Pharmacokinetics (ADME principles)

On this page
🧬 ADME Mastery: Your Pharmacokinetic Command Center
You'll master how drugs journey through the body-from the moment they enter until they exit-by understanding absorption across membranes, distribution through tissues, metabolic transformation in the liver, and elimination via kidneys. These ADME principles explain why dosing intervals matter, why some patients need adjustments, and how drug interactions occur. By integrating these four processes, you'll predict therapeutic outcomes, optimize regimens, and troubleshoot clinical scenarios with the precision every patient deserves.
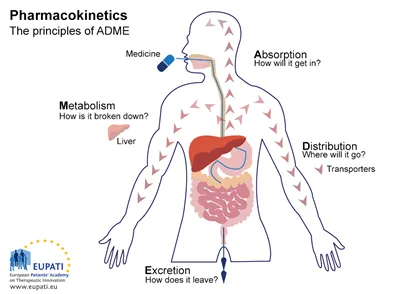
📌 Remember: ADME - Absorption gets it in, Distribution spreads it around, Metabolism breaks it down, Excretion kicks it out. Each phase follows quantifiable kinetics that predict clinical outcomes.
The ADME Framework Architecture
-
Absorption Phase
- Bioavailability: 0-100% depending on route and drug properties
- First-pass metabolism: Can reduce bioavailability by 30-90%
- Rate-limiting step: Often determines onset of action
- Oral absorption: 30 minutes to 4 hours
- IV administration: immediate bioavailability (100%)
- Sublingual: 15-30 minutes, bypasses first-pass
-
Distribution Phase
- Volume of distribution (Vd): 0.04-40 L/kg range
- Protein binding: 10-99% affects free drug concentration
- Tissue penetration: Determines site-specific efficacy
- CNS penetration: <1% for most drugs
- Adipose tissue: 10-50x higher for lipophilic drugs
| ADME Phase | Key Parameter | Normal Range | Clinical Impact | Monitoring |
|---|---|---|---|---|
| Absorption | Bioavailability (F) | 10-100% | Dose requirements | AUC comparison |
| Distribution | Volume (Vd) | 0.04-40 L/kg | Loading dose | Plasma levels |
| Metabolism | Clearance (CL) | 0.5-130 L/h | Maintenance dose | Half-life |
| Excretion | Renal clearance | 10-130 mL/min | Accumulation risk | Creatinine |
💡 Master This: ADME parameters are interconnected - half-life = 0.693 × Vd/CL. Understanding this relationship predicts dosing intervals and steady-state timing across all therapeutic classes.
Connect these foundational ADME principles through absorption mastery to understand how drugs enter systemic circulation with predictable kinetics.
🧬 ADME Mastery: Your Pharmacokinetic Command Center
🚪 Absorption Mastery: The Cellular Gateway
📌 Remember: PACT-ED - Passive diffusion, Active transport, Carrier-mediated, Transcytosis, Endocytosis, Diffusion through pores. Each mechanism has specific molecular size and polarity requirements.
Absorption Mechanism Hierarchy
-
Passive Diffusion (80% of oral drugs)
- Molecular weight: <500 Da optimal
- Lipophilicity: LogP 1-3 ideal range
- Rate equation: dC/dt = P × A × (C1-C2)
- P = permeability coefficient
- A = surface area (200 m² small intestine)
- Concentration gradient drives flux
-
Active Transport (15% of drugs)
- Carrier-mediated: Km values 10-100 μM
- Saturable kinetics: Vmax determines ceiling
- Energy-dependent: ATP or ion gradients
- P-glycoprotein: efflux pump reducing absorption
- OATP transporters: uptake enhancement
| Absorption Route | Bioavailability | Onset Time | First-Pass | Clinical Use |
|---|---|---|---|---|
| Intravenous | 100% | Immediate | None | Emergency, precise dosing |
| Sublingual | 75-95% | 5-15 min | Bypassed | Rapid onset needed |
| Oral | 10-95% | 30-120 min | Variable | Convenience, compliance |
| Rectal | 50-80% | 15-60 min | Partial bypass | Nausea, unconscious |
| Transdermal | 20-80% | 1-24 hours | Avoided | Sustained delivery |
💡 Master This: Bioequivalence requires AUC and Cmax within 80-125% of reference product. Understanding absorption kinetics predicts when generic substitution maintains therapeutic equivalence.
