Drug interactions and adverse effects

On this page
🎯 The Interaction Arsenal: Mastering Drug Combat Zones
Every prescription you write enters a complex battlefield where drugs compete for enzymes, hijack transporters, and clash at receptors-interactions that can amplify toxicity, erase efficacy, or trigger life-threatening syndromes. You'll master the mechanisms behind these collisions, from CYP450 induction and inhibition to P-glycoprotein blockade and pharmacodynamic synergy, then learn to spot emergencies like serotonin syndrome and systematically prevent harm before it unfolds. This lesson transforms drug interactions from unpredictable hazards into manageable, predictable clinical decisions.
Drug interactions represent one of medicine's most preventable causes of morbidity, affecting 15-20% of hospitalized patients and contributing to 3-5% of all hospital admissions. These interactions operate through four fundamental mechanisms: absorption interference, distribution competition, metabolism modulation, and excretion alteration.
📌 Remember: ADME-PD - Absorption, Distribution, Metabolism, Excretion, PharmacoDynamics. Every drug interaction fits into one of these five categories, with metabolism (CYP450) accounting for 60% of clinically significant interactions.
The interaction landscape divides into pharmacokinetic (what the body does to drugs) and pharmacodynamic (what drugs do to each other) mechanisms. Pharmacokinetic interactions alter drug concentrations through ADME processes, while pharmacodynamic interactions modify drug effects at receptor or cellular levels without changing plasma concentrations.
-
Pharmacokinetic Interactions
- Absorption: 25% of interactions, primarily chelation and pH changes
- Distribution: 10% of interactions, mainly protein binding displacement
- Warfarin displacement increases free fraction by 200-300%
- Phenytoin displacement triggers toxicity at therapeutic levels
- Metabolism: 60% of interactions, predominantly CYP450 modulation
- CYP3A4 metabolizes 50% of all medications
- CYP2D6 affects 25% of drugs with 7% population poor metabolizers
- Excretion: 15% of interactions, renal and biliary competition
-
Pharmacodynamic Interactions
- Synergistic: 1+1=3 effect, exponential toxicity risk
- Antagonistic: 1+1=0 effect, therapeutic failure potential
- Beta-blockers + calcium channel blockers = additive cardiac depression
- Warfarin + aspirin = multiplicative bleeding risk (RR 6.7)
| Interaction Type | Mechanism | Onset Time | Clinical Impact | Reversal Time | Monitoring Parameter |
|---|---|---|---|---|---|
| CYP450 Inhibition | Enzyme blockade | 2-5 days | ↑ Drug levels 200-500% | 5-7 days | Plasma concentrations |
| CYP450 Induction | Enzyme upregulation | 7-14 days | ↓ Drug levels 50-80% | 14-21 days | Therapeutic efficacy |
| P-gp Inhibition | Efflux pump blockade | 24-48 hours | ↑ Bioavailability 150-300% | 3-5 days | Drug-specific effects |
| Protein Displacement | Binding competition | Minutes-hours | ↑ Free fraction 200-400% | Hours-days | Free drug levels |
| Receptor Antagonism | Competitive binding | 30-60 minutes | Therapeutic failure | Drug half-life | Clinical response |
💡 Master This: High-risk populations amplify interaction severity - elderly patients (>65 years) show 3-fold increased interaction frequency due to polypharmacy (average 7.2 medications), reduced clearance (30-50% decline), and altered pharmacodynamics.
Connect these foundational interaction principles through specific mechanism analysis to understand how individual pathways create clinical consequences.
🎯 The Interaction Arsenal: Mastering Drug Combat Zones
⚙️ The CYP450 Command Center: Metabolic Warfare
📌 Remember: "Some Drugs Create Dangerous Liver Enzymes" - Some = CYP3A4 (most important), Drugs = CYP2D6, Create = CYP2C9, Dangerous = CYP2C19, Liver = CYP1A2, Enzymes = CYP2E1. These six enzymes metabolize 98% of all medications.
