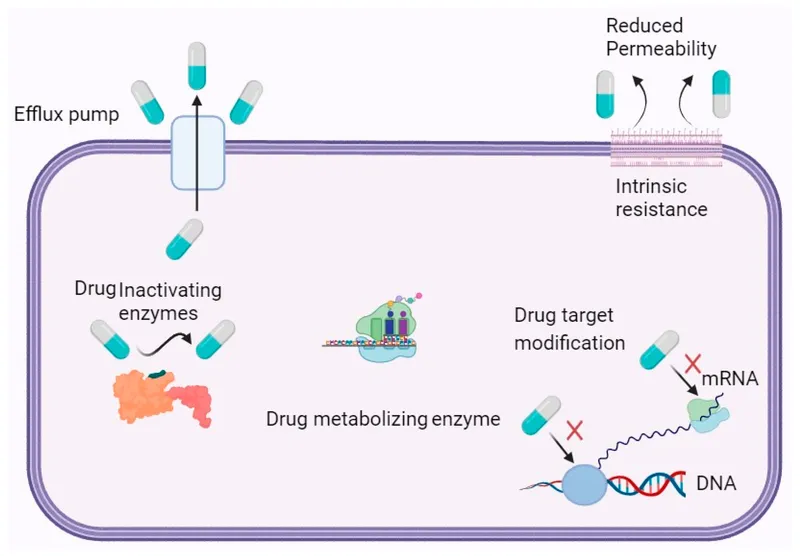
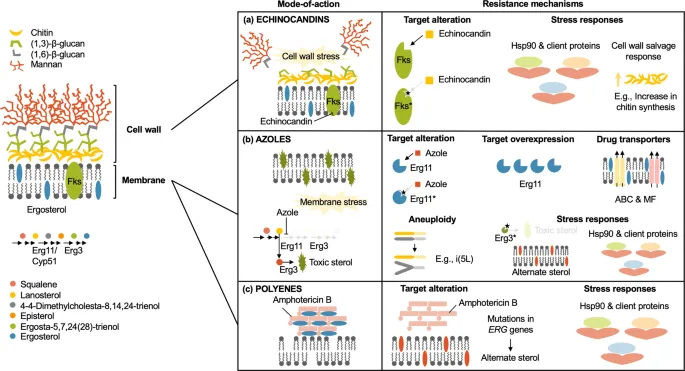
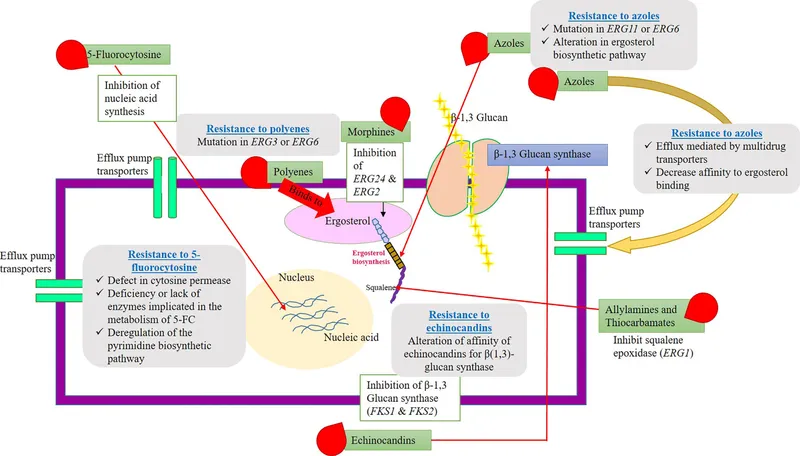
Antifungal resistance mechanisms US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Antifungal resistance mechanisms. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Antifungal resistance mechanisms US Medical PG Question 1: You are taking care of a patient with renal failure secondary to anti-fungal therapy. The patient is a 66-year-old male being treated for cryptococcal meningitis. This drug has a variety of known side effects including acute febrile reactions to infusions, anemia, hypokalemia and hypomagnesemia. What is the mechanism of action of this drug?