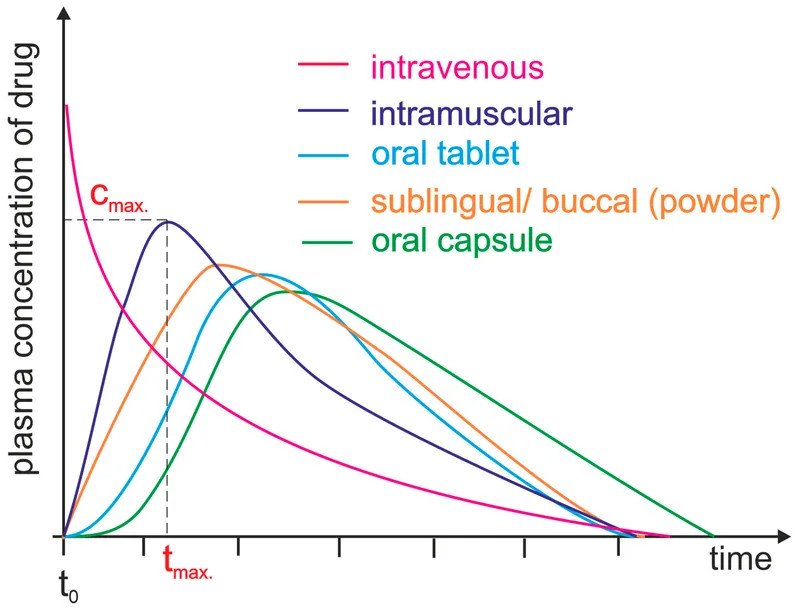
Bioavailability Determinants
-
Physicochemical Factors
- Dissolution rate: Rate-limiting for BCS Class II drugs
- Particle size: 10-fold reduction increases surface area 10-fold
- Crystal form: Polymorphs differ in solubility by 2-10x
- Amorphous forms: Higher energy, faster dissolution
- Crystalline forms: Stable but slower release
-
Physiological Variables
- Gastric pH: 1.5-3.5 affects weak acid/base ionization
- Intestinal transit: 3-5 hours determines contact time
- Blood flow: Splanchnic circulation 25% of cardiac output
- Exercise: Reduces GI blood flow by 50-80%
- Disease states: CHF reduces absorption 20-40%
⭐ Clinical Pearl: Enteric-coated formulations require pH >5.5 for dissolution. Proton pump inhibitors can delay release by 2-6 hours, affecting drugs like omeprazole and enteric aspirin.
Connect absorption mastery through distribution dynamics to understand how absorbed drugs reach target tissues with predictable concentration profiles.
🚪 Absorption Mastery: The Cellular Gateway
🌊 Distribution Dynamics: The Circulation Highway
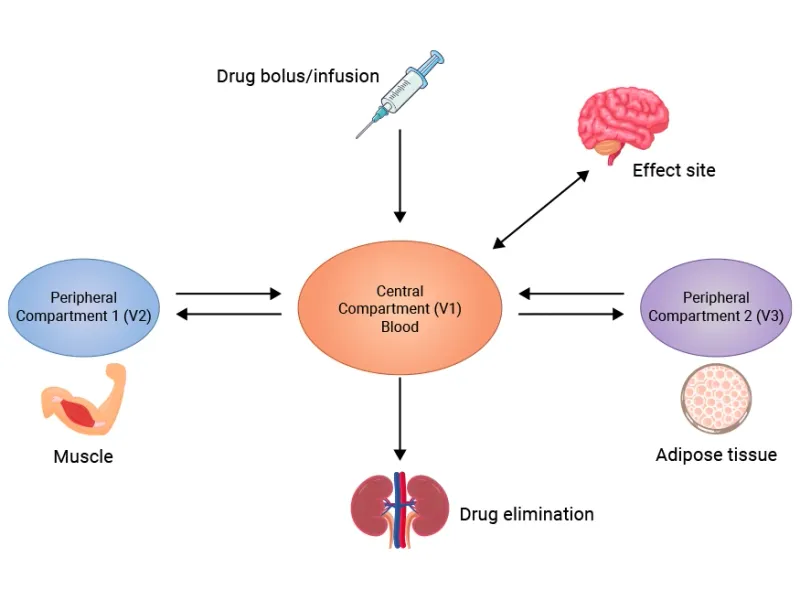
📌 Remember: VIP-BAT - Vd determines loading dose, Intravascular stays in blood, Plasma protein binding affects free drug, Barriers limit access, Adipose accumulates lipophilic drugs, Tissue binding prolongs action.
