Pharmacokinetic interaction mechanisms US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Pharmacokinetic interaction mechanisms. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Pharmacokinetic interaction mechanisms US Medical PG Question 1: A 61-year-old male is given acetazolamide to treat open-angle glaucoma. Upon diuresis, his urine is found to be highly alkaline. Which of the following accounts for the alkaline nature of this patient’s urine?
- A. Inhibition of bicarbonate reabsorption in the proximal tubule (Correct Answer)
- B. Inhibition of bicarbonate reabsorption in beta-intercalated cells
- C. Inhibition of acid secretion in alpha-intercalated cells
- D. Inhibition of chloride reabsorption in the distal convoluted tubule
- E. Inhibition of chloride reabsorption in the thick ascending loop of Henle
Pharmacokinetic interaction mechanisms Explanation: ***Inhibition of bicarbonate reabsorption in the proximal tubule***
- **Acetazolamide** is a **carbonic anhydrase inhibitor** that primarily acts on the **proximal tubule** of the kidney.
- Its action here prevents the reabsorption of **bicarbonate (HCO3-)**, leading to its increased excretion in the urine and thus making the urine alkaline.
*Inhibition of chloride reabsorption in the distal convoluted tubule*
- This effect is typically associated with **thiazide diuretics**, which inhibit the **Na-Cl cotransporter** in the distal convoluted tubule.
- While it affects electrolyte balance, it does not directly lead to the observed **alkaline urine** in the manner described.
*Inhibition of bicarbonate reabsorption in beta-intercalated cells*
- **Beta-intercalated cells** in the collecting duct secrete bicarbonate, and their inhibition would lead to **acidic urine**, not alkaline.
- They play a role in **bicarbonate secretion**, not reabsorption as seen with acetazolamide's primary action.
*Inhibition of acid secretion in alpha-intercalated cells*
- **Alpha-intercalated cells** secrete acid (H+) into the urine. Inhibiting their function would reduce acid excretion, making the urine less acidic or even alkaline.
- However, the primary mechanism of acetazolamide's effect on urine pH is through **bicarbonate wasting** in the proximal tubule, not direct inhibition of acid secretion in the collecting duct.
*Inhibition of chloride reabsorption in the thick ascending loop of Henle*
- This is the mechanism of action for **loop diuretics** like furosemide, which inhibit the **Na-K-2Cl cotransporter**.
- While loop diuretics cause significant diuresis, they do not directly lead to the pronounced **urinary alkalinization** seen with acetazolamide.
Pharmacokinetic interaction mechanisms US Medical PG Question 2: A 72-year-old man presents to the emergency department with a 1 hour history of bruising and bleeding. He says that he fell and scraped his knee on the ground. Since then, he has been unable to stop the bleeding and has developed extensive bruising around the area. He has a history of gastroesophageal reflux disease, hypertension, and atrial fibrillation for which he is taking an oral medication. He says that he recently started taking omeprazole for reflux. Which of the following processes is most likely inhibited in this patient?
- A. Sulfation
- B. Oxidation (Correct Answer)
- C. Filtration
- D. Acetylation
- E. Glucuronidation
Pharmacokinetic interaction mechanisms Explanation: ***Oxidation***
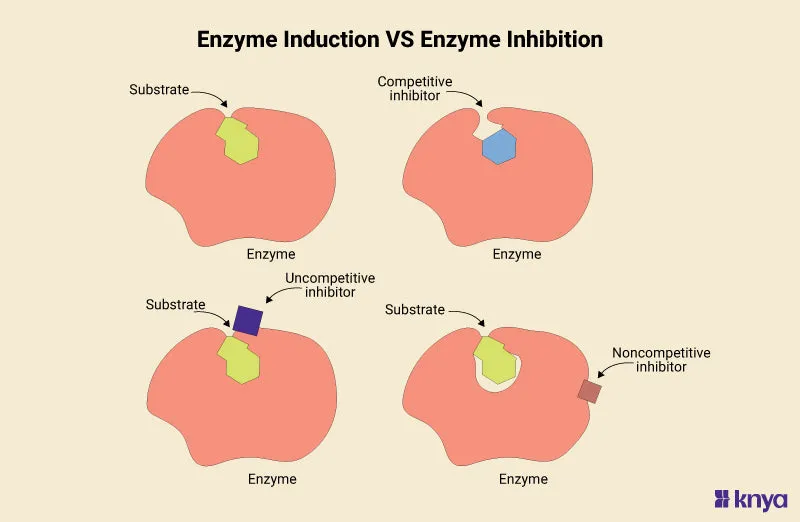
- The patient is taking **omeprazole**, a proton pump inhibitor, which is a known **CYP450 inhibitor**.