CYP450 interactions operate through inhibition (immediate effect, 2-5 days to steady-state) or induction (delayed effect, 7-14 days to maximum). Inhibition creates competitive, non-competitive, or mechanism-based (irreversible) enzyme blockade, while induction increases enzyme synthesis through nuclear receptor activation.
-
CYP450 Inhibition Mechanisms
- Competitive: Reversible active site competition (Ki values 1-100 μM)
- Fluconazole inhibits CYP2C9 (Ki = 5.2 μM), increases warfarin levels 300%
- Erythromycin inhibits CYP3A4 (Ki = 45 μM), increases simvastatin 1000%
- Non-competitive: Allosteric site binding, irreversible effect
- Paroxetine irreversibly inhibits CYP2D6, effect persists 14-21 days
- Clarithromycin forms metabolic intermediate complex with CYP3A4
- Competitive: Reversible active site competition (Ki values 1-100 μM)
-
CYP450 Induction Pathways
- PXR Activation (Pregnane X Receptor): CYP3A4 induction pathway
- Rifampin increases CYP3A4 expression 10-40 fold via PXR
- St. John's Wort activates PXR, reduces contraceptive efficacy 50%
- CAR Activation (Constitutive Androstane Receptor): CYP2B6 pathway
- Phenobarbital induces CYP2B6 5-10 fold through CAR activation
- PXR Activation (Pregnane X Receptor): CYP3A4 induction pathway
| CYP Isoform | Major Substrates | Potent Inhibitors | Strong Inducers | Genetic Polymorphism | Clinical Impact |
|---|---|---|---|---|---|
| CYP3A4 | Simvastatin, Cyclosporine | Ketoconazole, Ritonavir | Rifampin, Carbamazepine | Rare (<1%) | 50% of drug interactions |
| CYP2D6 | Codeine, Metoprolol | Paroxetine, Quinidine | None clinically | 7% poor metabolizers | Analgesic failure, β-blocker toxicity |
| CYP2C9 | Warfarin, Phenytoin | Fluconazole, Amiodarone | Rifampin, Phenytoin | 3% poor metabolizers | Bleeding, seizures |
| CYP2C19 | Omeprazole, Clopidogrel | Omeprazole, Fluvoxamine | Rifampin, St. John's Wort | 15-20% poor metabolizers | PPI failure, antiplatelet resistance |
| CYP1A2 | Caffeine, Theophylline | Fluvoxamine, Ciprofloxacin | Smoking, Charcoal | Rare (<1%) | Theophylline toxicity |
💡 Master This: Genetic polymorphisms create 10-100 fold differences in drug clearance. CYP2D6 poor metabolizers (7% Caucasians, 1% Asians) cannot convert codeine to morphine, experiencing zero analgesic effect, while ultra-rapid metabolizers risk life-threatening respiratory depression.
Connect CYP450 mastery through P-glycoprotein interactions to understand how efflux pumps create additional interaction complexity.
⚙️ The CYP450 Command Center: Metabolic Warfare
🚪 The P-Glycoprotein Gateway: Cellular Bouncers
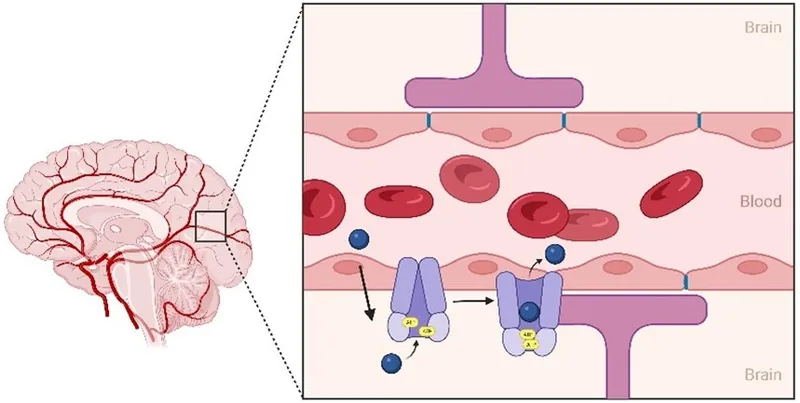
P-glycoprotein (P-gp, MDR1, ABCB1) functions as an ATP-dependent efflux pump, expressed at critical pharmacological barriers: blood-brain barrier (high density), intestinal epithelium (apical surface), hepatocytes (biliary canaliculi), and renal tubules (luminal membrane). This 170 kDa transmembrane protein determines bioavailability, tissue distribution, and elimination for 40% of marketed drugs.