- A. Inhibition of squalene epoxidase
- B. Binding of the 50S subunit
- C. Pore formation secondary to ergosterol binding (Correct Answer)
- D. Disruption of microtubule formation
- E. Inhibition of 1,3-beta-glucan synthase
Antifungal resistance mechanisms Explanation: ***Pore formation secondary to ergosterol binding***
- This describes the mechanism of action of **amphotericin B**, the antifungal agent used for cryptococcal meningitis.
- Amphotericin B binds to **ergosterol** in the fungal cell membrane, leading to the formation of pores, disruption of membrane integrity, and ultimately cell death.
- The side effects described—**nephrotoxicity with renal failure, hypokalemia, and hypomagnesemia**—are classic adverse effects of amphotericin B due to its effect on renal tubular cells and electrolyte wasting.
*Inhibition of squalene epoxidase*
- This is the mechanism of action for **terbinafine**, an antifungal primarily used for dermatophyte infections (e.g., onychomycosis), not systemic infections like cryptococcal meningitis.
- Terbinafine inhibits ergosterol synthesis at an earlier step but does not cause the severe nephrotoxicity and electrolyte disturbances described.
*Binding of the 50S subunit*
- This mechanism of action is characteristic of **macrolide antibiotics** like azithromycin or clarithromycin, which are antibacterial agents, not antifungals.
- These drugs inhibit bacterial protein synthesis and are ineffective against fungal infections.
*Disruption of microtubule formation*
- This is the mechanism of action for **griseofulvin**, an antifungal drug used for dermatophyte infections of the skin, hair, and nails.
- Griseofulvin interferes with fungal cell division and is not used for life-threatening systemic infections like cryptococcal meningitis.
*Inhibition of 1,3-beta-glucan synthase*
- This mechanism is associated with **echinocandins** (e.g., caspofungin, micafungin), which inhibit fungal cell wall synthesis.
- While echinocandins are used for some systemic fungal infections (particularly Candida and Aspergillus), they do not typically cause the severe renal failure and electrolyte disturbances characteristic of amphotericin B.
Antifungal resistance mechanisms US Medical PG Question 2: A 37-year-old woman with a history of anorectal abscesses complains of pain in the perianal region. Physical examination reveals mild swelling, tenderness, and erythema of the perianal skin. She is prescribed oral ampicillin and asked to return for follow-up. Two days later, the patient presents with a high-grade fever, syncope, and increased swelling. Which of the following would be the most common mechanism of resistance leading to the failure of antibiotic therapy in this patient?
- A. Intrinsic absence of a target site for the drug
- B. Use of an altered metabolic pathway
- C. Production of beta-lactamase enzyme (Correct Answer)
- D. Altered structural target for the drug
- E. Drug efflux pump
Antifungal resistance mechanisms Explanation: ***Production of beta-lactamase enzyme***
- The patient's symptoms of a rapidly worsening infection despite ampicillin treatment suggest the presence of a **beta-lactamase producing organism**. Ampicillin is a **beta-lactam antibiotic** that is inactivated by these enzymes.
- Anorectal abscesses and rapidly progressing soft tissue infections are often caused by **polymicrobial flora**, including staphylococci and enterococci, many of which can produce **beta-lactamase**.
*Intrinsic absence of a target site for the drug*
- While some bacteria inherently lack the target site for certain drugs (e.g., mycoplasma lacking a cell wall, thus being resistant to beta-lactams), this is less likely to be the **most common mechanism of acquired resistance** leading to treatment failure in a typical perianal infection.
- The rapid progression and failed initial treatment point towards an **acquired mechanism of resistance** rather than an intrinsic one.
*Use of an altered metabolic pathway*
- This mechanism, such as altered **folate synthesis pathways** in resistance to trimethoprim-sulfamethoxazole, is less common as the primary mechanism for ampicillin resistance.
- Ampicillin's mechanism of action primarily targets the **bacterial cell wall**, not a metabolic pathway in the same way.
*Altered structural target for the drug*
- This involves modifications to the **penicillin-binding proteins (PBPs)**, which are the targets of beta-lactam antibiotics like ampicillin. While a valid mechanism (e.g., in MRSA), the **production of beta-lactamase** is generally a more widespread and common cause of ampicillin failure, especially in infections involving mixed flora from the perianal region.