Volume of Distribution Architecture
-
Low Vd (0.04-0.2 L/kg) - Plasma-bound drugs
- Warfarin: Vd = 0.14 L/kg (99% protein bound)
- Furosemide: Vd = 0.1 L/kg (confined to extracellular)
- Clinical significance: Small loading doses, rapid equilibration
- Loading dose calculation: Dose = Vd × Target concentration
- Dialysis: Highly effective for removal
-
Medium Vd (0.2-1.0 L/kg) - Extracellular distribution
- Digoxin: Vd = 7 L/kg (tissue binding)
- Lithium: Vd = 0.8 L/kg (intracellular accumulation)
- Clinical significance: Moderate loading doses, tissue penetration
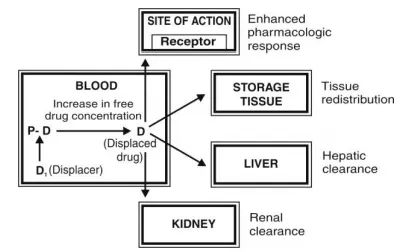
| Distribution Parameter | Low Value | High Value | Clinical Impact | Examples |
|---|---|---|---|---|
| Volume of Distribution | 0.04-0.2 L/kg | 10-40 L/kg | Loading dose needs | Warfarin vs Digoxin |
| Protein Binding | 10-50% | 90-99% | Drug interactions | Phenytoin, Warfarin |
| Tissue Penetration | <10% | >90% | Site-specific efficacy | CNS, Eye barriers |
| Clearance | 0.5-2 L/h | 50-130 L/h | Dosing frequency | Renal vs Hepatic |
| Half-life | 1-6 hours | 12-100 hours | Dosing intervals | Immediate vs Sustained |
Specialized Distribution Barriers
-
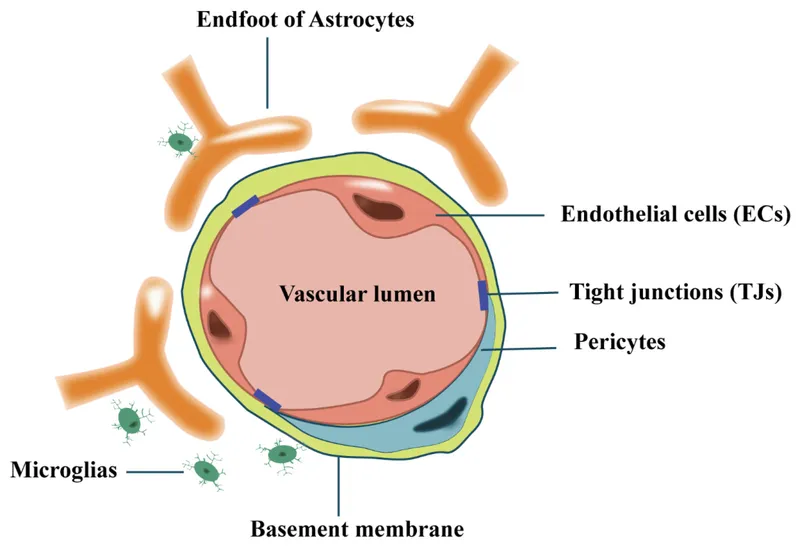
Blood-Brain Barrier
- P-glycoprotein efflux: Reduces CNS penetration 10-100x
- Tight junctions: Exclude hydrophilic molecules >180 Da
- Lipophilicity requirement: LogP >2 for significant penetration
- Morphine: CNS penetration 1-2%
- Fentanyl: CNS penetration 15-20%
- Propofol: CNS penetration 60-80%
-
Placental Barrier
- Molecular weight: <1000 Da crosses readily
- Protein binding: Reduces transfer by 50-90%
- Active transport: P-glycoprotein protection
- Category X drugs: Teratogenic risk >10%
- Warfarin: Crosses freely, teratogenic
- Heparin: Large molecule, doesn't cross
💡 Master This: Steady-state distribution requires 3-5 half-lives regardless of dosing frequency. Understanding this timing predicts when tissue concentrations equilibrate with plasma levels for optimal therapeutic monitoring.
- Tissue-Specific Accumulation
- Adipose tissue: Lipophilic drugs concentrate 10-50x
- Bone tissue: Bisphosphonates bind for months to years
- Thyroid gland: Iodine-containing drugs accumulate 100x
- Amiodarone: Thyroid half-life 100+ days
- Thiopental: Adipose redistribution terminates action
⭐ Clinical Pearl: Obesity increases Vd for lipophilic drugs by 20-200%, requiring higher loading doses but unchanged maintenance doses since clearance remains proportional to lean body weight.
Connect distribution dynamics through metabolic transformation to understand how drugs are chemically modified for elimination.
