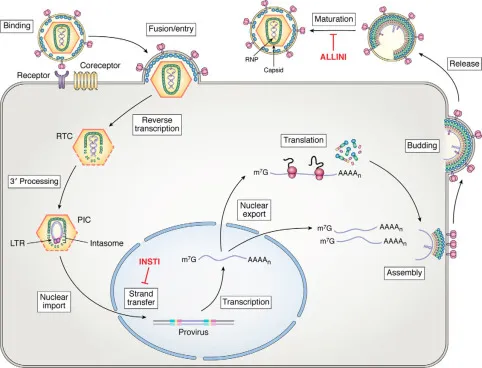
HIV integrase inhibitors US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for HIV integrase inhibitors. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
HIV integrase inhibitors US Medical PG Question 1: You are seeing a patient in clinic who recently started treatment for active tuberculosis. The patient is currently being treated with rifampin, isoniazid, pyrazinamide, and ethambutol. The patient is not used to taking medicines and is very concerned about side effects. Specifically regarding the carbohydrate polymerization inhibiting medication, which of the following is a known side effect?
- A. Vision loss (Correct Answer)
- B. Paresthesias of the hands and feet
- C. Cutaneous flushing
- D. Arthralgias
- E. Elevated liver enzymes
HIV integrase inhibitors Explanation: ***Vision loss***
- The "carbohydrate polymerization inhibiting medication" refers to **ethambutol**, which inhibits **arabinosyl transferase** (involved in mycobacterial cell wall arabinogalactan synthesis)
- **Ethambutol** causes **optic neuritis**, leading to **decreased visual acuity**, **red-green color blindness**, and potentially **irreversible vision loss**
- **Regular ophthalmologic monitoring** is essential during ethambutol therapy
*Paresthesias of the hands and feet*
- This describes **peripheral neuropathy** caused by **isoniazid**
- Isoniazid interferes with **pyridoxine (vitamin B6) metabolism**, leading to neurotoxicity
- Risk factors include malnutrition, diabetes, alcoholism, and pregnancy
- Prevented by **pyridoxine supplementation**
*Cutaneous flushing*
- Not a characteristic side effect of first-line anti-tuberculosis medications
- More commonly associated with niacin or certain allergic/vasodilatory reactions
*Arthralgias*
- Classic side effect of **pyrazinamide**, often affecting small joints
- Caused by **pyrazinamide-induced hyperuricemia** (inhibits renal uric acid excretion)
- May require dose adjustment or discontinuation if severe
*Elevated liver enzymes*
- **Hepatotoxicity** can occur with **rifampin**, **isoniazid**, and **pyrazinamide**
- Requires regular monitoring of liver function tests during TB treatment
- Most common serious adverse effect of combination TB therapy
HIV integrase inhibitors US Medical PG Question 2: A 25-year-old sexually active male presents to an internal medicine physician for a routine health check up after having several unprotected sexual encounters. After appropriate testing the physician discusses with the patient that he is HIV+ and must be started on anti-retroviral treatment. Which of the following medications prescribed acts on the gp41 subunit of the HIV envelope glycoprotein?
- A. Zidovudine
- B. Saquinavir
- C. Enfuvirtide (Correct Answer)
- D. Amantadine
- E. Rimantadine
HIV integrase inhibitors Explanation: ***Enfuvirtide***
- **Enfuvirtide** is a **fusion inhibitor** that binds specifically to the **gp41 subunit** of the HIV envelope glycoprotein.
- By binding to gp41, Enfuvirtide prevents the **fusion of the viral and host cell membranes**, thereby blocking viral entry and replication.
*Zidovudine*
- **Zidovudine** is a **nucleoside reverse transcriptase inhibitor (NRTI)**.
- It works by inhibiting the enzyme **reverse transcriptase**, which is responsible for converting viral RNA into DNA.
*Saquinavir*
- **Saquinavir** is a **protease inhibitor (PI)**.
- This drug works by inhibiting the **HIV protease enzyme**, which is crucial for cleaving viral polyproteins into functional proteins required for viral assembly and maturation.
*Amantadine*
- **Amantadine** is an **antiviral agent** primarily used to treat **influenza A**.
- It works by interfering with the **M2 proton channel** of the influenza A virus, thus inhibiting viral uncoating.
*Rimantadine*
- **Rimantadine** is another **antiviral agent** used for **influenza A treatment and prophylaxis**.
- Similar to amantadine, it targets the **M2 proton channel** of the influenza A virus, preventing the uncoating step necessary for viral replication.