- Given the abrupt failure of ampicillin, **beta-lactamase inactivation** is a more immediate and common cause than a rapid mutational change in PBPs.
*Drug efflux pump*
- **Efflux pumps** actively remove antibiotics from the bacterial cell, contributing to resistance against various drug classes.
- While efflux pumps can play a role, the dominant mechanism for resistance to **ampicillin** in many common perianal pathogens is the **enzymatic degradation by beta-lactamases**.
Antifungal resistance mechanisms US Medical PG Question 3: A 46-year-old man with HIV infection comes to the physician because of a 1-week history of severe retrosternal pain while swallowing. He has not been compliant with his antiretroviral drug regimen. His CD4+ T-lymphocyte count is 98/mm3 (N ≥ 500). Endoscopy shows white plaques in the esophagus. The most appropriate immediate treatment is a drug that inhibits which of the following enzymes?
- A. DNA polymerase
- B. Hydrogen-potassium ATPase
- C. Cytochrome p450 enzymes (Correct Answer)
- D. Phospholipase A2
- E. Squalene epoxidase
Antifungal resistance mechanisms Explanation: ***Cytochrome P450 enzymes***
- The patient's symptoms (retrosternal pain on swallowing, white plaques on endoscopy) and severely low **CD4+ count (98/mm³)** are highly suggestive of **esophageal candidiasis**, a common opportunistic infection in AIDS.
- **Fluconazole**, an azole antifungal, is the **first-line treatment** for esophageal candidiasis and works by inhibiting **14α-demethylase (lanosterol demethylase)**, a fungal **cytochrome P450 enzyme**.
- This inhibition prevents the conversion of lanosterol to ergosterol, disrupting **fungal cell membrane synthesis** and leading to fungal cell death.
*Squalene epoxidase*
- **Terbinafine** and **naftifine** (allylamine antifungals) inhibit squalene epoxidase in the ergosterol synthesis pathway.
- These agents are primarily used for **dermatophyte infections** (onychomycosis, tinea) and have **poor activity against Candida species**.
- They are not appropriate for treating esophageal candidiasis.
*DNA polymerase*
- Inhibitors of **DNA polymerase**, such as acyclovir or ganciclovir, are used to treat **herpesvirus infections** (HSV, CMV).
- While herpes esophagitis can occur in immunocompromised patients, it typically presents with **punched-out ulcers**, not white plaques.
*Hydrogen-potassium ATPase*
- **Proton pump inhibitors** (PPIs) target hydrogen-potassium ATPase in gastric parietal cells to reduce **acid secretion**.
- These are used to treat **GERD** or **peptic ulcers**, which do not present with white plaques on endoscopy.
- While PPIs may provide symptomatic relief, they do not treat the underlying fungal infection.
*Phospholipase A2*
- Phospholipase A2 inhibitors are used as **anti-inflammatory agents**, as PLA2 releases arachidonic acid, a precursor to inflammatory mediators.
- These drugs have no role in treating fungal infections like esophageal candidiasis.
Antifungal resistance mechanisms US Medical PG Question 4: An 18-year old college freshman presents to his university clinic because he has not been feeling well for the past two weeks. He has had a persistent headache, occasional cough, and chills without rigors. The patient’s vital signs are normal and physical exam is unremarkable. His radiograph shows patchy interstitial lung infiltrates and he is diagnosed with atypical pneumonia. The patient is prescribed azithromycin and takes his medication as instructed. Despite adherence to his drug regimen, he returns to the clinic one week later because his symptoms have not improved. The organism responsible for this infection is likely resistant to azithromycin through which mechanism?
- A. Mutation in topoisomerase II
- B. Methylation of ribosomal binding site
- C. Presence of a beta-lactamase
- D. Decreased binding to RNA polymerase
- E. Insertion of drug efflux pumps (Correct Answer)
Antifungal resistance mechanisms Explanation: ***Insertion of drug efflux pumps***
- **Azithromycin** is a macrolide antibiotic that inhibits bacterial protein synthesis by binding to the **50S ribosomal subunit**.
