Absorption factors and bioavailability US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Absorption factors and bioavailability. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Absorption factors and bioavailability US Medical PG Question 1: Which factor most strongly influences protein filtration at the glomerulus?
- A. Electrical charge
- B. Molecular size (Correct Answer)
- C. Shape
- D. Temperature
Absorption factors and bioavailability Explanation: ***Molecular size***
- The glomerular filtration barrier, particularly the **slit diaphragms** between podocytes, acts as a size-selective filter, restricting the passage of larger molecules.
- Proteins like **albumin** (molecular radius ~36 Å, molecular weight ~69 kDa) are significantly large, making them difficult to pass through the filtration barrier.
- Size selectivity is the **primary and most important** factor in protein filtration.
*Electrical charge*
- The glomerular basement membrane contains **negatively charged proteoglycans** (heparan sulfate), which repel negatively charged proteins like albumin, contributing to their retention.
- While important, the role of electrical charge is **secondary** to molecular size in preventing the bulk passage of most proteins.
*Shape*
- While abnormal protein shapes (e.g., **amyloid fibrils**) can impact filtration in specific disease states, the typical physiological filtration of most proteins is primarily governed by size and charge.
- The inherent shape of normal globular proteins plays a less direct role compared to their overall size.
*Temperature*
- **Physiological temperature** is relatively constant in the body and does not directly influence the molecular interactions and physical properties of the glomerular filtration barrier in a way that significantly alters protein filtration.
- Temperature changes would lead to denaturation or aggregation, which are not the primary determinants of normal protein filtration.
Absorption factors and bioavailability US Medical PG Question 2: A 56-year-old man with coronary artery disease agrees to participate in a pharmacological study. He takes an oral medication that leads to dephosphorylation of myosin light chains in venous smooth muscle cells. An investigator measures the plasma concentration of the drug over time after intravenous and then after oral administration. There is no statistically significant difference in the dose-corrected area under the curve for the 2 routes of administration. The patient most likely ingested which of the following drugs?
- A. Isosorbide mononitrate (Correct Answer)
- B. Nitroglycerine
- C. Nimodipine
- D. Nifedipine
- E. Nitroprusside
Absorption factors and bioavailability Explanation: ***Isosorbide mononitrate***
- **Isosorbide mononitrate** has nearly **100% oral bioavailability** due to minimal first-pass metabolism, which explains the comparable AUC between intravenous and oral administration.
- This drug acts by releasing **nitric oxide**, leading to dephosphorylation of myosin light chains and subsequent **venous smooth muscle relaxation**, a mechanism consistent with the question stem.
*Nitroglycerine*
- **Nitroglycerine** undergoes extensive **first-pass metabolism** when taken orally, resulting in very low oral bioavailability and a significantly smaller AUC compared to intravenous administration.
- It is typically administered sublingually or transdermally to avoid hepatic metabolism and achieve therapeutic effects.
*Nimodipine*
- **Nimodipine** is a **dihydropyridine calcium channel blocker** used for cerebral vasospasm, not primarily as a venous dilator acting via myosin light chain dephosphorylation.
- While it can be given orally, its mechanism of action and primary clinical use are different from the description.
*Nifedipine*
- **Nifedipine** is another **dihydropyridine calcium channel blocker** primarily affecting arterial smooth muscle, not venous smooth muscle via myosin light chain dephosphorylation.
- It undergoes significant first-pass metabolism, leading to variable oral bioavailability, which would likely result in a noticeable difference in AUC compared to IV administration.
*Nitroprusside*
- **Nitroprusside** is an **intravenously administered agent** that directly releases nitric oxide, but it has no oral formulation due to rapid degradation and toxicity.
- Its use is limited to acute hypertensive emergencies and it does not fit the description of an orally administered drug with high bioavailability.
Absorption factors and bioavailability US Medical PG Question 3: A 5-year-old boy undergoes MRI neuroimaging for the evaluation of worsening headaches and intermittent nausea upon awakening. He receives a bolus of intravenous thiopental for sedation during the procedure. Ten minutes after the MRI, the patient is awake and responsive. Which of the following pharmacological properties is most likely responsible for this patient's rapid recovery from this anesthetic agent?
- A. First-pass metabolism
- B. Redistribution (Correct Answer)
- C. Zero-order elimination
- D. Ion trapping
- E. Cytochrome P450 oxidation
Absorption factors and bioavailability Explanation: ***Redistribution***
- Thiopental is a highly **lipid-soluble** drug that rapidly crosses the **blood-brain barrier**, leading to quick onset of action.