- Since the patient is also on an **oral anticoagulant** for atrial fibrillation, inhibition of CYP450 enzymes can reduce the metabolism of the anticoagulant, leading to **increased anticoagulant effect** and subsequent bleeding and bruising.
*Sulfation*
- **Sulfation** is a phase II metabolic reaction that converts compounds into more polar and excretable forms, but omeprazole primarily affects phase I metabolism involving CYP450 enzymes.
- While sulfation can be important for the metabolism of some drugs, it is not the primary process inhibited by omeprazole to cause increased bleeding with oral anticoagulants.
*Filtration*
- **Filtration** is a renal process and not a metabolic enzyme pathway affected by omeprazole.
- Omeprazole's interaction with anticoagulants mainly occurs through hepatic metabolism, not renal filtration.
*Acetylation*
- **Acetylation** is a phase II metabolic reaction, primarily carried out by **N-acetyltransferases**.
- Omeprazole is primarily known to interact with **CYP450 enzymes** (phase I metabolism) rather than N-acetyltransferases.
*Glucuronidation*
- **Glucuronidation** is a phase II metabolic reaction involving **UGT enzymes** that typically inactivates and increases the excretion of drugs.
- While important for drug metabolism, omeprazole's primary drug interactions leading to increased anticoagulant effects are via **CYP450 inhibition** (phase I metabolism), not directly through glucuronidation.
Pharmacokinetic interaction mechanisms US Medical PG Question 3: A 72-year-old woman presents to the clinic complaining of diarrhea for the past week. She mentions intense fatigue and intermittent, cramping abdominal pain. She has not noticed any blood in her stool. She recalls an episode of pneumonia last month for which she was hospitalized and treated with antibiotics. She has traveled recently to Florida to visit her family and friends. Her past medical history is significant for hypertension, peptic ulcer disease, and hypercholesterolemia for which she takes losartan, esomeprazole, and atorvastatin. She also has osteoporosis, for which she takes calcium and vitamin D and occasional constipation for which she takes an over the counter laxative as needed. Physical examination shows lower abdominal tenderness but is otherwise insignificant. Blood pressure is 110/70 mm Hg, pulse is 80/min, and respiratory rate is 18/min. Stool testing is performed and reveals the presence of anaerobic, gram-positive bacilli. Which of the following increased this patient’s risk of developing this clinical presentation?
- A. Hypercholesterolemia treated with atorvastatin
- B. Constipation treated with laxatives
- C. Osteoporosis treated with calcium and vitamin D
- D. Peptic ulcer disease treated with esomeprazole
- E. Recent antibiotic use for pneumonia treatment (Correct Answer)
Pharmacokinetic interaction mechanisms Explanation: ***Recent antibiotic use for pneumonia treatment***
- **Antibiotic exposure** is the single most important risk factor for *Clostridioides difficile* infection (CDI), present in approximately 70% of cases.
- Antibiotics disrupt the normal protective gut microbiota, eliminating competitive bacteria and allowing *C. difficile* spores to germinate, colonize, and produce toxins.
- The patient's recent hospitalization and antibiotic treatment for pneumonia directly precipitated this infection by creating an ecological niche for *C. difficile* overgrowth.
- Common culprit antibiotics include fluoroquinolones, clindamycin, cephalosporins, and penicillins.
*Peptic ulcer disease treated with esomeprazole*
- **Proton pump inhibitors (PPIs)** like esomeprazole are an independent risk factor for CDI, increasing risk approximately 2-3 fold.
- PPIs reduce gastric acid production, which normally serves as a defense mechanism against ingested *C. difficile* spores.
- However, PPIs alone do not typically cause CDI without concurrent disruption of gut flora (usually by antibiotics).
- While this is a contributory risk factor in this patient, it is not the primary cause.
