drug distribution and protein binding Explanation: ***Warfarin***
- Warfarin inhibits **vitamin K epoxide reductase**, enzyme responsible for regenerating active vitamin K.
- Active vitamin K is a cofactor for the **gamma-carboxylation of glutamic acid residues** on factors II, VII, IX, X and protein C and S. Thus, warfarin blocks their activation, inhibiting coagulation.
*Steptokinase*
- **Streptokinase** is a **thrombolytic drug** that catalyzes the conversion of **plasminogen to plasmin**, an enzyme that degrades fibrin clots.
- Its mechanism of action is focused on **breaking down existing clots**, rather than preventing their formation by affecting coagulation factor synthesis.
*Bivalirudin*
- **Bivalirudin** is a direct **thrombin inhibitor**, binding directly to the active site and exosite I of thrombin to prevent its action.
- It does not interfere with the **carboxylation of glutamic acid residues** but rather directly inhibits the final common pathway of coagulation.
*Heparin*
- **Heparin** works by potentiating the action of **antithrombin III**, which in turn inactivates thrombin and factor Xa.
- Its mechanism involves accelerating the natural anticoagulant system, rather than inhibiting the **synthesis or activation of coagulation factors** through carboxylation.
*Rivaroxaban*
- **Rivaroxaban** is a **direct factor Xa inhibitor**, which blocks the activity of free and clot-bound factor Xa.
- It directly interferes with the coagulation cascade downstream of the carboxylation step, and does not affect the **vitamin K-dependent carboxylation process**.
Drug distribution and protein binding US Medical PG Question 7: A researcher is investigating the effects of a new antihypertensive medication on renal physiology. She gives a subject a dose of the new medication, and she then collects plasma and urine samples. She finds the following: Hematocrit: 40%; Serum creatinine: 0.0125 mg/mL; Urine creatinine: 1.25 mg/mL. Urinary output is 1 mL/min. Renal blood flow is 1 L/min. Based on the above information and approximating that the creatinine clearance is equal to the GFR, what answer best approximates filtration fraction in this case?
- A. 10%
- B. 17% (Correct Answer)
- C. 33%
- D. 50%
- E. 25%
Drug distribution and protein binding Explanation: ***17%***
- First, calculate **GFR** using the creatinine clearance formula: GFR = (Urine creatinine × Urinary output) / Serum creatinine = (1.25 mg/mL × 1 mL/min) / 0.0125 mg/mL = **100 mL/min**.
- Next, calculate **Renal Plasma Flow (RPF)** from Renal Blood Flow (RBF) and Hematocrit: RPF = RBF × (1 - Hematocrit) = 1000 mL/min × (1 - 0.40) = **600 mL/min**.
- Finally, calculate **Filtration Fraction (FF)** = GFR / RPF = 100 mL/min / 600 mL/min = 0.1667 = **16.7%, which approximates to 17%**.
- This is the correct answer based on the physiological calculations and represents a normal filtration fraction.
*10%*
- This would correspond to a filtration fraction of 0.10, which would require either a GFR of 60 mL/min (lower than calculated) or an RPF of 1000 mL/min (higher than calculated).
- This value is too low given the provided parameters and doesn't match the calculation from the given data.
*25%*
- This value would suggest FF = 0.25, requiring a GFR of 150 mL/min with the calculated RPF of 600 mL/min.
- This is higher than the calculated GFR of 100 mL/min and doesn't match the given creatinine values.
*33%*
- This would imply FF = 0.33, requiring a GFR of approximately 200 mL/min with RPF of 600 mL/min.
- This is significantly higher than the calculated GFR and would represent an abnormally elevated filtration fraction.
*50%*
- A filtration fraction of 50% is unphysiologically high and would indicate severe pathology.
- This would require a GFR of 300 mL/min with the calculated RPF, which is impossible given the provided creatinine clearance data.
Drug distribution and protein binding US Medical PG Question 8: A 65-year-old man with a history of myocardial infarction is admitted to the hospital for treatment of atrial fibrillation with rapid ventricular response. He is 180 cm (5 ft 11 in) tall and weighs 80 kg (173 lb). He is given an intravenous bolus of 150 mg of amiodarone. After 20 minutes, the amiodarone plasma concentration is 2.5 mcg/mL. Amiodarone distributes in the body within minutes, and its elimination half-life after intravenous administration is 30 days. Which of the following values is closest to the volume of distribution of the administered drug?
- A. 60 L (Correct Answer)
- B. 80 L
- C. 150 L
- D. 17 L
- E. 10 L
Drug distribution and protein binding Explanation: ***60 L***
- The **volume of distribution (Vd)** is calculated using the formula: **Vd = Dose / Plasma Concentration**.
- Given: Dose = 150 mg (150,000 mcg), Plasma concentration = 2.5 mcg/mL
- Calculation: Vd = 150,000 mcg / 2.5 mcg/mL = 60,000 mL = **60 L**
- Note: This calculation represents a simplified scenario. In clinical practice, amiodarone has an extremely large volume of distribution (60-100 L/kg or ~4,800-8,000 L in this patient) due to extensive tissue distribution, but the question tests the ability to apply the basic pharmacokinetic formula.
*80 L*
- This value would result if the plasma concentration were 1.875 mcg/mL (150,000 mcg / 80,000 mL), not the given 2.5 mcg/mL.
- This represents a common calculation error when working with pharmacokinetic parameters.
*150 L*
- This value would require a plasma concentration of 1 mcg/mL (150,000 mcg / 150,000 mL), which is lower than the measured 2.5 mcg/mL.