📌 Remember: "Please Go Pump Drugs Out" - P-gp locations: Gut (intestines), Pump (liver), Drain (kidneys), Out (brain barrier). These four sites control drug access to systemic circulation, hepatic elimination, renal excretion, and CNS penetration.
P-gp interactions create bidirectional effects: inhibition increases substrate bioavailability and tissue penetration (150-400%), while induction decreases drug exposure and efficacy (30-70% reduction). Unlike CYP450 interactions, P-gp effects occur rapidly (2-6 hours) and reverse quickly (24-48 hours) due to pump protein turnover.
-
P-gp Substrate Categories
- High-affinity substrates: Digoxin (Km = 5 μM), Dabigatran, Rivaroxaban
- Digoxin levels increase 200-300% with quinidine co-administration
- Dabigatran bioavailability rises from 6% to 25% with P-gp inhibition
- Moderate-affinity substrates: Fexofenadine, Loperamide, Talinolol
- Fexofenadine AUC increases 300% with grapefruit juice (P-gp inhibition)
- Low-affinity substrates: Morphine, Dexamethasone, Cyclosporine
- Morphine CNS penetration increases 150% with P-gp knockout
- High-affinity substrates: Digoxin (Km = 5 μM), Dabigatran, Rivaroxaban
-
P-gp Inhibitor Potency Classification
- Potent inhibitors (IC50 <10 μM): Quinidine, Verapamil, Cyclosporine
- Quinidine reduces digoxin clearance 40-60% within 6 hours
- Verapamil increases loperamide CNS toxicity risk 500%
- Moderate inhibitors (IC50 10-50 μM): Clarithromycin, Itraconazole
- Clarithromycin doubles dabigatran exposure in elderly patients
- Weak inhibitors (IC50 >50 μM): Omeprazole, Atorvastatin
- Potent inhibitors (IC50 <10 μM): Quinidine, Verapamil, Cyclosporine
| P-gp Location | Physiological Role | Clinical Substrates | Interaction Consequences | Onset Time | Recovery Time |
|---|---|---|---|---|---|
| Intestinal | Limits absorption | Digoxin, Dabigatran | ↑ Bioavailability 200-400% | 2-4 hours | 24-48 hours |
| Blood-Brain Barrier | CNS protection | Loperamide, Morphine | ↑ CNS toxicity risk | 1-2 hours | 12-24 hours |
| Hepatic | Biliary elimination | Doxorubicin, Paclitaxel | ↓ Hepatic clearance 50% | 4-6 hours | 48-72 hours |
| Renal | Tubular secretion | Digoxin, Metformin | ↓ Renal clearance 30% | 2-4 hours | 24-48 hours |
| Placental | Fetal protection | Saquinavir, Paclitaxel | ↑ Fetal exposure | 1-3 hours | 24-48 hours |
💡 Master This: Genetic polymorphisms in ABCB1 create 2-3 fold differences in P-gp expression. The C3435T variant (25% frequency in Caucasians) reduces P-gp function, increasing digoxin bioavailability 40% and CNS drug penetration.
Connect P-gp mastery through pharmacodynamic interactions to understand how receptor-level effects amplify or oppose transport-mediated changes.