*Hypercholesterolemia treated with atorvastatin*
- **Statins** like atorvastatin have no established association with increased risk of *Clostridioides difficile* infection.
- They work by inhibiting HMG-CoA reductase to lower cholesterol and do not affect gastric pH or gut microbiota composition.
*Constipation treated with laxatives*
- Occasional **over-the-counter laxative use** is not a risk factor for *Clostridioides difficile* infection.
- While laxatives affect gut motility, they do not disrupt the protective gut microbiota or increase susceptibility to CDI.
*Osteoporosis treated with calcium and vitamin D*
- **Calcium and vitamin D supplementation** has no association with increased risk of *Clostridioides difficile* infection.
- These supplements support bone health and calcium metabolism without affecting gut flora or gastric acid production.
Pharmacokinetic interaction mechanisms US Medical PG Question 4: A 76-year-old man comes to the physician for a follow-up examination. One week ago, he was prescribed azithromycin for acute bacterial sinusitis. He has a history of atrial fibrillation treated with warfarin and metoprolol. Physical examination shows no abnormalities. Compared to one month ago, laboratory studies show a mild increase in INR. Which of the following best explains this patient's laboratory finding?
- A. Drug-induced hepatotoxicity
- B. Depletion of intestinal flora
- C. Inhibition of cytochrome p450 (Correct Answer)
- D. Increased gastrointestinal absorption of warfarin
- E. Increased non-protein bound warfarin fraction
Pharmacokinetic interaction mechanisms Explanation: ***Inhibition of cytochrome p450***
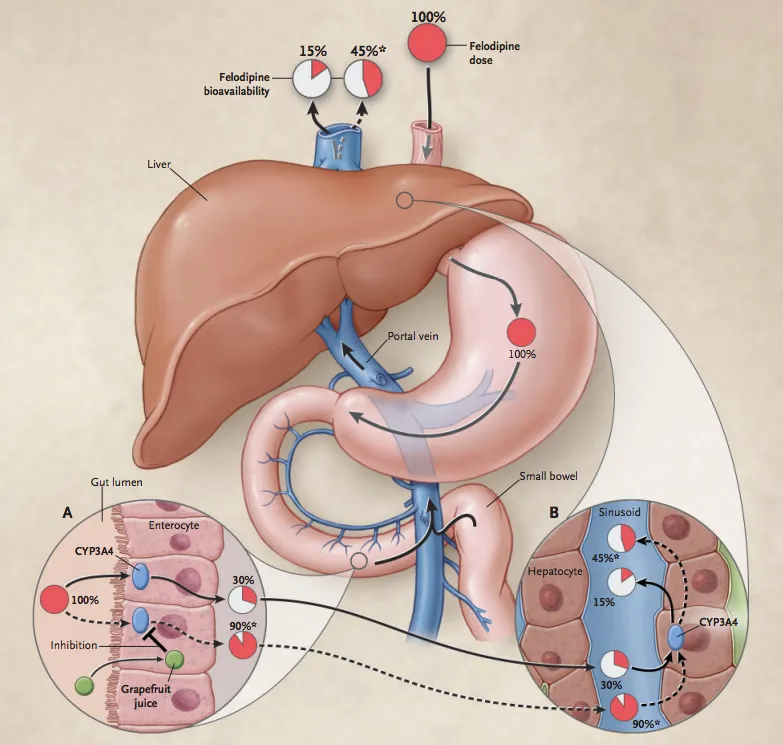
- **Azithromycin**, while a weaker inhibitor compared to erythromycin and clarithromycin, **does inhibit CYP3A4 and other cytochrome P450 enzymes** to a clinically significant degree.
- This inhibition **reduces warfarin metabolism**, leading to increased warfarin levels and **enhanced anticoagulant effect**, manifesting as an **increased INR**.
- This pharmacokinetic interaction is well-documented and is the **primary mechanism** for azithromycin-warfarin interaction.
*Depletion of intestinal flora*
- The theory that antibiotics deplete **vitamin K-producing gut bacteria** leading to increased warfarin effect is a **common misconception**.
- Humans obtain vitamin K primarily from **dietary sources** (leafy greens, vegetable oils), not from gut bacterial synthesis; intestinal bacteria contribute minimally to vitamin K stores.