- The drug then rapidly **redistributes** from the brain to other highly perfused tissues (e.g., muscle, fat) and then less perfused tissues, causing a rapid decrease in drug concentration at the site of action and thus termination of the anesthetic effect.
*First-pass metabolism*
- This refers to the **metabolism of a drug** before it reaches systemic circulation, typically after oral administration, and does not explain the termination of action for an intravenously administered drug like thiopental.
- While thiopental is ultimately metabolized by the liver, this process is slower than redistribution and does not account for the **rapid awakening**.
*Zero-order elimination*
- **Zero-order elimination** occurs when a constant amount of drug is eliminated per unit of time, regardless of the drug's concentration, often seen with drug saturation of elimination pathways.
- Thiopental elimination follows **first-order kinetics** at therapeutic doses, meaning a constant fraction of the drug is eliminated per unit time, and this describes slower, overall elimination, not rapid recovery.
*Ion trapping*
- **Ion trapping** occurs when a drug accumulates in a compartment due to differences in pH across a membrane and the drug's pKa, leading to ionization and reduced ability to diffuse back.
- This mechanism is important for drug excretion or distribution into specific compartments (e.g., accumulation of basic drugs in acidic urine) but does not explain the **rapid termination of CNS effects** via redistribution.
*Cytochrome P450 oxidation*
- **Cytochrome P450 (CYP450) oxidation** is a major pathway for drug metabolism in the liver, which is responsible for the eventual elimination of thiopental from the body.
- While important for overall drug clearance, the rate of CYP450 oxidation is too slow to account for the **rapid awakening** seen after a single bolus dose of thiopental; redistribution is the primary factor for rapid recovery.
Absorption factors and bioavailability US Medical PG Question 4: A drug discovery team is conducting research to observe the characteristics of a novel drug under different experimental conditions. The drug is converted into the inactive metabolites by an action of an enzyme E. After multiple experiments, the team concludes that as compared to physiologic pH, the affinity of the enzyme E for the drug decreases markedly in acidic pH. Co-administration of an antioxidant A increases the value of Michaelis-Menten constant (Km) for the enzyme reaction, while co-administration of a drug B decreases the value of Km. Assume the metabolism of the novel drug follows Michaelis-Menten kinetics at the therapeutic dose, and that the effects of different factors on the metabolism of the drug are first-order linear. For which of the following conditions will the metabolism of the drug be the slowest?
- A. Acidic pH, co-administration of antioxidant A and of drug B
- B. Acidic pH, co-administration of antioxidant A, no administration of drug B (Correct Answer)
- C. Physiologic pH, co-administration of antioxidant A, no administration of drug B
- D. Acidic pH, co-administration of drug B, no administration of antioxidant A
- E. Acidic pH, without administration of antioxidant A or drug B
Absorption factors and bioavailability Explanation: ***Acidic pH, co-administration of antioxidant A, no administration of drug B***
- **Decreased affinity** at acidic pH reduces the enzyme's ability to bind the drug, slowing metabolism.
- Co-administration of **antioxidant A increases Km**, indicating a further reduction in enzyme affinity and thus slower metabolism.
*Acidic pH, co-administration of antioxidant A and of drug B*
- While acidic pH and antioxidant A slow metabolism, co-administration of **drug B decreases Km**, which would increase enzyme affinity and counteract the slowing effect to some extent.
- The combination would result in a slower metabolism than baseline, but likely not the slowest possible due to the partially opposing effects of A and B.
*Physiologic pH, co-administration of antioxidant A, no administration of drug B*
- At **physiologic pH**, the enzyme's affinity for the drug is higher than at acidic pH, promoting faster metabolism.
- Although antioxidant A increases Km, the favorable pH for enzyme activity means metabolism will not be as slow as under acidic conditions.
*Acidic pH, co-administration of drug B, no administration of antioxidant A*
- **Acidic pH** reduces enzyme affinity, slowing metabolism.
- However, the co-administration of **drug B decreases Km**, which increases enzyme affinity and would partially offset the reduced affinity caused by the acidic pH, leading to a faster metabolism compared to when antioxidant A is present.
*Acidic pH, without administration of antioxidant A or drug B*
- At **acidic pH**, the enzyme's affinity for the drug decreases, which slows metabolism.