HIV integrase inhibitors US Medical PG Question 3: A 40-year-old man presents with problems with his vision. He says he has been experiencing blurred vision and floaters in his left eye for the past few days. He denies any ocular pain, fever, or headaches. Past medical history is significant for HIV infection a few years ago, for which he is noncompliant with his antiretroviral medications and his most recent CD4 count was 100 cells/mm3. His temperature is 36.5°C (97.7°F), the blood pressure is 110/89 mm Hg, the pulse rate is 70/min, and the respiratory rate is 14/min. Ocular exam reveals a decreased vision in the left eye, and a funduscopic examination is shown in the image. The patient is admitted and immediately started on intravenous ganciclovir. A few days after admission he is still complaining of blurry vision and floaters, so he is switched to a different medication. Inhibition of which of the following processes best describes the mechanism of action of the newly added medication?
- A. Protein synthesis
- B. Nucleic acid synthesis (Correct Answer)
- C. Progeny virus release
- D. Viral penetration into host cells
- E. Viral uncoating
HIV integrase inhibitors Explanation: ***Nucleic acid synthesis***
- This patient likely has **cytomegalovirus (CMV) retinitis**, characterized by **blurred vision**, **floaters**, and **necrotizing retinitis** in an HIV-positive individual with a **low CD4 count (100 cells/mm3)**.
- The initial drug, **ganciclovir**, targets nucleic acid synthesis by inhibiting viral DNA polymerase. If ganciclovir fails, a common second-line agent like **foscarnet** or **cidofovir** is used, and both also inhibit viral **nucleic acid (DNA) synthesis** through different mechanisms (foscarnet directly inhibits DNA polymerase, cidofovir is a nucleotide analog).
*Protein synthesis*
- This mechanism is targeted by certain antibacterial and antifungal drugs, but not typically by antiviral medications used for CMV.
- Antiviral drugs generally target specific viral processes, distinct from host protein synthesis, to limit toxicity.
*Progeny virus release*
- This mechanism is primarily targeted by **neuraminidase inhibitors** (e.g., oseltamivir, zanamivir) used to treat influenza, which prevent the release of new viral particles from infected cells.
- It is not a common mechanism for CMV antivirals.
*Viral penetration into host cells*
- Medications that inhibit viral penetration or entry, such as **fusion inhibitors** (e.g., enfuvirtide for HIV) or **CCR5 antagonists** (e.g., maraviroc for HIV), prevent the virus from entering the host cell.
- These mechanisms are not relevant to the treatment of CMV retinitis.
*Viral uncoating*
- **Amantadine** and **rimantadine** are examples of antiviral drugs that inhibit **viral uncoating** by interfering with the M2 ion channel in influenza A.
- This mechanism is specific to influenza viruses and is not involved in the action of CMV antiviral medications.
HIV integrase inhibitors US Medical PG Question 4: A 31-year-old man with untreated HIV infection is admitted to the hospital because of a 3-day history of blurred vision and flashing lights in his left eye. Indirect ophthalmoscopy shows retinal hemorrhages of the left eye. Treatment with a drug that directly inhibits viral DNA polymerases by binding to pyrophosphate-binding sites is initiated. Two days later, the patient has a generalized tonic-clonic seizure. This patient's seizure was most likely caused by which of the following?
- A. Hypoglycemia
- B. Demyelination
- C. Encephalitis
- D. Hypocalcemia (Correct Answer)
- E. Lactic acidosis
HIV integrase inhibitors Explanation: ***Hypocalcemia***
- The drug described is **foscarnet**, which inhibits viral DNA polymerase by binding to **pyrophosphate-binding sites** and is used to treat CMV retinitis, common in HIV patients.
- A known side effect of foscarnet is **electrolyte abnormalities**, including **hypocalcemia** and **hypomagnesemia**, which can precipitate seizures.
*Hypoglycemia*
- While hypoglycemia can cause seizures, it is not a direct known side effect of foscarnet or typically associated with the treatment of CMV retinitis.
- The clinical presentation does not suggest **low blood sugar** as the primary cause for the seizure.
*Demyelination*
- Demyelination can be seen in HIV infection (e.g., **PML**), but it's a slower process and less likely to cause an acute, sudden seizure following initiation of an antiviral drug for CMV retinitis.
- There is no direct link between foscarnet administration and acute demyelination leading to seizures.
*Encephalitis*
- Encephalitis can cause seizures, but the primary clinical picture describes **CMV retinitis** and a subsequent seizure after starting a specific antiviral medication.
- While HIV patients are susceptible to various CNS infections, the acute onset seizure directly linked to the initiation of foscarnet therapy points to a drug-related adverse effect rather than a new infection.