- This mechanism has been **debunked** in modern pharmacology literature and does not explain antibiotic-warfarin interactions.
*Drug-induced hepatotoxicity*
- While hepatotoxicity can impair **clotting factor synthesis** and increase INR, **azithromycin** rarely causes significant liver injury.
- The presentation shows only a **mild INR increase** one week after starting therapy, without other signs of liver dysfunction.
- This acute, mild change is more consistent with a **pharmacokinetic drug interaction** than hepatotoxicity.
*Increased gastrointestinal absorption of warfarin*
- **Warfarin** has high oral bioavailability (~100%) under normal conditions.
- **Azithromycin** does not enhance the **gastrointestinal absorption** of warfarin.
- This mechanism is not supported by pharmacological evidence for this drug interaction.
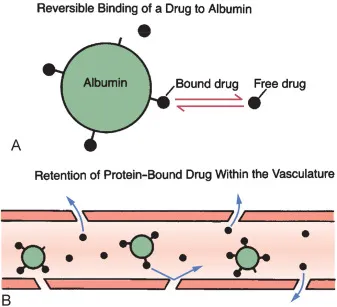
*Increased non-protein bound warfarin fraction*
- Displacement of warfarin from **plasma protein binding sites** can transiently increase free drug.
- However, **azithromycin** does not significantly displace warfarin from **albumin**.
- This mechanism does not explain the sustained INR elevation seen with azithromycin therapy.
Pharmacokinetic interaction mechanisms US Medical PG Question 5: A group of researchers wish to develop a clinical trial assessing the efficacy of a specific medication on the urinary excretion of amphetamines in intoxicated patients. They recruit 50 patients for the treatment arm and 50 patients for the control arm of the study. Demographics are fairly balanced between the two groups. The primary end points include (1) time to recovery of mental status, (2) baseline heart rate, (3) urinary pH, and (4) specific gravity. Which medication should they use in order to achieve a statistically significant result positively favoring the intervention?
- A. Potassium citrate
- B. Ascorbic acid (Correct Answer)
- C. Tap water
- D. Sodium bicarbonate
- E. Aluminum hydroxide
Pharmacokinetic interaction mechanisms Explanation: ***Ascorbic acid***
- Urinary excretion of **weak bases** like amphetamines is enhanced in an **acidic urine environment**. Ascorbic acid, or vitamin C, is an acidic substance that, when administered, can significantly **lower urinary pH**.
- By acidifying the urine, ascorbic acid promotes the **ionization of amphetamines** in the renal tubules, making them less lipid-soluble and decreasing their reabsorption, thereby **increasing their urinary excretion**.
*Potassium citrate*
- Potassium citrate is a **urinary alkalinizer**, meaning it would increase the pH of the urine.
- Increasing urinary pH would **decrease the excretion of acidic drugs** and **increase the reabsorption of basic drugs** like amphetamines, which is the opposite of the desired effect.
*Tap water*
- Administering tap water would primarily lead to **diuresis** (increased urine production) but would have a **negligible effect on urinary pH**.
- While increased urine volume can dilute the concentration of amphetamines, it does not significantly alter the **renal clearance rate based on pH**, which is crucial for weak bases.
*Sodium bicarbonate*
- Sodium bicarbonate is a potent **urinary alkalinizer**, used to increase the pH of the urine.
- Just like potassium citrate, a higher urinary pH would **inhibit the excretion of amphetamines** by promoting their non-ionized, lipid-soluble form and increasing their reabsorption.
*Aluminum hydroxide*
- Aluminum hydroxide is primarily an **antacid** and phosphate binder, used for conditions like GERD or hyperphosphatemia; it has **no significant direct effect on urinary pH or amphetamine excretion**.
- Its action is largely confined to the gastrointestinal tract, and it does not get absorbed in a way that would acidify the urine.
Pharmacokinetic interaction mechanisms US Medical PG Question 6: A 55-year-old male presents to his primary care physician for a normal check-up. He has a history of atrial fibrillation for which he takes metoprolol and warfarin. During his last check-up, his international normalized ratio (INR) was 2.5. He reports that he recently traveled to Mexico for a business trip where he developed a painful red rash on his leg. He was subsequently prescribed an unknown medication by a local physician. The rash resolved after a few days and he currently feels well. His temperature is 98.6°F (37°C), blood pressure is 130/80 mmHg, pulse is 95/min, and respirations are 18/min. Laboratory analysis reveals that his current INR is 4.5. Which of the following is the most likely medication this patient took while in Mexico?