- However, in this condition, there are no additional factors (like antioxidant A) further increasing Km, nor factors (like drug B) decreasing Km, so the metabolic rate would be slower than physiologic pH but not as slow as when antioxidant A is also present.
Absorption factors and bioavailability US Medical PG Question 5: A scientist is studying the excretion of a novel toxin X by the kidney in order to understand the dynamics of this new substance. He discovers that this new toxin X has a clearance that is half that of inulin in a particular patient. This patient's filtration fraction is 20% and his para-aminohippuric acid (PAH) dynamics are as follows:
Urine volume: 100 mL/min
Urine PAH concentration: 30 mg/mL
Plasma PAH concentration: 5 mg/mL
Given these findings, what is the clearance of the novel toxin X?
- A. 1,500 mL/min
- B. 600 mL/min
- C. 300 mL/min
- D. 60 mL/min (Correct Answer)
- E. 120 mL/min
Absorption factors and bioavailability Explanation: ***60 ml/min***
- First, calculate the **renal plasma flow (RPF)** using PAH clearance: RPF = (Urine PAH conc. × Urine vol.) / Plasma PAH conc. = (30 mg/mL × 100 mL/min) / 5 mg/mL = 600 mL/min.
- Next, calculate the **glomerular filtration rate (GFR)**, which is the clearance of inulin. GFR = RPF × Filtration Fraction = 600 mL/min × 0.20 = 120 mL/min. Toxin X clearance is half of inulin clearance, so 120 mL/min / 2 = **60 mL/min**.
*1,500 ml/min*
- This value is likely obtained if an incorrect formula or conversion was made, possibly by misinterpreting the units or the relationship between GFR, RPF, and filtration fraction.
- It significantly overestimates the clearance for a substance that is cleared at half the rate of inulin.
*600 ml/min*
- This value represents the **renal plasma flow (RPF)**, calculated using the PAH clearance data.
- It does not account for the filtration fraction or the fact that toxin X clearance is half of inulin clearance (GFR).
*300 ml/min*
- This value would be obtained if the renal plasma flow (RPF) was incorrectly halved, or if an intermediate calculation was misinterpreted as the final answer.
- It does not align with the given filtration fraction and the relationship between toxin X and inulin clearance.
*120 ml/min*
- This value represents the **glomerular filtration rate (GFR)**, which is equal to the clearance of inulin (RPF × Filtration Fraction = 600 mL/min × 0.20 = 120 mL/min).
- The question states that the clearance of toxin X is **half** that of inulin, so this is an intermediate step, not the final answer.
Absorption factors and bioavailability US Medical PG Question 6: An investigator is studying the effect of antihypertensive drugs on cardiac output and renal blood flow. For comparison, a healthy volunteer is given a placebo and a continuous infusion of para-aminohippuric acid (PAH) to achieve a plasma concentration of 0.02 mg/ml. His urinary flow rate is 1.5 ml/min and the urinary concentration of PAH is measured to be 8 mg/ml. His hematocrit is 50%. Which of the following values best estimates cardiac output in this volunteer?
- A. 8 L/min
- B. 3 L/min
- C. 4 L/min
- D. 1.2 L/min
- E. 6 L/min (Correct Answer)
Absorption factors and bioavailability Explanation: ***6 L/min***
- This value represents the estimated **cardiac output** based on the calculated renal blood flow.
- Step 1: Calculate renal plasma flow (RPF) using PAH clearance: RPF = (Urinary PAH × Urine flow rate) / Plasma PAH = (8 mg/ml × 1.5 ml/min) / 0.02 mg/ml = 600 ml/min = 0.6 L/min
- Step 2: Calculate renal blood flow (RBF): Since hematocrit is 50%, RBF = RPF / (1 - Hematocrit) = 0.6 / 0.5 = 1.2 L/min
- Step 3: Estimate cardiac output: The kidneys normally receive approximately **20-25% of cardiac output**. Using 20%: Cardiac Output = RBF / 0.20 = 1.2 / 0.20 = **6 L/min**
- This is consistent with normal resting cardiac output in a healthy adult.
*8 L/min*
- This value overestimates cardiac output based on the renal blood flow calculation.
- While some individuals may have higher cardiac output during exercise, the calculated RBF of 1.2 L/min suggests a resting cardiac output closer to 6 L/min.
*3 L/min*
- This value significantly underestimates cardiac output.
