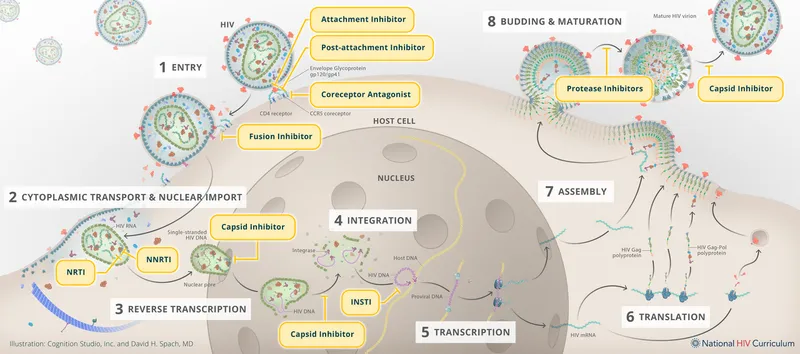
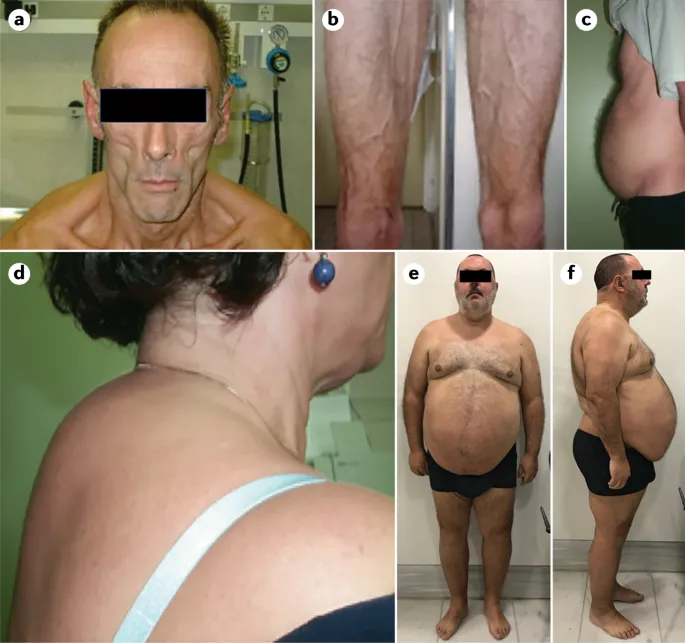
HIV protease inhibitors US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for HIV protease inhibitors. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
HIV protease inhibitors US Medical PG Question 1: A 37-year-old man comes to the physician because of a 6-month history of progressive breast enlargement. Two years ago, he was diagnosed with HIV infection and started treatment with antiretroviral medications. Examination shows a soft, non-tender, ill-defined swelling at the nape of the neck. The cheeks appear hollowed. Serum studies show increased total cholesterol and LDL concentration. Which of the following medications is the most likely cause of these findings?
- A. Nevirapine
- B. Indinavir (Correct Answer)
- C. Enfuvirtide
- D. Abacavir
- E. Raltegravir
HIV protease inhibitors Explanation: ***Indinavir***
- This patient presents with signs of **lipodystrophy**, specifically **lipoaccumulation** (breast enlargement, "buffalo hump" at the nape of the neck) and **lipoatrophy** (hollow cheeks), along with **dyslipidemia**.
- **Protease inhibitors (PIs)**, such as indinavir, are well-known to cause these metabolic complications, including **lipodystrophy** and **hyperlipidemia**, in patients with HIV.
*Nevirapine*
- Nevirapine is a **non-nucleoside reverse transcriptase inhibitor (NNRTI)**.
- While NNRTIs can be associated with some metabolic side effects, they are less commonly implicated in severe **lipodystrophy** and **dyslipidemia** compared to protease inhibitors.
*Enfuvirtide*
- Enfuvirtide is a **fusion inhibitor** and generally has a favorable metabolic profile.
- It is not typically associated with **lipodystrophy** or significant **dyslipidemia**.
*Abacavir*
- Abacavir is a **nucleoside reverse transcriptase inhibitor (NRTI)**.