- A. Griseofulvin
- B. Rifampin
- C. St. John’s wort
- D. Trimethoprim-sulfamethoxazole (Correct Answer)
- E. Phenobarbital
Pharmacokinetic interaction mechanisms Explanation: ***Trimethoprim-sulfamethoxazole***
- **Trimethoprim-sulfamethoxazole** is a potent inhibitor of **CYP2C9**, the primary enzyme responsible for metabolizing **warfarin**, leading to significantly increased INR and bleeding risk.
- The patient's **elevated INR (4.5)** from a previous stable level of 2.5 strongly suggests an interaction with a medication that inhibits warfarin metabolism, and trimethoprim-sulfamethoxazole is a common culprit.
- TMP-SMX is commonly used to treat **cellulitis** and other skin infections, which aligns with the clinical presentation of a painful red rash.
*Griseofulvin*
- **Griseofulvin** is an antifungal agent that acts as a **CYP inducer**, which would *increase* warfarin metabolism and lead to a *decreased* INR, not the elevated INR seen in this patient.
- While it could treat fungal skin infections (e.g., tinea), it would cause the opposite effect on warfarin levels.
*Rifampin*
- **Rifampin** is a strong **CYP inducer**, meaning it would *increase* warfarin metabolism and thus *decrease* INR, leading to a higher risk of clotting, which is the opposite of what is seen in this patient.
- It is often used for tuberculosis or serious bacterial infections, not typically for a simple skin rash.
*St. John's wort*
- **St. John's wort** is a known **CYP inducer**, similar to rifampin, and would lead to a *decrease* in warfarin levels and INR.
- It is an herbal supplement primarily used for depression and would not typically be prescribed by a physician for a rash.
*Phenobarbital*
- **Phenobarbital** is a potent **CYP inducer**, which would *accelerate* warfarin metabolism and result in a *decreased* INR, increasing the risk of thrombosis.
- It is an anticonvulsant and sedative, not a medication typically prescribed for a rash.
Pharmacokinetic interaction mechanisms US Medical PG Question 7: A 29-year-old woman presents with shortness of breath and chest pain for the past week. She says her chest pain is aggravated by deep breathing and she becomes short of breath while walking upstairs in her home. She also has been feeling feverish and fatigued for the past week, as well as pain in her wrists, hands, and left knee. Review of systems is significant for a 4.5 kg (10.0 lb) weight loss over the previous month. Past medical history consists of 2 spontaneous abortions, both of which occurred in the 1st trimester. On physical examination, there is a pink rash present over her face, which is aggravated by exposure to sunlight. There are decreased breath sounds on the right. A chest radiograph is performed which reveals evidence of a right pleural effusion. Serum ANA and anti-dsDNA autoantibodies are positive. Urinalysis is unremarkable. Errors with which of the following is most likely to lead to her disease?
- A. Intrinsic pathway
- B. Cytotoxic CD8+ T cells
- C. Bcl-2 overexpression
- D. Necrosis
- E. Fas-FasL interaction (Correct Answer)
Pharmacokinetic interaction mechanisms Explanation: ***Fas-FasL interaction***
- This patient presents with multiple symptoms suggestive of **systemic lupus erythematosus (SLE)**, including photosensitive rash, arthritis, serositis (pleural effusion), weight loss, recurrent spontaneous abortions, and positive ANA/anti-dsDNA.
- Genetic defects in the **Fas** or **Fas ligand (FasL) apoptotic pathway** are strongly associated with increased risk of autoimmunity, particularly SLE, as they impair the deletion of autoreactive lymphocytes.
*Intrinsic pathway*
- The intrinsic apoptotic pathway is primarily activated by intracellular stress and mitochondria-dependent signals.
- While essential for cell death, defects in the intrinsic pathway are not as specifically implicated in the pathogenesis of SLE as the Fas-FasL (extrinsic) pathway.