🌊 Distribution Dynamics: The Circulation Highway
⚗️ Metabolic Transformation: The Chemical Processing Plant
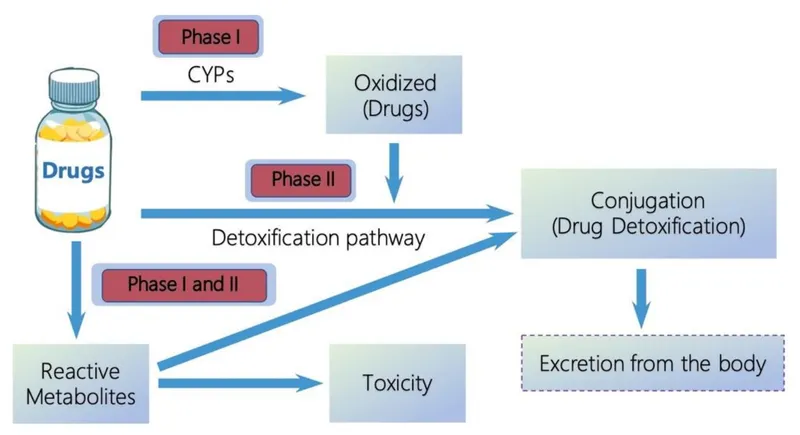
📌 Remember: PHASE-12 - Phase I makes Hydrophilic through Addition of polar groups, Synthetic reactions in Phase II Eliminate through conjugation. 1 = Oxidation/Reduction/Hydrolysis, 2 = Conjugation reactions.
Phase I Metabolism Architecture
-
Cytochrome P450 System (75% of Phase I reactions)
- CYP3A4: 50% of drug metabolism, intestinal and hepatic
- CYP2D6: 25% of drugs, genetic polymorphisms in 7-10%
- CYP2C9: 15% of drugs, warfarin metabolism
- Poor metabolizers: 2-10% of population
- Ultra-rapid metabolizers: 1-5% of population
- Activity range: 0-300% of normal
-
Non-P450 Enzymes (25% of Phase I reactions)
- Alcohol dehydrogenase: Zero-order kinetics at high concentrations
- Monoamine oxidase: Neurotransmitter metabolism
- Esterases: Rapid hydrolysis of esters and amides
| Metabolic Pathway | Enzyme System | Substrate Examples | Genetic Variation | Clinical Impact |
|---|---|---|---|---|
| CYP3A4 | Phase I Oxidation | Midazolam, Simvastatin | 5-20x variation | Drug interactions |
| CYP2D6 | Phase I Oxidation | Codeine, Metoprolol | 0-300% activity | Efficacy/toxicity |
| UGT1A1 | Phase II Conjugation | Bilirubin, SN-38 | Gilbert's syndrome | Hyperbilirubinemia |
| NAT2 | Phase II Acetylation | Isoniazid, Hydralazine | Slow/fast acetylators | Toxicity risk |
| TPMT | Phase II Methylation | 6-Mercaptopurine | 1:300 deficiency | Severe toxicity |
Phase II Conjugation Systems
-
Glucuronidation (UGT enzymes, 60% of Phase II)
- UGT1A1 deficiency: Gilbert's syndrome (5-10% population)
- Substrate capacity: High-capacity, low-affinity system
- Age effects: Reduced in neonates by 50-90%
- Chloramphenicol: Gray baby syndrome
- Morphine: Prolonged effects in newborns
-
Sulfation (SULT enzymes, 25% of Phase II)
- High-affinity, low-capacity system
- Saturable at therapeutic doses
- Acetaminophen: Sulfation saturates at >1g doses
💡 Master This: First-pass metabolism can be bypassed through sublingual, rectal, or transdermal routes. Nitroglycerin has 90% first-pass metabolism, requiring sublingual administration for acute effects.
Metabolic Drug Interactions
-
Enzyme Induction (Increased metabolism)
- Timeline: 3-7 days onset, 7-14 days maximum
- Magnitude: 2-10x increase in clearance
- Clinical effect: Reduced efficacy, withdrawal symptoms
- Carbamazepine: Auto-induction over 2-4 weeks
- Phenytoin: Induces own metabolism and other drugs
-
Enzyme Inhibition (Decreased metabolism)
- Timeline: Hours to days for competitive inhibition
- Magnitude: 50-95% reduction in clearance
- Clinical effect: Increased toxicity, prolonged effects
- Ketoconazole: Potent CYP3A4 inhibitor
- Grapefruit juice: Irreversible intestinal CYP3A4 inhibition
⭐ Clinical Pearl: Genetic polymorphisms in CYP2D6 affect codeine efficacy. Poor metabolizers (7% Caucasians) get no analgesia, while ultra-rapid metabolizers (1-5%) risk morphine toxicity from excessive conversion.