- In **Mycoplasma pneumoniae** (the most common cause of atypical pneumonia in young adults), the **most common** mechanism of macrolide resistance is through **efflux pumps**, particularly the **mef genes**.
- These efflux pumps actively transport macrolides out of the bacterial cell, reducing intracellular drug concentration and conferring resistance.
- This mechanism is responsible for the majority of macrolide-resistant *M. pneumoniae* isolates worldwide.
*Methylation of ribosomal binding site*
- **Methylation** of the ribosomal binding site (specifically the **23S rRNA** via erm genes) does prevent azithromycin from binding effectively.
- While this is a valid macrolide resistance mechanism seen in organisms like *Streptococcus pneumoniae* and *Streptococcus pyogenes*, it is **less common** in *Mycoplasma pneumoniae*.
- Efflux pumps (mef) are the predominant mechanism in *M. pneumoniae* resistant strains.
*Mutation in topoisomerase II*
- **Topoisomerase II** (DNA gyrase) is the target of **fluoroquinolone antibiotics**, not macrolides.
- Mutations in this enzyme lead to resistance against fluoroquinolones, such as **ciprofloxacin**.
*Presence of a beta-lactamase*
- **Beta-lactamase enzymes** inactivate **beta-lactam antibiotics** (e.g., penicillin, cephalosporins) by hydrolyzing their beta-lactam ring.
- Additionally, *Mycoplasma pneumoniae* **lacks a cell wall**, making it inherently resistant to all beta-lactam antibiotics regardless of beta-lactamase production.
*Decreased binding to RNA polymerase*
- **RNA polymerase** is the target for antibiotics like **rifampin**, which inhibits bacterial transcription.
- Decreased binding to RNA polymerase would lead to rifampin resistance, not azithromycin resistance.
Antifungal resistance mechanisms US Medical PG Question 5: A 32-year-old man presents to an outpatient clinic for tuberculosis prophylaxis before leaving for a trip to Asia, where tuberculosis is endemic. The Mantoux test is positive, but the chest X-ray and AFB sputum culture are negative. He was started on isoniazid. What is the most likely mechanism of resistance to isoniazid?
- A. Methylation of the RNA binding site
- B. Plasmid-mediated resistance
- C. Reduction of drug binding to RNA polymerase
- D. Increased efflux from the cell
- E. Mutations in katG (Correct Answer)
Antifungal resistance mechanisms Explanation: ***Mutations in katG***
- The **katG gene** encodes **catalase-peroxidase**, an enzyme essential for activating isoniazid into its active form within *Mycobacterium tuberculosis*.
- Mutations in *katG* prevent the activation of isoniazid, thereby conferring **resistance**.
*Methylation of the RNA binding site*
- This mechanism is primarily associated with **aminoglycoside resistance**, where methylation of ribosomal RNA prevents antibiotic binding.
- It is not a known mechanism for resistance to **isoniazid**.
*Plasmid-mediated resistance*
- While common in many bacteria for antibiotic resistance, **plasmid-mediated resistance** is rare for **first-line anti-tuberculosis drugs** like isoniazid in *Mycobacterium tuberculosis*.
- Most *M. tuberculosis* resistance mechanisms involve **chromosomal mutations**.
*Reduction of drug binding to RNA polymerase*
- This mechanism is typically associated with resistance to **rifamycins** (e.g., rifampin), which target the **bacterial RNA polymerase**.
- Isoniazid's mechanism of action involves **mycolic acid synthesis inhibition**, not RNA polymerase binding.
*Increased efflux from the cell*
- While efflux pumps contribute to antibiotic resistance in many bacteria, they are less commonly the primary mechanism for high-level **isoniazid resistance** in *M. tuberculosis*.
- Resistance is predominantly linked to target modification or enzyme deficits, like those involving **katG**.
Antifungal resistance mechanisms US Medical PG Question 6: A 74-year-old man is admitted to the medical ward after he developed a fungal infection. He has aplastic anemia. The most recent absolute neutrophil count was 450/µL. An anti-fungal agent is administered that inhibits the fungal enzyme, (1→3)-β-D-glucan synthase, and thereby disrupts the integrity of the fungal cell wall. He responds well to the treatment. Although amphotericin B is more efficacious for his condition, it was not used because of the side effect profile. What was the most likely infection?