*Cytotoxic CD8+ T cells*
- **CD8+ T cells** are primarily involved in killing virally infected or cancerous cells and are crucial for cellular immunity.
- While involved in some autoimmune processes, their dysfunction is not the primary or most common error leading to the development of SLE, which is largely mediated by autoantibodies.
*Bcl-2 overexpression*
- **Bcl-2** is an anti-apoptotic protein, and its overexpression inhibits apoptosis.
- While Bcl-2 overexpression could theoretically prevent the deletion of autoreactive cells, specific defects in the direct Fas-FasL signaling pathway are more directly and commonly linked to the immune dysregulation seen in SLE.
*Necrosis*
- **Necrosis** is an uncontrolled form of cell death often associated with inflammation and tissue damage.
- While certainly present in tissues affected by SLE due to inflammation, necrosis itself is a consequence of the disease process, not an upstream error in cell death regulation that leads to the autoimmunity of SLE.
Pharmacokinetic interaction mechanisms US Medical PG Question 8: A 66-year-old man was referred for endoscopic evaluation due to iron deficiency anemia. He has had anorexia and weight loss for two months. Three years ago, the patient had coronary artery bypass grafting and aortic mechanical valve replacement. He has a 12-year history of diabetes mellitus and hypertension. He takes warfarin, lisinopril, amlodipine, metformin, aspirin, and carvedilol. His blood pressure is 115/65 mm Hg, pulse is 68/min, respirations are 14/min, temperature is 36.8°C (98.2°F), and blood glucose is 220 mg/dL. Conjunctivae are pale. Heart examination reveals a metallic click just before the carotid pulse. Which of the following is the most appropriate switch in this patient’s drug therapy before the endoscopy?
- A. Metformin to empagliflozin
- B. Aspirin to clopidogrel
- C. Lisinopril to losartan
- D. Warfarin to heparin (Correct Answer)
- E. Amlodipine to diltiazem
Pharmacokinetic interaction mechanisms Explanation: ***Warfarin to heparin***
- The patient is on **warfarin** due to his **mechanical aortic valve**, which increases his risk of bleeding during endoscopy.
- Switching to **heparin (bridging therapy)** allows for a shorter half-life and easier reversal if bleeding occurs, making it safer for the procedure.
*Metformin to empagliflozin*
- This change in **antidiabetic medication** does not address the immediate concern of bleeding risk for endoscopy.
- **Empagliflozin** can cause **euglycemic diabetic ketoacidosis** and its benefits related to cardiovascular outcomes are long term, not relevant to peri-procedural management.
*Aspirin to clopidogrel*
- Both **aspirin** and **clopidogrel** are **antiplatelet agents** that increase bleeding risk.
- Switching from one to the other does not mitigate the bleeding risk for endoscopy; often, one or both are held before such procedures if possible.
*Lisinopril to losartan*
- Both **lisinopril** and **losartan** are **antihypertensive medications** (ACE inhibitor and ARB, respectively) with similar effects on blood pressure.
- This change would not impact the **bleeding risk** or the need for peri-procedural anticoagulation management.
*Amlodipine to diltiazem*
- Both **amlodipine** and **diltiazem** are **calcium channel blockers** used for hypertension and angina.
- While they have different mechanisms, switching between them does not address the immediate safety concern of **bleeding risk** during endoscopy.
Pharmacokinetic interaction mechanisms US Medical PG Question 9: A 17-year-old woman is rushed into the emergency department by her father who found her collapsed in her bedroom 15 minutes before the ambulance's arrival. There was an empty bottle of clomipramine in her bedroom which her mother takes for her depression. Vital signs include the following: respiratory rate 8/min, pulse 130/min, and blood pressure 100/60 mm Hg. On physical examination, the patient is unresponsive to vocal and tactile stimuli. Oral mucosa and tongue are dry, and the bladder is palpable. A bedside electrocardiogram (ECG) shows widening of the QRS complexes. Which of the following would be the best course of treatment in this patient?
- A. Norepinephrine
- B. Sodium bicarbonate (Correct Answer)
- C. Diazepam
- D. Lidocaine
- E. Induced vomiting
Pharmacokinetic interaction mechanisms Explanation: ***Sodium bicarbonate***
- The patient presents with classic signs of **tricyclic antidepressant (TCA) overdose**, including coma, tachycardia, hypotension, dry oral mucosa (anticholinergic effects), urinary retention, and significantly, **widened QRS complexes** on ECG.