Connect metabolic transformation through excretion mechanisms to understand how drugs and metabolites are eliminated from the body.
⚗️ Metabolic Transformation: The Chemical Processing Plant
🚰 Excretion Mechanisms: The Elimination Highway
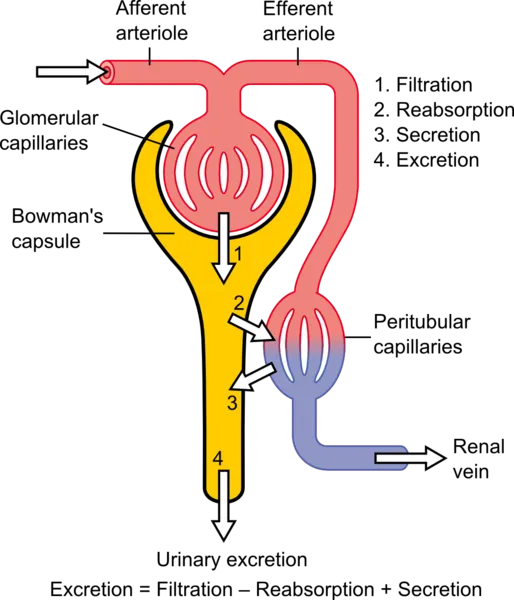
📌 Remember: FSR-CLEAR - Filtration is passive, Secretion is active, Reabsorption recovers drugs. Clearance = Load eliminated, Excretion = Active + passive, Renal function determines dosing.
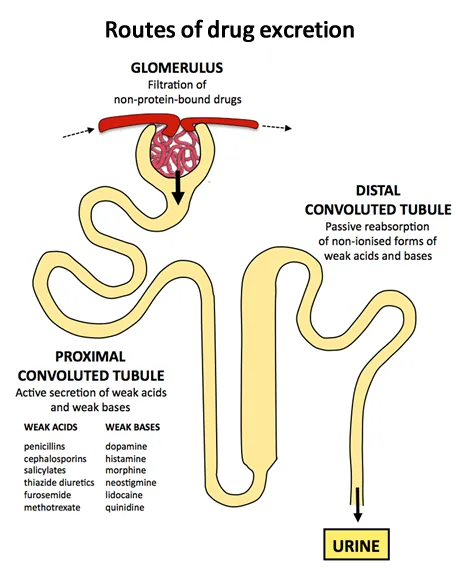
Renal Excretion Architecture
-
Glomerular Filtration (Passive process)
- GFR normal: 120-130 mL/min (180 L/day)
- Molecular weight cutoff: <20,000 Da filtered freely
- Protein binding: Only free drug filtered
- Filtration clearance = GFR × fu (fraction unbound)
- Creatinine clearance: Marker of GFR
- Age decline: 1 mL/min/year after age 30
-
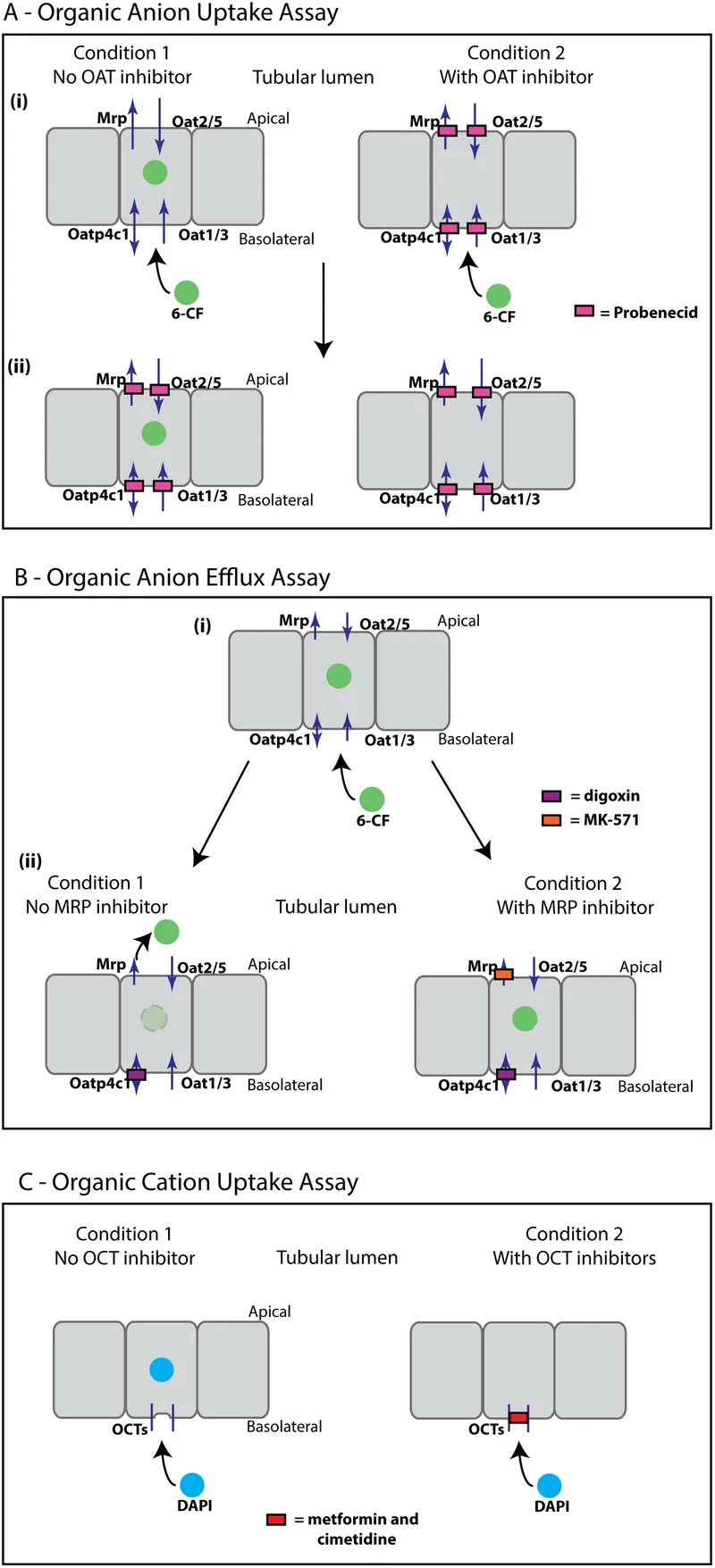
Active Tubular Secretion (Energy-dependent)
- Organic anion transporters (OAT): PAH, furosemide
- Organic cation transporters (OCT): Metformin, morphine
- P-glycoprotein: Digoxin efflux pump
- Secretion clearance: Can exceed GFR
- Saturable kinetics: Competition between drugs
- Probenecid: Blocks OAT, increases penicillin levels 4x
| Excretion Mechanism | Process Type | Capacity | Drug Examples | Clinical Significance |
|---|---|---|---|---|
| Glomerular Filtration | Passive | 120 mL/min | Creatinine, Inulin | GFR-dependent dosing |
| Tubular Secretion | Active | Variable | PAH, Furosemide | Drug interactions |
| Tubular Reabsorption | Active/Passive | pH-dependent | Weak acids/bases | Urine pH effects |