🚪 The P-Glycoprotein Gateway: Cellular Bouncers
⚡ The Receptor Battlefield: Pharmacodynamic Warfare
📌 Remember: "Receptors Always Compete Seriously" - Receptor competition, Additive effects, Competitive antagonism, Synergistic toxicity. Four fundamental pharmacodynamic interaction patterns that determine clinical outcomes within minutes to hours.
Pharmacodynamic interactions demonstrate concentration-response relationships following Hill equation kinetics: Effect = (Emax × [Drug]^n) / (EC50^n + [Drug]^n). When multiple drugs target the same pathway, their combined effect depends on receptor affinity (Kd values), intrinsic activity (efficacy), and concentration ratios.
-
Additive Interactions (Independent pathways, combined effects)
- Antihypertensive combinations: ACE inhibitor + thiazide diuretic
- Individual BP reduction: 10-15 mmHg each
- Combined reduction: 20-30 mmHg (true addition)
- Mechanism: Different pathways (RAAS vs. volume depletion)
- Analgesic combinations: Acetaminophen + ibuprofen
- Pain reduction: 30% + 40% = 70% total relief
- Mechanism: Central vs. peripheral pain pathways
- Antihypertensive combinations: ACE inhibitor + thiazide diuretic
-
Synergistic Interactions (Amplified combined effects)
- CNS depressants: Alcohol + benzodiazepines
- Individual sedation: Mild + Moderate = Severe/Fatal
- Mechanism: GABA-A receptor positive allosteric modulation
- Risk increase: 50-fold higher respiratory depression
- Bleeding risk: Warfarin + aspirin
- Individual bleeding risk: 2% + 1% = 6.7% combined (RR 6.7)
- Mechanism: Anticoagulation + antiplatelet synergy
- CNS depressants: Alcohol + benzodiazepines
- Antagonistic Interactions (Opposing or competing effects)
- Competitive receptor antagonism: Beta-agonist + beta-blocker
- Albuterol bronchodilation completely blocked by propranolol
- Mechanism: Competitive binding at β2-adrenergic receptors
- Physiological antagonism: Insulin + glucagon
- Opposing effects on blood glucose through different receptors
- Insulin decreases glucose 50-100 mg/dL, glucagon increases 50-150 mg/dL
- Competitive receptor antagonism: Beta-agonist + beta-blocker
| Interaction Type | Mechanism | Mathematical Relationship | Clinical Example | Onset Time | Predictability |
|---|---|---|---|---|---|
| Additive | Independent pathways | Effect = A + B | ACE-I + Diuretic | 30-60 min | High |
| Synergistic | Pathway amplification | Effect > A + B | Alcohol + Benzos | 15-30 min | Moderate |
| Competitive | Same receptor | Effect = A/(1+B/Ki) | Agonist + Antagonist | 5-15 min | High |
| Physiological | Opposing systems | Effect = A - B | Insulin + Glucagon | 10-30 min | Moderate |
| Chemical | Direct inactivation | Effect = 0 | Protamine + Heparin | Immediate | High |
💡 Master This: Allosteric modulation creates non-competitive interactions that cannot be overcome by increasing agonist concentration. Benzodiazepines increase GABA affinity 10-fold at GABA-A receptors, creating ceiling-independent enhancement of CNS depression.
Connect pharmacodynamic principles through clinical syndrome recognition to identify life-threatening interaction patterns in real-time.
⚡ The Receptor Battlefield: Pharmacodynamic Warfare
🚨 The Syndrome Spotters: Recognizing Interaction Emergencies
📌 Remember: "Some Neurological Accidents" - Serotonin syndrome (hyperthermia + hyperreflexia + clonus), Neuroleptic malignant syndrome (rigidity + hyperthermia + altered mental status), Anticholinergic crisis (hot, dry, blind, mad). Three interaction emergencies with overlapping presentations but different treatments.