- A. Invasive aspergillosis
- B. Mucormycosis
- C. Histoplasmosis
- D. Paracoccidioidomycosis
- E. Candidemia (Correct Answer)
Antifungal resistance mechanisms Explanation: ***Candidemia***
- The patient's **neutropenia** (absolute neutrophil count of 450/µL) due to aplastic anemia is a major risk factor for invasive candidiasis, including candidemia.
- The antifungal agent's mechanism of action, targeting **(1→3)-β-D-glucan synthase**, is characteristic of **echinocandins**, which are first-line agents for candidemia, especially in critically ill or neutropenic patients, and often preferred over amphotericin B due to a better side effect profile.
*Invasive aspergillosis*
- While neutropenia is a significant risk factor for invasive aspergillosis, the primary antifungal drugs for this condition are typically **voriconazole** or **isavuconazole**, although echinocandins may be used as salvage therapy or in combination.
- The description of the drug's mechanism specifically targeting **(1→3)-β-D-glucan synthase** does not make aspergillosis the *most likely* infection, as some Aspergillus species may have less β-D-glucan in their cell walls compared to *Candida*.
*Mucormycosis*
- This aggressive fungal infection is often seen in immunocompromised patients, particularly those with **diabetes** or profound neutropenia, but the primary treatment is usually **amphotericin B**.
- Mucorales fungi typically **lack ergosterol** and their cell walls do not contain **(1→3)-β-D-glucan**, making echinocandins ineffective.
*Histoplasmosis*
- This is a dimorphic fungal infection endemic to certain geographic regions, primarily affecting the lungs and disseminating in immunocompromised individuals.
- The drug of choice for severe or disseminated histoplasmosis is **amphotericin B**, followed by azoles; echinocandins are generally not active against *Histoplasma*.
*Paracoccidioidomycosis*
- This is a chronic systemic mycosis found in Latin America, primarily affecting the lungs, skin, and lymph nodes.
- Treatment for severe forms typically involves **amphotericin B**, followed by sulfonamides or azoles for maintenance; echinocandins are not effective against *Paracoccidioides*.
Antifungal resistance mechanisms US Medical PG Question 7: A 30-year-old man is admitted to the hospital with a presumed pneumonia and started on antibiotics. Two days later, the patient shows no improvement. Blood cultures reveal yeast with pseudophyphae. Which of the following cell types is most likely deficient or dysfunctional in this patient?
- A. Eosinophils
- B. Macrophages
- C. Neutrophils (Correct Answer)
- D. T-cells
- E. B-cells
Antifungal resistance mechanisms Explanation: ***Neutrophils***
- The presence of **yeast with pseudohyphae** in blood cultures, particularly *Candida*, indicates a fungal infection.
- **Neutrophils** are crucial for the host defense against *Candida* and other fungal pathogens, so their deficiency or dysfunction would predispose to candidemia.
- Neutropenia or neutrophil dysfunction (e.g., chronic granulomatous disease) significantly increases risk of invasive candidiasis.
*Eosinophils*
- **Eosinophils** are primarily involved in defense against **parasitic infections** and in allergic reactions.
- They play a minimal role in the immune response to systemic fungal infections like candidemia.
*Macrophages*
- **Macrophages** are phagocytic cells that contribute to antifungal immunity, particularly in tissue surveillance and chronic infection control.
- However, **neutrophils** are the primary and most critical defense against acute *Candida* bloodstream infections.
- Macrophage deficiency alone does not typically predispose to candidemia as severely as neutrophil deficiency.
*T-cells*
- **T-cells** are important for cell-mediated immunity, particularly against **intracellular pathogens** and viral infections.
- While they play a role in modulating antifungal responses, their deficiency typically leads to infections with *Pneumocystis jirovecii* or severe mucocutaneous candidiasis, rather than disseminated candidemia.
*B-cells*
- **B-cells** are responsible for **humoral immunity** through antibody production, which is primarily effective against extracellular bacteria and toxins.
- They are not the primary line of defense against fungal infections such as candidemia.