- This error might occur if the dose value were confused with the volume of distribution.
*17 L*
- This value would be obtained with a plasma concentration of approximately 8.8 mcg/mL (150,000 mcg / 17,000 mL), significantly higher than the measured 2.5 mcg/mL.
- This represents a significant underestimation of Vd and would suggest limited drug distribution.
*10 L*
- This value would require a plasma concentration of 15 mcg/mL (150,000 mcg / 10,000 mL), which is 6-fold higher than the given 2.5 mcg/mL.
- Such a small Vd would suggest drug confined primarily to plasma, which is inappropriate for lipophilic drugs with extensive tissue distribution.
Drug distribution and protein binding US Medical PG Question 9: An experimental drug, ES 62, is being studied. It prohibits the growth of vancomycin-resistant Staphylococcus aureus. It is highly lipid-soluble. The experimental design is dependent on a certain plasma concentration of the drug. The target plasma concentration is 100 mmol/dL. Which of the following factors is most important for calculating the appropriate loading dose?
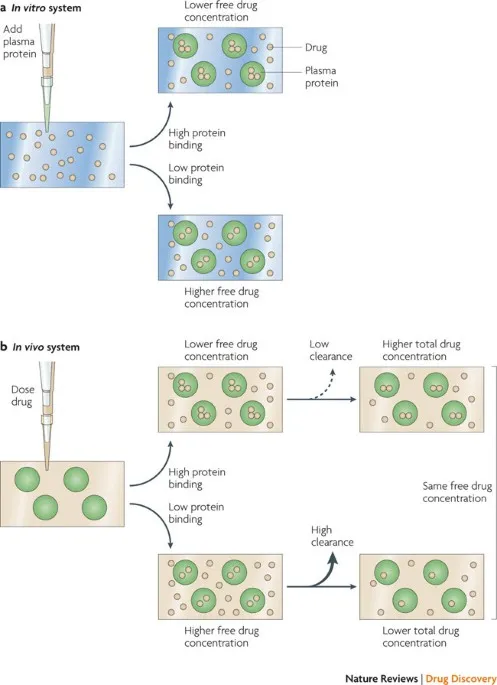
- A. Volume of distribution (Correct Answer)
- B. Half-life of the drug
- C. Therapeutic index
- D. Clearance of the drug
- E. Rate of administration
Drug distribution and protein binding Explanation: **Volume of distribution**
- The **loading dose** is primarily determined by the desired **plasma concentration** and the **volume of distribution (Vd)**, as it reflects how extensively a drug is distributed in the body.
- The formula for loading dose is: Loading Dose = (Target Plasma Concentration × Vd).
*Half-life of the drug*
- The **half-life** is crucial for determining the **dosing interval** and the time it takes to reach **steady-state concentrations**, not the initial loading dose.
- It reflects the rate at which the drug is eliminated from the body.
*Therapeutic index*
- The **therapeutic index** is a measure of a drug's relative safety, indicating the ratio between the **toxic dose** and the **effective dose**.
- While important for drug safety, it does not directly determine the magnitude of the loading dose itself.
*Clearance of the drug*
- **Clearance** is the rate at which the drug is removed from the body and is a primary determinant of the **maintenance dose** required to sustain a desired plasma concentration.
- It does not directly calculate the initial loading dose needed to achieve an immediate target concentration.
*Rate of administration*
- The **rate of administration** (e.g., infusion rate) primarily influences how quickly the drug reaches its target concentration, but not the total quantity of drug needed for the initial loading dose.
- It affects the kinetics of how the loading dose achieves the target concentration, rather than defining the dose amount.
Drug distribution and protein binding US Medical PG Question 10: An investigator is developing a drug for muscle spasms. The drug inactivates muscular contraction by blocking the site where calcium ions bind to regulate actin-myosin interaction. Which of the following is the most likely site of action of this drug?
- A. Troponin C (Correct Answer)
- B. Myosin-binding site
- C. Acetylcholine receptor
- D. Ryanodine receptor
- E. Myosin head
Drug distribution and protein binding Explanation: ***Troponin C***
- **Calcium ions** bind to **Troponin C**, initiating a conformational change in the troponin-tropomyosin complex, which exposes the **myosin-binding sites on actin**.
- Blocking this site directly prevents the **calcium-mediated regulation** of muscle contraction, thus inactivating it.
*Myosin-binding site*
- The **myosin-binding site** is located on the **actin filament** and is where the **myosin head** attaches to form cross-bridges.
- While essential for contraction, this site doesn't directly bind calcium ions to initiate the process.
*Acetylcholine receptor*
- The **acetylcholine receptor** is located on the **neuromuscular junction** and mediates the transmission of a nerve impulse to the muscle fiber.
- Blocking this receptor would prevent muscle depolarization, but it's not the direct site where calcium ions regulate actin-myosin interaction.
*Ryanodine receptor*
- The **ryanodine receptor** is located on the **sarcoplasmic reticulum** and controls the release of calcium ions into the sarcoplasm.
- While it's involved in calcium signaling, it doesn't represent the site where calcium binds to *regulate* the actin-myosin interaction itself.
*Myosin head*
- The **myosin head** contains the **ATPase activity** and binds to actin to form cross-bridges, enabling muscle contraction.
- It does not directly bind **calcium ions** to regulate the initiation of contraction; instead, its binding to actin is regulated by the troponin-tropomyosin complex.
More Drug distribution and protein binding US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.