- **Sodium bicarbonate** is the treatment of choice for TCA overdose-induced cardiotoxicity, as it alkalinizes the blood, reducing the binding of TCAs to myocardial fast sodium channels and improving cardiac conduction.
- The primary indication is **QRS widening >100 ms**, which indicates severe sodium channel blockade and increased risk of ventricular arrhythmias.
*Norepinephrine*
- While the patient is hypotensive, **norepinephrine** (a vasopressor) primarily addresses blood pressure and not the underlying cardiotoxicity or arrhythmias caused by TCA overdose.
- Using vasopressors without addressing the membrane-stabilizing effects of TCAs on the heart may not resolve the critical cardiac issues.
- Sodium bicarbonate should be administered first to address the cardiotoxicity.
*Diazepam*
- **Diazepam** is a benzodiazepine used to treat seizures or agitation, which can sometimes occur in TCA overdose.
- However, it does not address the vital signs or the severe cardiotoxicity (widened QRS) which is the most life-threatening complication here.
*Lidocaine*
- **Lidocaine** is an antiarrhythmic drug; however, it is relatively contraindicated in TCA overdose as it blocks cardiac sodium channels, which could worsen the existing sodium channel blockade caused by TCAs.
- This could exacerbate QRS widening and increase the risk of malignant arrhythmias.
*Induced vomiting*
- **Induced vomiting** (e.g., with ipecac syrup) is contraindicated in cases of overdose, particularly with altered mental status, due to the high risk of **aspiration pneumonitis**.
- In a patient who is unresponsive, attempts at gastric decontamination via emesis are dangerous and ineffective.
Pharmacokinetic interaction mechanisms US Medical PG Question 10: A 45-year-old woman presents to the emergency department with fever, cough, tonsillar enlargement, and bleeding lips. She has a diffuse blistering rash that encompasses the palms and soles of her feet, in total covering 55% of her total body surface area (TBSA). The upper epidermal layer easily slips away with slight rubbing. Within 24 hours the rash progresses to 88% TBSA involvement and the patient requires mechanical ventilation for respiratory distress. Which of the following is the most likely etiology of this patient’s condition?
- A. Cytomegalovirus
- B. Deficiency of C-1 esterase inhibitor
- C. Exposure to carbamazepine (Correct Answer)
- D. Herpes simplex virus
- E. Molluscum contagiosum
Pharmacokinetic interaction mechanisms Explanation: ***Exposure to carbamazepine***
- The rapid progression of a widespread blistering rash, **positive Nikolsky's sign** (skin slipping away), significant TBSA involvement (55% rapidly increasing to 88%), and systemic symptoms (fever, respiratory distress) are highly characteristic of **Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN)**.
- **Carbamazepine** is a well-known medication trigger for SJS/TEN, a severe cutaneous adverse drug reaction.
*Cytomegalovirus*
- While CMV can cause a rash and systemic symptoms, it typically manifests as a **maculopapular rash** or purpura, not extensive blistering with a positive Nikolsky's sign.
- CMV infection usually presents with features like mononucleosis-like syndrome, hepatitis, or retinitis, which are not described here as the primary concern.
*Deficiency of C-1 esterase inhibitor*
- This deficiency causes **hereditary angioedema**, characterized by recurrent episodes of localized swelling, typically affecting the face, airways, and gastrointestinal tract.
- It does not cause a blistering rash or the extensive epidermal detachment seen in this patient.
*Herpes simplex virus*
- HSV can cause blistering lesions, but these are typically **localized vesicles** that progress to ulcers, such as cold sores or genital herpes.
- While widespread HSV infection can occur in immunocompromised patients, it does not typically present as a diffuse blistering rash with such extensive epidermal detachment and high TBSA involvement as described.
*Molluscum contagiosum*
- This is a viral skin infection that causes characteristic **dome-shaped, umbilicated papules**.
- It does not cause a widespread blistering rash, fever, or the severe systemic symptoms and epidermal detachment seen in this patient.
More Pharmacokinetic interaction mechanisms US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.