Recognition depends on temporal relationships (symptom onset 2-24 hours after drug changes), characteristic triads, and specific physical findings. Serotonin syndrome shows hyperreflexia and clonus, NMS demonstrates lead-pipe rigidity, while anticholinergic toxicity presents with mydriasis and dry mucous membranes.
-
Serotonin Syndrome (Excess serotonergic activity)
- Classic triad: Altered mental status + autonomic instability + neuromuscular abnormalities
- Mental status: Agitation, confusion, delirium (100% of cases)
- Autonomic: Hyperthermia (>38.5°C), tachycardia (>100 bpm), diaphoresis
- Neuromuscular: Hyperreflexia (>3+), clonus (sustained >5 beats), tremor
- Hunter Criteria (Sensitivity 84%, Specificity 97%)
- Spontaneous clonus OR inducible clonus + agitation/diaphoresis
- Ocular clonus + agitation/diaphoresis OR tremor + hyperreflexia
- Causative combinations: SSRI + MAOI, Tramadol + SSRI, Linezolid + SSRI
- Classic triad: Altered mental status + autonomic instability + neuromuscular abnormalities
-
Neuroleptic Malignant Syndrome (Dopamine receptor blockade)
- Cardinal features: Hyperthermia + rigidity + altered consciousness + autonomic dysfunction
- Hyperthermia: >38°C in 100%, often >40°C (severe cases)
- Rigidity: Lead-pipe rigidity (100%), cogwheel rigidity (75%)
- Mental status: Stupor to coma (75%), mutism (50%)
- Autonomic: Tachycardia (>120 bpm), labile BP, diaphoresis
- Laboratory findings: CK elevation >1000 U/L (90%), often >10,000 U/L
- Triggers: Antipsychotic initiation/increase, dopamine agonist withdrawal
- Cardinal features: Hyperthermia + rigidity + altered consciousness + autonomic dysfunction
- Anticholinergic Toxicity (Muscarinic receptor blockade)
- Classic presentation: "Hot as a hare, dry as a bone, blind as a bat, mad as a hatter"
- Hyperthermia: >38°C without diaphoresis (dry heat)
- Dry mucous membranes: No saliva, dry axillae (100%)
- Mydriasis: Fixed dilated pupils (>6mm), photophobia
- Altered mental status: Delirium, hallucinations, agitation
- Peripheral signs: Decreased bowel sounds, urinary retention, flushed skin
- Common causes: TCA overdose, antihistamines, scopolamine, jimsonweed
- Classic presentation: "Hot as a hare, dry as a bone, blind as a bat, mad as a hatter"
| Syndrome | Onset Time | Temperature | Muscle Tone | Reflexes | Pupils | Skin | Mortality |
|---|---|---|---|---|---|---|---|
| Serotonin | 2-6 hours | 38-40°C | Hypertonicity | Hyperreflexia 4+ | Normal | Diaphoretic | 10-15% |
| NMS | 24-72 hours | >40°C | Lead-pipe rigidity | Normal/decreased | Normal | Diaphoretic | 20-30% |
| Anticholinergic | 1-4 hours | 38-39°C | Normal | Normal | Mydriasis >6mm | Hot, dry | 5-10% |
| Malignant Hyperthermia | Minutes | >41°C | Severe rigidity | Hyperreflexia | Normal | Diaphoretic | 70% untreated |
💡 Master This: Antidote specificity determines survival - cyproheptadine (4-8 mg PO q6h) for serotonin syndrome, dantrolene (1-2.5 mg/kg IV) for NMS, physostigmine (0.5-2 mg IV) for anticholinergic crisis. Wrong antidote worsens outcome.
Connect syndrome recognition through systematic management protocols to transform emergency identification into life-saving interventions.
🚨 The Syndrome Spotters: Recognizing Interaction Emergencies
🎛️ The Management Matrix: Systematic Interaction Control
📌 Remember: "Prevent Problems Before Patients Pay" - Prescribing alerts, Pharmacy screening, Biomarker monitoring, Patient education, Protocol adherence. Five systematic checkpoints that intercept 95% of dangerous interactions before clinical harm occurs.