- While some NRTIs (especially stavudine and zidovudine) were strongly linked to lipoatrophy, abacavir is much less likely to cause this severe form of **lipodystrophy** or **hyperlipidemia**.
*Raltegravir*
- Raltegravir is an **integrase strand transfer inhibitor (INSTI)**.
- INSTIs are increasingly used due to their generally good metabolic profile and are not a common cause of **lipodystrophy** or **dyslipidemia**.
HIV protease inhibitors US Medical PG Question 2: A 60-year-old man comes to the physician’s office with jaundice. Liver ultrasound reveals a shrunken liver and biopsy reveals cirrhosis. Hepatitis serologies are below:
Anti-HAV: negative
HBsAg: negative
HBsAb: positive
HBeAg: negative
Anti-HBe: negative
Anti-HBc: negative
Anti-HCV: positive
The hepatitis C viral load is 1,000,000 copies/mL. The patient is started on an antiviral regimen including sofosbuvir. What is the mechanism of action of this drug?
- A. Inhibits reverse transcriptase
- B. Inhibits integrase
- C. Inhibits synthesis of DNA-dependent DNA polymerase
- D. Inhibits RNA-dependent RNA polymerase (Correct Answer)
- E. Inhibits hepatitis C protease
HIV protease inhibitors Explanation: ***Inhibits RNA-dependent RNA polymerase***
- Sofosbuvir is a **nucleotide analog** that targets the **HCV RNA-dependent RNA polymerase (NS5B)**, essential for viral replication.
- By inhibiting NS5B, it acts as a **chain terminator**, preventing the synthesis of new viral RNA strands.
*Inhibits reverse transcriptase*
- This mechanism is characteristic of drugs used to treat **HIV infection**, as reverse transcriptase is an enzyme found in retroviruses.
- Hepatitis C virus (HCV) is an **RNA virus** that replicates via an RNA intermediate, not DNA, and thus does not utilize reverse transcriptase.
*Inhibits integrase*
- Integrase inhibitors are a class of drugs primarily used in the treatment of **HIV infection**, preventing the viral DNA from integrating into the host genome.
- HCV replication does not involve an integration step into the host DNA, making this mechanism irrelevant for HCV treatment.
*Inhibits synthesis of DNA-dependent DNA polymerase*
- Inhibition of DNA-dependent DNA polymerase primarily targets organisms that replicate their DNA, such as **herpesviruses** or host cell processes.
- HCV is an RNA virus and does not synthesize or rely on a DNA-dependent DNA polymerase for its replication cycle.
*Inhibits hepatitis C protease*
- While **protease inhibitors (e.g., -previr drugs)** are an important class of anti-HCV drugs, sofosbuvir specifically targets the viral **RNA polymerase (NS5B)**.
- Protease inhibitors block the **NS3/4A protease**, which is responsible for cleaving the large HCV polyprotein into functional proteins.
HIV protease inhibitors US Medical PG Question 3: An investigator is studying a drug that acts on the thyroid hormone pathway. Levels of serum free T3 and T4 in healthy participants are measured before and after administration of the drug. After administration, there is a decrease in the average serum free T3 level, while the average serum free T4 level is increased compared to initial serum studies. Inhibition of which of the following is the most likely mechanism of action of this drug?
- A. Thyroid-stimulating hormone
- B. Follicular iodotyrosine deiodinase
- C. Follicular thyroid peroxidase
- D. Peripheral 5'-deiodinase (Correct Answer)
- E. Follicular thyroid proteases
HIV protease inhibitors Explanation: ***Peripheral 5'-deiodinase***
- Inhibition of **peripheral 5'-deiodinase** would decrease the conversion of **T4 to T3** in the periphery, resulting in lower **free T3** and higher **free T4** levels.
- This enzyme is crucial for activating T4 into the more potent T3, and its blockade explains the observed changes in hormone levels.
*Thyroid-stimulating hormone*
- Inhibition of **TSH** would lead to a decrease in the production and release of both **T3 and T4** from the thyroid gland.
- This contradicts the observed increase in **free T4** levels.