| Biliary Excretion | Active | MW >300 Da | Rifampin, Contrast | Enterohepatic cycling |
| Pulmonary Excretion | Passive | Volatile drugs | Anesthetics, Alcohol | Rapid elimination |
Tubular Reabsorption Dynamics
-
pH-Dependent Reabsorption
- Weak acids: Reabsorbed in acidic urine (pH <7)
- Weak bases: Reabsorbed in alkaline urine (pH >7)
- Henderson-Hasselbalch equation: Predicts ionization
- Aspirin overdose: Alkalinize urine to pH 8-8.5
- Amphetamine toxicity: Acidify urine to pH 5-6
- Urine pH range: 4.5-8.0 affects reabsorption 10-1000x
-
Transporter-Mediated Reabsorption
- Glucose transporters: SGLT2 inhibitors block reabsorption
- Uric acid transporters: URAT1 targeted by febuxostat
- Amino acid transporters: Gabapentin, pregabalin
💡 Master This: Renal clearance calculation: CLr = (Urine concentration × Urine flow) / Plasma concentration. Understanding this relationship enables dosing adjustments based on creatinine clearance estimates.
Non-Renal Excretion Pathways
-
Biliary Excretion (Molecular weight >300-500 Da)
- Active transport: BCRP, MRP2 transporters
- Enterohepatic circulation: Prolongs half-life
- Clinical examples: Rifampin (60% biliary), morphine glucuronides
- Cholestasis: Impairs biliary excretion
- Antibiotics: Disrupt enterohepatic cycling
-
Pulmonary Excretion (Volatile compounds)
- Partition coefficient: Blood:gas ratio determines elimination
- Ventilation-dependent: Hyperventilation accelerates
- Examples: Isoflurane, ethanol (5% exhaled)
⭐ Clinical Pearl: Creatinine clearance overestimates GFR by 10-40% due to tubular secretion. Cimetidine blocks creatinine secretion, providing more accurate GFR estimates for drug dosing.