Management protocols stratify interactions by severity levels: contraindicated (avoid combination), major (monitor closely), moderate (consider alternatives), and minor (document awareness). Each level triggers specific interventions with defined monitoring parameters and quantitative thresholds for action.
-
Contraindicated Interactions (Absolute avoidance required)
- MAOI + SSRI: 14-day washout mandatory, serotonin syndrome risk >90%
- Fluoxetine requires 5-week washout due to long half-life (7 days)
- Phenelzine + sertraline: 100% serotonin syndrome incidence
- Warfarin + Miconazole: INR increases >10 in 80% of patients
- Bleeding risk increases 20-fold within 48-72 hours
- Simvastatin >20mg + Strong CYP3A4 inhibitors
- Rhabdomyolysis risk >100-fold increase with ketoconazole
- MAOI + SSRI: 14-day washout mandatory, serotonin syndrome risk >90%
-
Major Interactions (Close monitoring essential)
- Digoxin + Quinidine: Monitor digoxin levels every 2-3 days
- Target reduction: 50% digoxin dose when starting quinidine
- Therapeutic range: 0.8-2.0 ng/mL (lower end with quinidine)
- Warfarin + Antibiotics: INR monitoring every 2-3 days
- Ciprofloxacin increases INR 2-3 fold within 3-5 days
- Metronidazole prolongs PT 50-100% through CYP2C9 inhibition
- Digoxin + Quinidine: Monitor digoxin levels every 2-3 days
- Monitoring Parameter Selection
- Pharmacokinetic interactions: Drug levels, clearance markers
- CYP450 substrates: Plasma concentrations every 3-5 days initially
- Renal elimination: Creatinine clearance weekly during initiation
- Pharmacodynamic interactions: Physiological endpoints
- Anticoagulants: INR/aPTT every 2-3 days for 2 weeks
- Antihypertensives: BP monitoring daily for 1 week
- CNS depressants: Mental status assessment every 4-6 hours
- Pharmacokinetic interactions: Drug levels, clearance markers
| Interaction Severity | Monitoring Frequency | Laboratory Tests | Clinical Assessment | Intervention Threshold | Documentation Required |
|---|---|---|---|---|---|
| Contraindicated | N/A (Avoid) | N/A | N/A | Immediate substitution | Alternative rationale |
| Major | Daily-Weekly | Every 2-3 days | Every shift | >50% parameter change | Monitoring plan |
| Moderate | Weekly-Monthly | Weekly initially | Daily initially | >25% parameter change | Risk acknowledgment |
| Minor | Monthly-PRN | Baseline + PRN | PRN symptoms | Patient-reported changes | Patient education |
💡 Master This: Quantitative thresholds trigger interventions - >2-fold increase in drug levels mandates dose reduction, >50% change in physiological parameters requires immediate assessment, >25% laboratory abnormalities warrant increased monitoring frequency.
Connect systematic management through evidence-based prevention strategies to create comprehensive interaction safety protocols.
🎛️ The Management Matrix: Systematic Interaction Control
🛡️ The Prevention Protocols: Mastering Interaction Safety
📌 Remember: "Every Doctor Should Prevent Harm" - Education (prescriber training), Decision support (alerts), Screening (pharmacy), Patient involvement (education), Hazard elimination (formulary restrictions). Five prevention layers that reduce interaction-related ADEs by 85%.
Risk stratification identifies high-risk patients requiring intensive monitoring: elderly (>65 years), polypharmacy (>5 medications), renal impairment (CrCl <60 mL/min), hepatic dysfunction, and genetic polymorphisms. These populations show 3-5 fold higher interaction rates and 10-fold increased severity.