*Follicular iodotyrosine deiodinase*
- This enzyme is involved in recycling iodine from **monoiodotyrosine (MIT)** and **diiodotyrosine (DIT)** within the thyroid follicular cells, which is important for efficient thyroid hormone synthesis.
- Its inhibition would primarily affect iodine availability and synthesis, not directly lead to increased T4 and decreased T3 in the periphery.
*Follicular thyroid peroxidase*
- **Thyroid peroxidase (TPO)** is critical for the **iodination of tyrosine residues** on thyroglobulin and the **coupling of MIT and DIT** to form T3 and T4.
- Inhibition of TPO would decrease the synthesis of both **T3 and T4**, contrary to the observed increase in **free T4**.
*Follicular thyroid proteases*
- **Thyroid proteases** cleave thyroglobulin to release mature **T3 and T4** into the bloodstream.
- Inhibition of these proteases would lead to a decrease in the release of both **T3 and T4**, which does not align with the observed increase in **free T4**.
HIV protease inhibitors US Medical PG Question 4: A 27-year-old woman consults an obstetrician as she is planning to become pregnant. She has been diagnosed with HIV (human immunodeficiency virus) infection recently and is currently taking antiretroviral therapy (HAART), as prescribed by her physician. The obstetrician emphasizes the importance of antenatal and peripartum antiretroviral therapy for reducing the risk of mother-to-child transmission of HIV. She also tells the patient that certain antiretroviral drugs, if taken during pregnancy, increase the risk of birth defects in the fetus. She gives a printed list of such drugs to the woman for educational and informational purposes. Which of the following drugs are most likely to be present on the list?
- A. Nelfinavir and Saquinavir
- B. Abacavir and Didanosine
- C. Efavirenz and Delavirdine (Correct Answer)
- D. Lopinavir and Ritonavir
- E. Lamivudine and Nevirapine
HIV protease inhibitors Explanation: ***Efavirenz and Delavirdine***
- Both **efavirenz** and **delavirdine** are **non-nucleoside reverse transcriptase inhibitors (NNRTIs)** and have been associated with an increased risk of **teratogenicity**, particularly neural tube defects, in early pregnancy.
- Due to these potential risks, they are generally **avoided during the first trimester of pregnancy** or when pregnancy is being planned, unless no other suitable alternative exists.
*Nelfinavir and Saquinavir*
- **Nelfinavir** and **saquinavir** are **protease inhibitors (PIs)** which are generally considered **safe for use during pregnancy** and are often part of recommended regimens for HIV-positive pregnant women.
- They do not carry the same significant teratogenic risks as some other antiretroviral drugs.
*Abacavir and Didanosine*
- **Abacavir** and **didanosine** are **nucleoside reverse transcriptase inhibitors (NRTIs)** commonly used in HIV treatment.
- While didanosine can be associated with lactic acidosis and pancreatitis, neither drug is typically considered to significantly increase the risk of birth defects.
*Lopinavir and Ritonavir*
- **Lopinavir/ritonavir** is a commonly used **protease inhibitor (PI)** combination that is generally considered **safe and effective for use throughout pregnancy** to prevent mother-to-child transmission.
- It does not have known significant teratogenic effects.
*Lamivudine and Nevirapine*
- **Lamivudine** is an **NRTI** and **nevirapine** is an **NNRTI**. Lamivudine is generally considered safe during pregnancy.
- Nevirapine is used in pregnancy, particularly if started after the first trimester, and generally has a more favorable safety profile regarding birth defects compared to efavirenz and delavirdine.
HIV protease inhibitors US Medical PG Question 5: Drug A is an experimental compound being investigated for potential use as a protectant against venous thrombosis. Binding assays reveal that the drug’s primary mechanism of action is to block carboxylation of glutamic acid residues in certain serum proteins. Drug A is most similar to which of the following:
- A. Streptokinase
- B. Bivalirudin
- C. Warfarin (Correct Answer)
- D. Heparin
- E. Rivaroxaban
HIV protease inhibitors Explanation: ***Warfarin***
- Warfarin inhibits **vitamin K epoxide reductase**, enzyme responsible for regenerating active vitamin K.