Antifungal resistance mechanisms US Medical PG Question 8: A 45-year-old man presents to the emergency department with difficulties swallowing food. He states that he experiences pain when he attempts to swallow his medications or when he drinks water. He reveals that he was diagnosed with HIV infection five years ago. He asserts that he has been taking his antiretroviral regimen, including emtricitabine, rilpivirine, and tenofovir. His temperature is 98°F (37°C), blood pressure is 100/60 mmHg, pulse is 90/min, respirations are 22/min, and oxygen saturation is 99% on room air. His physical exam is notable for a clear oropharynx, no lymphadenopathy, and a normal cardiac and pulmonary exam. No rashes are noted throughout his body. His laboratory results are displayed below:
Hemoglobin: 12 g/dL
Hematocrit: 37 %
Leukocyte count: 8,000/mm^3 with normal differential
Platelet count: 160,000/mm^3
Serum:
Na+: 138 mEq/L
Cl-: 108 mEq/L
K+: 3.5 mEq/L
HCO3-: 26 mEq/L
BUN: 35 mg/dL
Glucose: 108 mg/dL
Creatinine: 1.1 mg/dL
CD4+ count: 90/mm^3
HIV viral load: 59,000 copies/mL
What is the best next step in management?
- A. Fluconazole (Correct Answer)
- B. Nystatin
- C. Oral swab and microscopy
- D. Methylprednisolone
- E. Esophageal endoscopy and biopsy
Antifungal resistance mechanisms Explanation: ***Fluconazole***
- The patient's **odynophagia**, low **CD4+ count**, and high **HIV viral load** are highly suggestive of **esophageal candidiasis**.
- **Fluconazole** is the initial empiric treatment of choice for suspected esophageal candidiasis in HIV-positive patients, given its high efficacy and good tolerability.
*Nystatin*
- **Nystatin** is typically used for **oral candidiasis (thrush)**, which presents with white plaques in the mouth.
- The patient has a **clear oropharynx** and **odynophagia**, indicating esophageal involvement, for which nystatin is less effective.
*Oral swab and microscopy*
- While an **oral swab** can confirm oral candidiasis, it is not sufficient for diagnosing **esophageal candidiasis**.
- Given the patient's symptoms of odynophagia and high clinical suspicion in an immunocompromised patient, empiric treatment is preferred over initial diagnostic testing for uncomplicated esophageal candidiasis.
*Methylprednisolone*
- **Methylprednisolone** is a corticosteroid used to reduce inflammation and is not indicated for the treatment of **candidal infections**.
- Using corticosteroids in an immunocompromised patient with an active opportunistic infection could worsen his condition.
*Esophageal endoscopy and biopsy*
- **Esophageal endoscopy and biopsy** are typically reserved for patients who **fail empiric antifungal therapy** or present with **atypical symptoms** not consistent with candidiasis.
- Given the clear clinical picture, initial empiric treatment with fluconazole is the standard first step.
Antifungal resistance mechanisms US Medical PG Question 9: A 64-year-old female with type 2 diabetes mellitus comes to the physician because of a 1-week history of painful red swelling on her left thigh. Examination shows a 3- x 4-cm, tender, fluctuant mass. Incision and drainage of the abscess are performed. Culture of the abscess fluid grows gram-positive, coagulase-positive cocci that are resistant to oxacillin. Which of the following best describes the mechanism of resistance of the causal organism to oxacillin?
- A. Degradation of the antibiotic
- B. Decreased uptake of the antibiotic
- C. Decreased activation of the antibiotic
- D. Altered target of the antibiotic (Correct Answer)
- E. Acetylation of the antibiotic
Antifungal resistance mechanisms Explanation: ***Altered target of the antibiotic***
- The organism described (gram-positive, coagulase-positive cocci, oxacillin-resistant) is **methicillin-resistant *Staphylococcus aureus* (MRSA)**.
- MRSA achieves oxacillin (and other beta-lactam) resistance by acquiring the ***mecA* gene**, which encodes for a **modified penicillin-binding protein (PBP2a)** with reduced affinity for beta-lactam antibiotics.
*Degradation of the antibiotic*
- This mechanism, primarily through the production of **beta-lactamase enzymes**, can degrade beta-lactam antibiotics.