Connect excretion mechanisms through integration mastery to understand how ADME processes interact in complex clinical scenarios.
🚰 Excretion Mechanisms: The Elimination Highway
🔄 Integration Mastery: The Pharmacokinetic Orchestra
📌 Remember: STEADY-CLEAR - Steady state in 5 half-lives, Time to equilibrium predictable, Elimination follows first-order, Accumulation depends on dosing interval, Dosing adjustments use Yield calculations, Clearance determines maintenance dose, Loading dose uses Effective volume, Adjustments for Renal/hepatic function.
Steady-State Kinetics Integration
-
Time to Steady State (Universal principle)
- 5 half-lives: 97% of steady state achieved
- Independent of dose or dosing frequency
- Clinical applications: Therapeutic monitoring timing
- Digoxin: t½ = 36 hours, steady state in 7-8 days
- Warfarin: t½ = 40 hours, steady state in 8-10 days
- Amiodarone: t½ = 50 days, steady state in 8-10 months
-
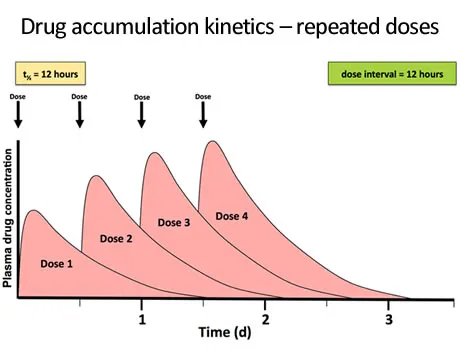
Accumulation Factor (Dosing interval effects)
- R = 1/(1-e^(-kτ)) where τ = dosing interval
- τ = t½: Accumulation factor = 2
- τ = 2×t½: Accumulation factor = 1.33
- Twice-daily dosing: Minimal accumulation if τ ≤ t½
- Once-daily dosing: Significant accumulation if t½ > 12 hours
| Integration Parameter | Mathematical Relationship | Clinical Application | Normal Range | Adjustment Factors |
|---|---|---|---|---|
| Half-life | t½ = 0.693 × Vd/CL | Dosing interval | 1-100 hours | Age, disease, genetics |
| Clearance | CL = Dose/AUC | Maintenance dose | 0.5-130 L/h | Renal/hepatic function |
| Bioavailability | F = AUCoral/AUCIV | Dose conversion | 10-100% | Route, formulation |
| Steady State | 5 × t½ | Monitoring timing | Hours to months | Half-life dependent |
| Loading Dose | LD = Vd × Ctarget/F | Rapid onset | Variable | Volume, target level |
Multi-System Integration Patterns
-
Hepatic-Renal Interactions
- Dual elimination: Requires both function assessments
- Metabolite accumulation: Active metabolites in renal failure
- Protein synthesis: Hypoalbuminemia increases free drug fraction
- Phenytoin: Renal failure increases free fraction 2-3x
- Morphine-6-glucuronide: Active metabolite accumulates
- Child-Pugh Class C: Reduce dose 50-75%
-
Age-Related Integration Changes
- Pediatric differences: Higher clearance/kg, different Vd
- Geriatric changes: Reduced clearance, altered distribution
- Physiological aging: GFR decline, hepatic blood flow reduction
- Neonates: Immature metabolism, prolonged half-lives
- Elderly: 30-50% reduction in hepatic clearance
- Creatinine clearance: Declines 1 mL/min/year after age 30
💡 Master This: Therapeutic drug monitoring requires understanding when to sample (steady state), what to measure (free vs total), and how to interpret (population vs individual kinetics).
Personalized Pharmacokinetics
-
Genetic Polymorphisms (Population variability)
- CYP2D6: Poor metabolizers 7%, ultra-rapid 1-5%
- CYP2C19: Poor metabolizers 15-20% in Asians
- TPMT deficiency: 1:300 risk of severe toxicity
- Pharmacogenetic testing: Guides initial dosing
- Phenotyping: Probe drugs assess enzyme activity
- Population kinetics: Bayesian estimation improves dosing
-
Disease State Modifications
- Heart failure: Reduced hepatic blood flow 30-50%
- Cirrhosis: Portosystemic shunting bypasses first-pass
- Obesity: Altered Vd for lipophilic drugs
- Dosing weight: Ideal vs actual vs adjusted
- Clearance scaling: Lean body weight for most drugs
⭐ Clinical Pearl: Population pharmacokinetics provides starting doses, but individual optimization requires therapeutic monitoring and dose titration based on clinical response and measured concentrations.