- Active vitamin K is a cofactor for the **gamma-carboxylation of glutamic acid residues** on factors II, VII, IX, X and protein C and S. Thus, warfarin blocks their activation, inhibiting coagulation.
*Steptokinase*
- **Streptokinase** is a **thrombolytic drug** that catalyzes the conversion of **plasminogen to plasmin**, an enzyme that degrades fibrin clots.
- Its mechanism of action is focused on **breaking down existing clots**, rather than preventing their formation by affecting coagulation factor synthesis.
*Bivalirudin*
- **Bivalirudin** is a direct **thrombin inhibitor**, binding directly to the active site and exosite I of thrombin to prevent its action.
- It does not interfere with the **carboxylation of glutamic acid residues** but rather directly inhibits the final common pathway of coagulation.
*Heparin*
- **Heparin** works by potentiating the action of **antithrombin III**, which in turn inactivates thrombin and factor Xa.
- Its mechanism involves accelerating the natural anticoagulant system, rather than inhibiting the **synthesis or activation of coagulation factors** through carboxylation.
*Rivaroxaban*
- **Rivaroxaban** is a **direct factor Xa inhibitor**, which blocks the activity of free and clot-bound factor Xa.
- It directly interferes with the coagulation cascade downstream of the carboxylation step, and does not affect the **vitamin K-dependent carboxylation process**.
HIV protease inhibitors US Medical PG Question 6: A 35-year-old man comes to the physician because of a 6-month history of fatigue and increased sweating at night. He says that he feels “constantly tired” and needs more rest than usual although he sleeps well. In the morning, his sheets are often wet and his skin is clammy. He has not had any sore throat, runny nose, or cough recently. He has not traveled anywhere. Over the past 4 months, he has had a 6.8-kg (15-lb) weight loss, despite having a normal appetite. He does not drink or urinate more than usual. He is 181 cm (5 ft 11 in) tall and weighs 72 kg (159 lb); BMI is 22 kg/m2. His temperature is 37.9°C (100.2°F), pulse is 65/min, and blood pressure is 120/70 mm Hg. Physical examination shows no abnormalities. An HIV screening test and confirmatory test are both positive. The CD4 count is 600 cells/μl and the viral load is 104 copies/mL. Treatment with lamivudine, zidovudine, and indinavir is begun. The patient is at greatest risk for which of the following adverse effects?
- A. Urolithiasis (Correct Answer)
- B. Stevens-Johnson syndrome
- C. Hypersensitivity reaction
- D. Chronic kidney disease
- E. Pancreatitis
HIV protease inhibitors Explanation: ***Urolithiasis***
- The patient is receiving **indinavir**, a protease inhibitor known to cause **nephrolithiasis** (kidney stones) due to the drug's poor solubility.
- Patients on indinavir should be well-hydrated to reduce the risk of stone formation.
*Stevens-Johnson syndrome*
- This severe skin reaction is more commonly associated with non-nucleoside reverse transcriptase inhibitors (NNRTIs) like **nevirapine** and **efavirenz**, or with sulfonamide antibiotics, rather than indinavir.
- While possible with many drugs, it is not the *greatest risk* among the options for this specific regimen.
*Hypersensitivity reaction*
- While hypersensitivity can occur with many drugs, particularly abacavir (an NRTI not included in this regimen), it is not the most prominent or specific adverse effect for the given combination, especially indinavir.
- Symptoms usually include fever, rash, and multi-organ involvement, which can be acute.
*Chronic kidney disease*
- While some antiretrovirals, particularly **tenofovir disoproxil fumarate (TDF)**, can cause renal tubular dysfunction and lead to chronic kidney disease, TDF is not part of this patient's regimen.
- Indinavir's primary renal complication is acute stone formation, not typically chronic kidney disease in the absence of pre-existing conditions or other nephrotoxic drugs.
*Pancreatitis*
- Pancreatitis is a known adverse effect of some NRTIs, particularly **didanosine** and **stavudine**, neither of which are in this patient's treatment plan.
- Lamivudine and zidovudine have a lower risk of pancreatitis compared to other NRTIs.