- While *Staphylococcus aureus* can produce beta-lactamases, oxacillin (a **penicillinase-resistant penicillin**) is specifically engineered to be stable against these enzymes.
*Decreased uptake of the antibiotic*
- Reduced permeability of the bacterial cell wall can lead to decreased uptake, a mechanism more commonly associated with **gram-negative bacteria** due to their outer membrane.
- This is not the primary mechanism of resistance for MRSA to oxacillin.
*Decreased activation of the antibiotic*
- Some antibiotics are prodrugs that require activation by bacterial enzymes, and resistance can arise from mutations affecting this activation.
- Oxacillin is active in its administered form and does not require bacterial activation.
*Acetylation of the antibiotic*
- **Enzymatic modification**, such as acetylation, adenylylation, or phosphorylation, is a common mechanism of resistance, particularly against **aminoglycoside antibiotics**.
- This specific mechanism is not responsible for oxacillin resistance in MRSA.
Antifungal resistance mechanisms US Medical PG Question 10: A 41-year-old man comes to the physician because of a 3-week history of fatigue, cough, and a 4.5-kg (10-lb) weight loss. He does not smoke or drink alcohol. He appears emaciated. A chest x-ray shows a calcified nodule in the left lower lobe and left hilar lymphadenopathy. The physician initiates therapy for the condition and informs him that he will have to return for monthly ophthalmologic examination for the next 2 months. These examinations are most likely to evaluate the patient for an adverse effect of a drug with which of the following mechanisms of action?
- A. Impaired synthesis of mycolic acids
- B. Impaired protein synthesis due to binding to 50S ribosomes
- C. Impaired production of hemozoin from heme
- D. Impaired synthesis of cell wall polysaccharides (Correct Answer)
- E. Impaired protein synthesis due to binding to 30S ribosomes
Antifungal resistance mechanisms Explanation: ***Impaired synthesis of cell wall polysaccharides***
- The patient's clinical presentation (fatigue, cough, weight loss, calcified nodule, hilar lymphadenopathy) is classic for **tuberculosis**.
- The requirement for **monthly ophthalmologic examinations** is pathognomonic for **ethambutol** therapy, as this drug causes **optic neuritis** (decreased visual acuity, red-green color blindness).
- **Ethambutol** inhibits **arabinosyl transferase**, which impairs the synthesis of **arabinogalactan**, a key polysaccharide component of the mycobacterial cell wall.
- Due to the risk of optic neuritis, patients on ethambutol require baseline and monthly ophthalmologic monitoring, especially during the first 2 months of therapy.
*Impaired synthesis of mycolic acids*
- This describes the mechanism of **isoniazid (INH)**, a first-line anti-TB drug that inhibits mycolic acid synthesis.
- The main adverse effects of isoniazid are **peripheral neuropathy** (prevented with pyridoxine/vitamin B6) and **hepatotoxicity**, not optic neuritis.
- Isoniazid does not require routine ophthalmologic monitoring.
*Impaired protein synthesis due to binding to 50S ribosomes*
- This mechanism describes **macrolides** (e.g., clarithromycin, azithromycin) and **chloramphenicol**.
- While macrolides may be used for atypical mycobacterial infections, they are not first-line TB therapy and do not cause optic neuritis requiring monthly eye exams.
*Impaired protein synthesis due to binding to 30S ribosomes*
- This mechanism describes **aminoglycosides** (e.g., streptomycin) and **tetracyclines**.
- While streptomycin is a second-line anti-TB drug, its main adverse effects are **ototoxicity** (hearing loss, vestibular dysfunction) and **nephrotoxicity**, not optic neuritis.
- These drugs do not require ophthalmologic monitoring.
*Impaired production of hemozoin from heme*
- This is the mechanism of **chloroquine** and **hydroxychloroquine**, which are antimalarial drugs.
- While chloroquine can cause retinopathy requiring ophthalmologic monitoring, this patient has **tuberculosis**, not malaria.
- The clinical scenario (calcified lung nodule, hilar lymphadenopathy) and TB treatment context make this mechanism incorrect for this case.
More Antifungal resistance mechanisms US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.