- If cardiac output were 3 L/min, the kidneys would be receiving 40% of cardiac output (1.2/3), which is physiologically implausible at rest.
*4 L/min*
- This value underestimates cardiac output based on the renal data.
- This would mean kidneys receive 30% of cardiac output (1.2/4), which is higher than the typical 20-25%.
*1.2 L/min*
- This is the calculated **renal blood flow**, not cardiac output.
- While this calculation is correct for RBF, the question specifically asks for cardiac output estimation, which requires accounting for the fact that kidneys receive only about 20-25% of total cardiac output.
Absorption factors and bioavailability US Medical PG Question 7: An experimental drug, ES 62, is being studied. It prohibits the growth of vancomycin-resistant Staphylococcus aureus. It is highly lipid-soluble. The experimental design is dependent on a certain plasma concentration of the drug. The target plasma concentration is 100 mmol/dL. Which of the following factors is most important for calculating the appropriate loading dose?
- A. Volume of distribution (Correct Answer)
- B. Half-life of the drug
- C. Therapeutic index
- D. Clearance of the drug
- E. Rate of administration
Absorption factors and bioavailability Explanation: **Volume of distribution**
- The **loading dose** is primarily determined by the desired **plasma concentration** and the **volume of distribution (Vd)**, as it reflects how extensively a drug is distributed in the body.
- The formula for loading dose is: Loading Dose = (Target Plasma Concentration × Vd).
*Half-life of the drug*
- The **half-life** is crucial for determining the **dosing interval** and the time it takes to reach **steady-state concentrations**, not the initial loading dose.
- It reflects the rate at which the drug is eliminated from the body.
*Therapeutic index*
- The **therapeutic index** is a measure of a drug's relative safety, indicating the ratio between the **toxic dose** and the **effective dose**.
- While important for drug safety, it does not directly determine the magnitude of the loading dose itself.
*Clearance of the drug*
- **Clearance** is the rate at which the drug is removed from the body and is a primary determinant of the **maintenance dose** required to sustain a desired plasma concentration.
- It does not directly calculate the initial loading dose needed to achieve an immediate target concentration.
*Rate of administration*
- The **rate of administration** (e.g., infusion rate) primarily influences how quickly the drug reaches its target concentration, but not the total quantity of drug needed for the initial loading dose.
- It affects the kinetics of how the loading dose achieves the target concentration, rather than defining the dose amount.
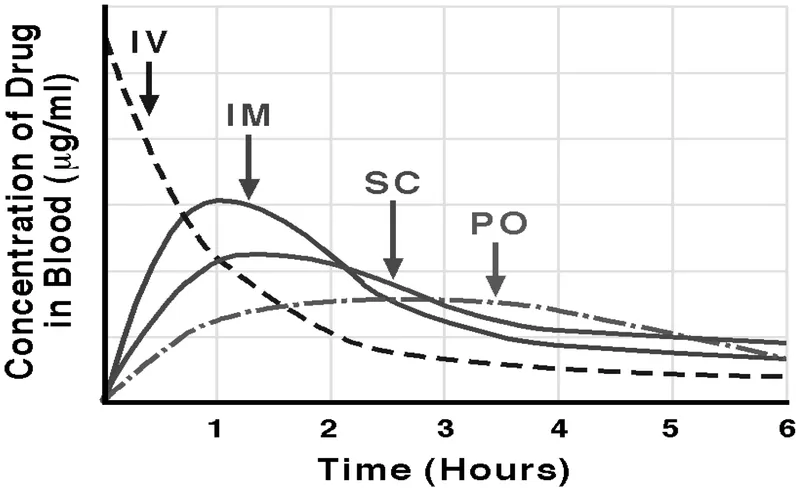
Absorption factors and bioavailability US Medical PG Question 8: A 45-year-old Caucasian man is given nitroglycerin for the management of his stable angina. Nitroglycerin given for the rapid relief of acute angina would most likely be given through what route of administration?