HIV protease inhibitors US Medical PG Question 7: A 43-year-old man with HIV infection comes to the physician because of a 2-week history of progressive diarrhea and a 3-kg (6.6-lb) weight loss. During this period, he has had 3–4 episodes of watery stools daily, with multiple instances of blood in the stool. He is currently receiving antiretroviral therapy with zidovudine, lamivudine, and dolutegravir. Physical examination shows pallor and dry mucous membranes. A colonoscopy shows multiple linear ulcers. Polymerase chain reaction of a stool sample is positive for cytomegalovirus. Treatment with valganciclovir is begun. Adding this drug to his current medication regimen puts this patient at greatest risk for which of the following adverse effects?
- A. Hepatic steatosis
- B. Abnormal dreams
- C. Pancytopenia (Correct Answer)
- D. Orthostatic dysregulation
- E. Hyperglycemia
HIV protease inhibitors Explanation: ***Pancytopenia***
- **Valganciclovir** is a known cause of **bone marrow suppression**, leading to **pancytopenia** (low red blood cells, white blood cells, and platelets).
- The patient is also on **zidovudine**, an antiretroviral that can cause **myelosuppression**, thus the combined use significantly increases the risk of pancytopenia.
*Hepatic steatosis*
- **Hepatic steatosis** (fatty liver) is a rare but known adverse effect of some nucleoside reverse transcriptase inhibitors (NRTIs), particularly older ones.
- While lamivudine is an NRTI, **valganciclovir** is not primarily associated with hepatic steatosis, and the combination does not specifically heighten this risk more than other options.
*Abnormal dreams*
- **Abnormal dreams** are a common side effect associated with certain antiretroviral drugs, particularly the non-nucleoside reverse transcriptase inhibitor **efavirenz**.
- This patient is on dolutegravir (an integrase inhibitor), zidovudine, and lamivudine, none of which are primarily known for causing abnormal dreams as a prominent side effect, and valganciclovir does not contribute to this.
*Orthostatic dysregulation*
- **Orthostatic dysregulation** (orthostatic hypotension) can be a side effect of various medications, but it is not a prominent adverse effect of either **valganciclovir** or the patient's current antiretroviral regimen.
- While dehydration from diarrhea can cause it, the medication itself does not directly increase this risk in particular.
*Hyperglycemia*
- **Hyperglycemia** can be a side effect of certain antiretroviral drugs, particularly some **protease inhibitors** and older NRTIs.
- However, the patient's current regimen (zidovudine, lamivudine, dolutegravir) and **valganciclovir** are not strongly associated with hyperglycemia as a primary adverse effect compared to other options.
HIV protease inhibitors US Medical PG Question 8: A 63-year-old HIV-positive man comes to the physician for a routine health maintenance examination. Four years ago, he was diagnosed with HIV and was started on cART therapy. He tells the physician that he has been having difficulty adhering to his medication regimen. He has been unemployed for the past couple of years and relies on unemployment benefits to cover the costs of daily living. His father died of lymphoma at the age of 60 years. He wants more information about his risk of developing DLBCL. Which of the following is the greatest risk factor for the development of DLBCL in HIV-positive patients?
- A. Poor adherence to cART (Correct Answer)
- B. Income below $30,000 per year
- C. Male sex
- D. Positive family history of cancer
- E. Age over 55 years
HIV protease inhibitors Explanation: **Poor adherence to cART**
- **Poor adherence** to cART leads to **uncontrolled HIV replication** and persistent **immunosuppression**, which significantly increases the risk of developing **DLBCL**.
- **Immune dysregulation** caused by HIV directly contributes to a higher incidence of **AIDS-defining malignancies**, including DLBCL.
*Income below $30,000 per year*
- While **socioeconomic factors** can impact access to care and medication adherence, low income itself is not a direct biological risk factor for DLBCL.
- Its influence is secondary to its effect on adherence and overall health status, rather than a primary risk factor for the malignancy.
*Positive family history of cancer*
- Although a family history of cancer can increase the risk for some malignancies, it is generally **not a significant risk factor** for **HIV-associated DLBCL**.
- The primary drivers of HIV-associated DLBCL are linked to HIV-induced immunodeficiency, not specific inherited genetic predispositions for lymphoma.