*Lactic acidosis*
- Lactic acidosis can occur in HIV patients, particularly with certain antiretroviral therapies (**NRTIs**), but it is not a direct or common side effect of foscarnet.
- While severe lactic acidosis can cause neurological symptoms, it primarily manifests with other systemic signs (e.g., nausea, vomiting, tachypnea) not described here.
HIV integrase inhibitors US Medical PG Question 5: The drug cilostazol is known for its ability to relax vascular smooth muscle and therefore cause vasodilation through its inhibition of phosphodiesterase 3. Given this mechanism of action, what other effect would be expected?
- A. Antiarrhythmic action
- B. Negative chronotropy
- C. Angioedema
- D. Increased left ventricular end-diastolic volume
- E. Positive inotropy (Correct Answer)
HIV integrase inhibitors Explanation: ***Positive inotropy***
- **Cilostazol** inhibits **phosphodiesterase 3 (PDE3)**, leading to an increase in **cAMP** levels within cardiac myocytes.
- Increased **cAMP** in the heart results in enhanced calcium influx and release, which strengthens myocardial contraction, leading to **positive inotropy**.
*Antiarrhythmic action*
- While some drugs affecting **cAMP** can have antiarrhythmic effects, **PDE3 inhibitors** like cilostazol can sometimes **increase heart rate** and potentially cause or worsen arrhythmias due to increased conduction and excitability.
- Their primary mechanism of action for cardiac effects is **inotropy**, not rhythm stabilization.
*Negative chronotropy*
- **Negative chronotropy** refers to a decrease in heart rate, which is typically seen with drugs that reduce **cAMP** or directly inhibit the **sinoatrial node**.
- As a **PDE3 inhibitor**, cilostazol leads to increased **cAMP**, which usually causes a modest increase in heart rate (**positive chronotropy**), not a decrease.
*Angioedema*
- **Angioedema** is a side effect often associated with **ACE inhibitors** or certain allergic reactions, mediated by bradykinin or histamine release.
- It is not a known or expected effect of **cilostazol** based on its mechanism of **PDE3 inhibition** and cAMP modulation.
*Increased left ventricular end-diastolic volume*
- **Cilostazol** causes **vasodilation**, which **reduces peripheral vascular resistance** and thus **afterload**.
- This reduction in afterload, combined with its **positive inotropic** effects, tends to improve cardiac output and can actually lead to a **decrease** in **left ventricular end-diastolic volume** due to more efficient ejection, rather than an increase.
HIV integrase inhibitors US Medical PG Question 6: A 49-year-old woman presents to her primary care doctor in late December with malaise. She reports worsening fatigue, myalgias, headache, and malaise that started 1 day ago. She works as a lunch lady at an elementary school. Her past medical history is notable for a distal radius fracture after a fall 2 years ago, but she is otherwise healthy and takes no medications. She does not smoke or drink alcohol. She is married and has 3 adult children who are healthy. Her temperature is 102.9°F (39.4°C), blood pressure is 101/61 mmHg, pulse is 112/min, and respirations are 21/min. On exam, she appears lethargic and uncomfortable but is able to answer questions appropriately. Breath sounds are normal bilaterally. She is started on intravenous fluids and a pharmacologic agent for treatment. Which of the following is the most likely mechanism of action of the drug being used to treat this patient?
- A. Neuraminidase inhibitor (Correct Answer)
- B. Reverse transcriptase inhibitor
- C. RNA-dependent polymerase inhibitor
- D. DNA polymerase inhibitor
- E. Protease inhibitor
HIV integrase inhibitors Explanation: ***Neuraminidase inhibitor***
- The patient's symptoms (malaise, fatigue, myalgias, headache, fever) with rapid onset in **late December**, especially given her exposure to children in an elementary school, are highly suggestive of **influenza**.
- **Neuraminidase inhibitors** (e.g., oseltamivir, zanamivir) are the primary antiviral treatment for influenza, preventing the release of new viral particles from infected cells.
*Reverse transcriptase inhibitor*
- **Reverse transcriptase inhibitors** are primarily used in the treatment of **HIV infection**, which typically presents with a different constellation of symptoms and has a chronic rather than acute course.
- This class of drugs targets the enzyme **reverse transcriptase**, which is not central to the influenza virus replication cycle.
*RNA-dependent polymerase inhibitor*
- While **baloxavir marboxil** (an RNA polymerase inhibitor) is FDA-approved for influenza treatment, **neuraminidase inhibitors** remain the most commonly used first-line agents.
- In this clinical scenario without specific contraindications to neuraminidase inhibitors, oseltamivir or zanamivir would be the most likely agents prescribed.