- A. Subcutaneous injection
- B. Intramuscular injection
- C. Intravenous injection
- D. Sublingual (Correct Answer)
- E. Oral
Absorption factors and bioavailability Explanation: ***Sublingual***
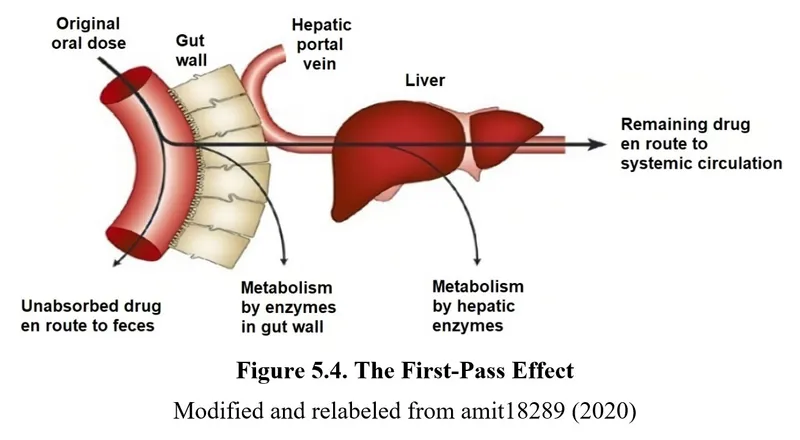
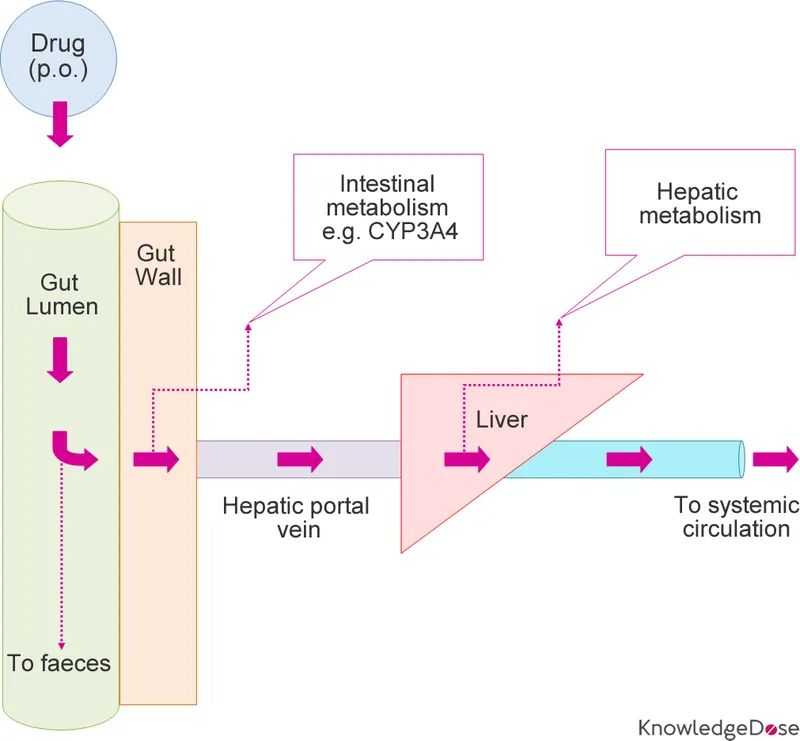
- **Sublingual** administration provides rapid absorption into the bloodstream through the oral mucosa, bypassing first-pass metabolism, which is crucial for quick relief of acute angina.
- This route allows the medication to exert its vasodilatory effects within 1-3 minutes, alleviating chest pain efficiently.
- It is the **standard of care** for outpatient management of acute angina episodes due to ease of self-administration.
*Subcutaneous injection*
- **Subcutaneous injection** has a slower onset of action compared to sublingual administration, making it unsuitable for rapid relief of acute angina.
- While it avoids first-pass metabolism, the absorption rate is not fast enough for emergency situations.
*Intramuscular injection*
- **Intramuscular injection** also has a relatively slower onset of action and is less predictable for rapid relief compared to sublingual routes.
- It is not a standard route for acute angina management due to the need for immediate action.
*Intravenous injection*
- **Intravenous administration** provides immediate systemic availability and is used for continuous infusion in unstable angina or acute coronary syndromes in hospital settings.
- However, it is **not practical for outpatient or self-administered rapid relief** due to the need for IV access, medical personnel, and monitoring.
- While highly effective in critical care, it is not the route for typical acute angina episodes outside the hospital.
*Oral*
- **Oral administration** undergoes significant **first-pass metabolism** in the liver, which delays the onset of action and reduces bioavailability, rendering it ineffective for rapid relief of acute angina.
- The delayed absorption (typically 30-60 minutes) makes it impractical for emergency situations where immediate vasodilation is needed.