*Age over 55 years*
- While the incidence of many cancers increases with **age**, for **HIV-associated DLBCL**, age is less prominent than the degree of **immunodeficiency** caused by HIV.
- The stronger prognostic factor remains the state of the immune system, particularly a **low CD4 count**, which is often exacerbated by poor cART adherence.
*Male sex*
- While there are minor differences in cancer incidence between sexes, **male sex** is not a primary or significant independent risk factor for **HIV-associated DLBCL**.
- The risk is predominantly driven by factors related to HIV infection itself and the resulting immune dysfunction.
HIV protease inhibitors US Medical PG Question 9: Administration of which of the following drugs would increase the bioavailability of saquinavir?
- A. Cimetidine
- B. Vitamin C
- C. Ritonavir (Correct Answer)
- D. Ganciclovir
HIV protease inhibitors Explanation: **Ritonavir**
- **Ritonavir** is a potent **CYP3A4 inhibitor**, which is the primary enzyme responsible for the metabolism of saquinavir.
- By inhibiting **saquinavir** metabolism, ritonavir significantly **increases its plasma concentrations and bioavailability**, making it an effective pharmacokinetic enhancer.
- This combination (saquinavir/ritonavir) is a clinically established strategy in antiretroviral therapy.
*Cimetidine*
- **Cimetidine** inhibits various cytochrome P450 enzymes but is a less potent and more general inhibitor compared to ritonavir, particularly for **CYP3A4**.
- While it could theoretically have some effect on drug metabolism, its impact on saquinavir's bioavailability would be **clinically insignificant** compared to ritonavir.
*Vitamin C*
- **Vitamin C** (ascorbic acid) is an antioxidant and plays various roles in the body.
- It has **no significant interaction** with cytochrome P450 enzymes and would not affect the metabolism or bioavailability of saquinavir.
*Ganciclovir*
- **Ganciclovir** is an antiviral drug primarily used to treat cytomegalovirus (CMV) infections.
- It does not significantly inhibit or induce cytochrome P450 enzymes and would therefore **not affect the bioavailability** of saquinavir.
HIV protease inhibitors US Medical PG Question 10: A physician scientist is looking for a more efficient way to treat HIV. Patients infected with HIV mount a humoral immune response by producing antibodies against the HIV envelope proteins. These antibodies are the same antibodies detected by the ELISA and western blot assays used to diagnose the disease. The physician scientist is trying to generate a new, more potent antibody against the same HIV envelope proteins targeted by the natural humoral immune response. Of the following proteins, which is the most likely target of the antibody he is designing?
- A. p24
- B. CXCR4
- C. CCR5
- D. p17
- E. gp120 (Correct Answer)
HIV protease inhibitors Explanation: ***gp120***
- **gp120** is an **envelope glycoprotein** on the surface of HIV, responsible for binding to CD4 receptors on host cells.
- Antibodies against **gp120** are generated during natural infection and are detected by diagnostic assays, making it a primary target for therapeutic antibody development.
*p24*
- **p24** is a **capsid protein** of HIV, forming the conical core of the virus, but it is not an envelope protein.
- While antibodies against **p24** are produced during infection and are detectable, it's an internal protein, not exposed on the viral surface for direct neutralization.
*CXCR4*
- **CXCR4** is a **chemokine co-receptor** found on the surface of host cells (e.g., T-lymphocytes), used by some HIV strains (T-tropic) for entry.
- It is a host cell protein, not an HIV viral protein, so it would not be a target for antibodies aiming to directly neutralize the virus.
*CCR5*
- **CCR5** is another **chemokine co-receptor** on host cells (e.g., macrophages, T-lymphocytes) used by other HIV strains (M-tropic) for viral entry.
- Similar to CXCR4, it is a host cell protein, not an HIV envelope protein, and therefore not a direct target for neutralizing antibodies against the virus itself.
*p17*
- **p17** is an HIV **matrix protein** located just beneath the viral envelope, playing a role in viral assembly and budding.
- Similar to p24, it is an internal structural protein, not an external envelope protein, making it less accessible for neutralizing antibodies.
More HIV protease inhibitors US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.