-
High-Risk Patient Identification
- Elderly patients (>65 years): Average 7.2 medications, 40% inappropriate prescribing
- Beers Criteria: 53 potentially inappropriate medications in elderly
- STOPP/START criteria: Identifies inappropriate prescribing in 35% of elderly
- Interaction risk increases exponentially: 2 drugs = 6%, 5 drugs = 50%, 8 drugs = 100%
- Renal impairment: CrCl <60 mL/min affects 40% of drug elimination
- Dose adjustment required for 60% of renally eliminated drugs
- Drug accumulation increases interaction severity 2-5 fold
- Hepatic impairment: Child-Pugh Class B/C reduces metabolism 50-90%
- CYP450 activity decreases proportionally to liver function
- Protein binding altered in hypoalbuminemia (<3.5 g/dL)
- Elderly patients (>65 years): Average 7.2 medications, 40% inappropriate prescribing
-
Technology-Enabled Prevention
- Clinical Decision Support Systems (CDSS): Real-time interaction screening
- Sensitivity: 85-95% for major interactions
- Specificity: 70-85% (high false-positive rates)
- Alert fatigue: >90% of alerts overridden without review
- Pharmacogenomic testing: Personalized interaction risk
- CYP2D6 genotyping: Identifies poor metabolizers (7% population)
- CYP2C19 testing: Predicts clopidogrel resistance (30% reduced function)
- ABCB1 variants: 2-3 fold differences in P-gp substrate exposure
- Clinical Decision Support Systems (CDSS): Real-time interaction screening
- Evidence-Based Prevention Tools
- Medication Reconciliation: Reduces ADEs by 70% at transitions
- Best Possible Medication History (BPMH): Gold standard approach
- Discrepancy rates: 60-70% at hospital admission
- Clinically significant errors: 15-20% of all discrepancies
- Deprescribing protocols: Systematic medication reduction
- Inappropriate polypharmacy: >5 medications without indication
- Deprescribing reduces interactions 40-60% in elderly
- Clinical outcomes: Improved cognition, reduced falls (30%)
- Medication Reconciliation: Reduces ADEs by 70% at transitions
| Prevention Strategy | Implementation Rate | ADE Reduction | Cost-Effectiveness | Barriers to Adoption | Success Metrics |
|---|---|---|---|---|---|
| CDSS Alerts | >90% hospitals | 30-50% | $3-7 per $1 invested | Alert fatigue, workflow | Override rates <50% |
| Pharmacist Review | 60% hospitals | 60-80% | $4-16 per $1 invested | Staffing, integration | Interventions >5 per day |
| Medication Reconciliation | 75% hospitals | 70% | $2-5 per $1 invested | Time, training | Discrepancy detection >60% |
| Patient Education | 40% systematic | 25-40% | $2-4 per $1 invested | Health literacy | Adherence >80% |
| Genetic Testing | <10% routine | 40-60% (selected) | Variable | Cost, interpretation | Actionable results >20% |
💡 Master This: Patient engagement amplifies prevention effectiveness - educated patients identify 40% of medication errors missed by providers, report 60% of adverse effects within 24 hours, and demonstrate 80% adherence to monitoring recommendations.
Understanding these prevention protocols transforms reactive interaction management into proactive patient safety systems, creating the foundation for optimal therapeutic outcomes across all clinical settings.
🛡️ The Prevention Protocols: Mastering Interaction Safety
Practice Questions: Drug interactions and adverse effects
Test your understanding with these related questions
A 61-year-old man with a history of type 1 diabetes mellitus and depression is brought to the emergency department because of increasing confusion and fever over the past 14 hours. Four days ago, he was prescribed metoclopramide by his physician for the treatment of diabetic gastroparesis. His other medications include insulin and paroxetine. His temperature is 39.9°C (103.8°F), pulse is 118/min, and blood pressure is 165/95 mm Hg. Physical examination shows profuse diaphoresis and flushed skin. There is generalized muscle rigidity and decreased deep tendon reflexes. His serum creatine kinase is 1250 U/L. Which of the following drugs is most likely to also cause this patient's current condition?