- Oral nitrates are used for prophylaxis, not acute relief.
Absorption factors and bioavailability US Medical PG Question 9: A 47-year-old African-American woman presents to her primary care physician for a general checkup appointment. She works as a middle school teacher and has a 25 pack-year smoking history. She has a body mass index (BMI) of 22 kg/m^2 and is a vegetarian. Her last menstrual period was 1 week ago. Her current medications include oral contraceptive pills. Which of the following is a risk factor for osteoporosis in this patient?
- A. Smoking history (Correct Answer)
- B. Race
- C. Estrogen therapy
- D. Age
- E. Body mass index
Absorption factors and bioavailability Explanation: ***Smoking history***
- **Smoking** is a well-established risk factor for osteoporosis due to its negative effects on bone density and **calcium absorption**.
- Smokers have lower bone density and increased fracture risk due to direct toxic effects on osteoblasts and accelerated estrogen metabolism.
*Race*
- **African-American women** typically have higher bone mineral density and a lower risk of osteoporosis compared to Caucasians and Asians.
- This patient's racial background is considered a protective factor, not a risk factor, for osteoporosis.
*Estrogen therapy*
- **Oral contraceptive pills** contain estrogen, which helps maintain bone density and is protective against osteoporosis.
- Estrogen deficiency, not estrogen therapy, is a risk factor for osteoporosis, especially after menopause.
*Age*
- While **advancing age** is a significant risk factor for osteoporosis, this patient is 47 years old and still having regular menstrual periods, indicating pre-menopausal status.
- The effects of age on bone density become more pronounced after menopause due to declining estrogen levels.
*Body mass index*
- A **BMI of 22 kg/m^2** is within the normal range, and higher BMI is generally associated with greater bone density due to increased weight bearing and higher estrogen levels in adipose tissue.
- Being underweight (low BMI) is a risk factor for osteoporosis, as it often correlates with poorer nutritional status and lower bone mass.
Absorption factors and bioavailability US Medical PG Question 10: A 65-year-old man with a history of myocardial infarction is admitted to the hospital for treatment of atrial fibrillation with rapid ventricular response. He is 180 cm (5 ft 11 in) tall and weighs 80 kg (173 lb). He is given an intravenous bolus of 150 mg of amiodarone. After 20 minutes, the amiodarone plasma concentration is 2.5 mcg/mL. Amiodarone distributes in the body within minutes, and its elimination half-life after intravenous administration is 30 days. Which of the following values is closest to the volume of distribution of the administered drug?
- A. 60 L (Correct Answer)
- B. 80 L
- C. 150 L
- D. 17 L
- E. 10 L
Absorption factors and bioavailability Explanation: ***60 L***
- The **volume of distribution (Vd)** is calculated using the formula: **Vd = Dose / Plasma Concentration**.
- Given: Dose = 150 mg (150,000 mcg), Plasma concentration = 2.5 mcg/mL
- Calculation: Vd = 150,000 mcg / 2.5 mcg/mL = 60,000 mL = **60 L**
- Note: This calculation represents a simplified scenario. In clinical practice, amiodarone has an extremely large volume of distribution (60-100 L/kg or ~4,800-8,000 L in this patient) due to extensive tissue distribution, but the question tests the ability to apply the basic pharmacokinetic formula.
*80 L*
- This value would result if the plasma concentration were 1.875 mcg/mL (150,000 mcg / 80,000 mL), not the given 2.5 mcg/mL.
- This represents a common calculation error when working with pharmacokinetic parameters.
*150 L*
- This value would require a plasma concentration of 1 mcg/mL (150,000 mcg / 150,000 mL), which is lower than the measured 2.5 mcg/mL.
- This error might occur if the dose value were confused with the volume of distribution.
*17 L*
- This value would be obtained with a plasma concentration of approximately 8.8 mcg/mL (150,000 mcg / 17,000 mL), significantly higher than the measured 2.5 mcg/mL.
- This represents a significant underestimation of Vd and would suggest limited drug distribution.
*10 L*
- This value would require a plasma concentration of 15 mcg/mL (150,000 mcg / 10,000 mL), which is 6-fold higher than the given 2.5 mcg/mL.
- Such a small Vd would suggest drug confined primarily to plasma, which is inappropriate for lipophilic drugs with extensive tissue distribution.
More Absorption factors and bioavailability US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.