*DNA polymerase inhibitor*
- **DNA polymerase inhibitors** are primarily used to treat **DNA viral infections** such as herpes viruses (e.g., acyclovir for HSV/VZV) or cytomegalovirus (e.g., ganciclovir).
- Influenza is an **RNA virus** and therefore does not have a DNA polymerase for replication.
*Protease inhibitor*
- **Protease inhibitors** are a class of antiviral drugs predominantly used in the treatment of **HIV** and **Hepatitis C virus** infections.
- Influenza viruses do not have a protease target that is typically inhibited by these drugs for therapeutic purposes.
HIV integrase inhibitors US Medical PG Question 7: An HIV-positive patient with a CD4+ count of 45 is receiving recommended first-line treatment for a case of cytomegalovirus retinitis. Coadministration with which of the following agents would be most likely to precipitate a deficiency of neutrophils in this patient?
- A. Ritonavir
- B. Raltegravir
- C. Foscarnet
- D. Efavirenz
- E. Zidovudine (Correct Answer)
HIV integrase inhibitors Explanation: ***Zidovudine***
- **Zidovudine (AZT)** is a nucleoside reverse transcriptase inhibitor (NRTI) that is well-known for causing **myelosuppression**, particularly **neutropenia** and **anemia**.
- In an HIV-positive patient with a low **CD4+ count** and concurrent treatment for **CMV retinitis** (which often involves drugs like ganciclovir that can also cause myelosuppression), adding zidovudine significantly increases the risk of severe neutropenia.
*Ritonavir*
- **Ritonavir** is a protease inhibitor primarily known for its role as a **pharmacokinetic booster** in HIV therapy, enhancing the levels of other antiretrovirals.
- While it can cause gastrointestinal side effects and hepatotoxicity, **myelosuppression** and specifically neutropenia are not its primary or common adverse effects.
*Raltegravir*
- **Raltegravir** is an integrase strand transfer inhibitor (INSTI) generally well-tolerated with a favorable side effect profile.
- Common side effects include headache, nausea, and fatigue, but it is **not typically associated with significant myelosuppression** or neutropenia.
*Foscarnet*
- **Foscarnet** is an antiviral agent used for treating CMV retinitis, particularly in cases of ganciclovir resistance.
- Its major dose-limiting toxicities include **nephrotoxicity** and **electrolyte disturbances** (e.g., hypocalcemia, hypomagnesemia), not primarily neutropenia.
*Efavirenz*
- **Efavirenz** is a non-nucleoside reverse transcriptase inhibitor (NNRTI) associated with central nervous system side effects such as dizziness, insomnia, and vivid dreams.
- While skin rash and hepatotoxicity can occur, **bone marrow suppression** leading to neutropenia is not a characteristic or frequent adverse effect of efavirenz.
HIV integrase inhibitors US Medical PG Question 8: A 63-year-old HIV-positive man comes to the physician for a routine health maintenance examination. Four years ago, he was diagnosed with HIV and was started on cART therapy. He tells the physician that he has been having difficulty adhering to his medication regimen. He has been unemployed for the past couple of years and relies on unemployment benefits to cover the costs of daily living. His father died of lymphoma at the age of 60 years. He wants more information about his risk of developing DLBCL. Which of the following is the greatest risk factor for the development of DLBCL in HIV-positive patients?
- A. Poor adherence to cART (Correct Answer)
- B. Income below $30,000 per year
- C. Male sex
- D. Positive family history of cancer
- E. Age over 55 years
HIV integrase inhibitors Explanation: **Poor adherence to cART**
- **Poor adherence** to cART leads to **uncontrolled HIV replication** and persistent **immunosuppression**, which significantly increases the risk of developing **DLBCL**.
- **Immune dysregulation** caused by HIV directly contributes to a higher incidence of **AIDS-defining malignancies**, including DLBCL.
*Income below $30,000 per year*
- While **socioeconomic factors** can impact access to care and medication adherence, low income itself is not a direct biological risk factor for DLBCL.
- Its influence is secondary to its effect on adherence and overall health status, rather than a primary risk factor for the malignancy.
*Positive family history of cancer*
- Although a family history of cancer can increase the risk for some malignancies, it is generally **not a significant risk factor** for **HIV-associated DLBCL**.
- The primary drivers of HIV-associated DLBCL are linked to HIV-induced immunodeficiency, not specific inherited genetic predispositions for lymphoma.
*Age over 55 years*
- While the incidence of many cancers increases with **age**, for **HIV-associated DLBCL**, age is less prominent than the degree of **immunodeficiency** caused by HIV.