Connect integration mastery through clinical optimization to understand how pharmacokinetic principles guide therapeutic decision-making.
🔄 Integration Mastery: The Pharmacokinetic Orchestra
🎯 Clinical Optimization: The Therapeutic Precision Toolkit
📌 Remember: TARGET-DOSE - Timing of samples critical, Adjust for patient factors, Reach steady state first, Guide with population data, Evaluate response, Titrate systematically, Document changes, Optimize outcomes, Safety monitoring, Effectiveness assessment.
Therapeutic Drug Monitoring Mastery
-
Sampling Strategy (Timing determines accuracy)
- Steady state: 5 half-lives minimum before sampling
- Peak levels: 1-2 hours post-dose for most oral drugs
- Trough levels: Pre-dose for maintenance monitoring
- Digoxin: 6-8 hours post-dose (distribution complete)
- Vancomycin: Trough <20 mg/L, peak 25-40 mg/L
- Phenytoin: Any time at steady state (long half-life)
-
Interpretation Framework (Population vs individual)
- Therapeutic range: Population-derived guidelines
- Individual optimization: Clinical response guides targets
- Free drug monitoring: Hypoalbuminemia, renal failure
- Phenytoin free fraction: Normal 10%, uremia 20-25%
- Valproic acid: Free levels in hepatic dysfunction
- Protein binding displacement: Clinically significant >90% bound
| Drug Class | Target Range | Sampling Time | Adjustment Factor | Monitoring Frequency |
|---|---|---|---|---|
| Digoxin | 1.0-2.0 ng/mL | 6-8h post-dose | Renal function | Weekly initially |
| Phenytoin | 10-20 mg/L | Any (steady state) | Albumin, genetics | 2-3 times/week |
| Vancomycin | Trough 10-20 mg/L | Pre-dose | Renal function | Every 3rd dose |
| Lithium | 0.6-1.2 mEq/L | 12h post-dose | Renal, sodium | Weekly initially |
| Warfarin | INR 2.0-3.0 | Any time | CYP2C9, VKORC1 | Daily initially |
Population-Based Dosing Algorithms
-
Renal Function Adjustments
- Cockcroft-Gault equation: Most validated for drug dosing
- MDRD/CKD-EPI: More accurate GFR, less validated for dosing
- Adjustment methods: Dose reduction vs interval extension
- CrCl 50-80 mL/min: Reduce dose 25%
- CrCl 10-50 mL/min: Reduce dose 50%
- CrCl <10 mL/min: Reduce dose 75% or extend interval
-
Hepatic Function Adjustments
- Child-Pugh Score: Class A (5-6), B (7-9), C (10-15)
- Dose reductions: Class A: 25%, Class B: 50%, Class C: 75%
- High hepatic extraction: Greater dose reduction needed
- Propranolol: First-pass 70%, major reduction needed
- Morphine: Hepatic metabolism 90%, significant adjustment
💡 Master This: Bayesian dosing combines population pharmacokinetics with individual patient data to predict optimal doses. Software programs use measured concentrations to refine estimates and improve precision.
Advanced Optimization Strategies
-
Pharmacokinetic-Pharmacodynamic Integration
- Effect compartment modeling: Delays between concentration and effect
- Hysteresis loops: Concentration-effect relationships
- Tolerance development: Receptor desensitization
- Warfarin: 3-5 day delay for anticoagulant effect
- Furosemide: Tolerance develops with continuous infusion
- Morphine: Tolerance requires dose escalation
-
Special Population Considerations
- Obesity: Dosing weight selection critical
- Pregnancy: Increased clearance, altered distribution
- Critical illness: Altered protein binding, organ dysfunction
- Aminoglycosides: Ideal body weight for dosing
- Vancomycin: Actual body weight if obese
- Pregnancy: Increase doses 25-50% for many drugs
⭐ Clinical Pearl: Model-informed precision dosing (MIPD) uses real-time data and population models to continuously optimize dosing regimens, improving therapeutic outcomes by 20-40% compared to standard approaches.
Understanding ADME mastery through clinical optimization provides the quantitative foundation for evidence-based therapeutics, enabling precision medicine approaches that maximize therapeutic benefit while minimizing adverse effects through systematic application of pharmacokinetic principles.
🎯 Clinical Optimization: The Therapeutic Precision Toolkit
Practice Questions: Pharmacokinetics (ADME principles)
Test your understanding with these related questions
What is the primary mechanism for iron absorption in the duodenum?