- The stronger prognostic factor remains the state of the immune system, particularly a **low CD4 count**, which is often exacerbated by poor cART adherence.
*Male sex*
- While there are minor differences in cancer incidence between sexes, **male sex** is not a primary or significant independent risk factor for **HIV-associated DLBCL**.
- The risk is predominantly driven by factors related to HIV infection itself and the resulting immune dysfunction.
HIV integrase inhibitors US Medical PG Question 9: A 43-year-old man with HIV infection comes to the physician because of a 2-week history of progressive diarrhea and a 3-kg (6.6-lb) weight loss. During this period, he has had 3–4 episodes of watery stools daily, with multiple instances of blood in the stool. He is currently receiving antiretroviral therapy with zidovudine, lamivudine, and dolutegravir. Physical examination shows pallor and dry mucous membranes. A colonoscopy shows multiple linear ulcers. Polymerase chain reaction of a stool sample is positive for cytomegalovirus. Treatment with valganciclovir is begun. Adding this drug to his current medication regimen puts this patient at greatest risk for which of the following adverse effects?
- A. Hepatic steatosis
- B. Abnormal dreams
- C. Pancytopenia (Correct Answer)
- D. Orthostatic dysregulation
- E. Hyperglycemia
HIV integrase inhibitors Explanation: ***Pancytopenia***
- **Valganciclovir** is a known cause of **bone marrow suppression**, leading to **pancytopenia** (low red blood cells, white blood cells, and platelets).
- The patient is also on **zidovudine**, an antiretroviral that can cause **myelosuppression**, thus the combined use significantly increases the risk of pancytopenia.
*Hepatic steatosis*
- **Hepatic steatosis** (fatty liver) is a rare but known adverse effect of some nucleoside reverse transcriptase inhibitors (NRTIs), particularly older ones.
- While lamivudine is an NRTI, **valganciclovir** is not primarily associated with hepatic steatosis, and the combination does not specifically heighten this risk more than other options.
*Abnormal dreams*
- **Abnormal dreams** are a common side effect associated with certain antiretroviral drugs, particularly the non-nucleoside reverse transcriptase inhibitor **efavirenz**.
- This patient is on dolutegravir (an integrase inhibitor), zidovudine, and lamivudine, none of which are primarily known for causing abnormal dreams as a prominent side effect, and valganciclovir does not contribute to this.
*Orthostatic dysregulation*
- **Orthostatic dysregulation** (orthostatic hypotension) can be a side effect of various medications, but it is not a prominent adverse effect of either **valganciclovir** or the patient's current antiretroviral regimen.
- While dehydration from diarrhea can cause it, the medication itself does not directly increase this risk in particular.
*Hyperglycemia*
- **Hyperglycemia** can be a side effect of certain antiretroviral drugs, particularly some **protease inhibitors** and older NRTIs.
- However, the patient's current regimen (zidovudine, lamivudine, dolutegravir) and **valganciclovir** are not strongly associated with hyperglycemia as a primary adverse effect compared to other options.
HIV integrase inhibitors US Medical PG Question 10: Administration of which of the following drugs would increase the bioavailability of saquinavir?
- A. Cimetidine
- B. Vitamin C
- C. Ritonavir (Correct Answer)
- D. Ganciclovir
HIV integrase inhibitors Explanation: **Ritonavir**
- **Ritonavir** is a potent **CYP3A4 inhibitor**, which is the primary enzyme responsible for the metabolism of saquinavir.
- By inhibiting **saquinavir** metabolism, ritonavir significantly **increases its plasma concentrations and bioavailability**, making it an effective pharmacokinetic enhancer.
- This combination (saquinavir/ritonavir) is a clinically established strategy in antiretroviral therapy.
*Cimetidine*
- **Cimetidine** inhibits various cytochrome P450 enzymes but is a less potent and more general inhibitor compared to ritonavir, particularly for **CYP3A4**.
- While it could theoretically have some effect on drug metabolism, its impact on saquinavir's bioavailability would be **clinically insignificant** compared to ritonavir.
*Vitamin C*
- **Vitamin C** (ascorbic acid) is an antioxidant and plays various roles in the body.
- It has **no significant interaction** with cytochrome P450 enzymes and would not affect the metabolism or bioavailability of saquinavir.
*Ganciclovir*
- **Ganciclovir** is an antiviral drug primarily used to treat cytomegalovirus (CMV) infections.
- It does not significantly inhibit or induce cytochrome P450 enzymes and would therefore **not affect the bioavailability** of saquinavir.
More HIV integrase inhibitors US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.