Vancomycin and other glycopeptides US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Vancomycin and other glycopeptides. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Vancomycin and other glycopeptides US Medical PG Question 1: A 57-year-old woman is brought to the emergency department because of crampy abdominal pain and foul-smelling, watery diarrhea. One week ago, she underwent treatment of cellulitis with clindamycin. She has developed shortness of breath and urticaria after treatment with vancomycin in the past. Her temperature is 38.4°C (101.1°F). Abdominal examination shows mild tenderness in the left lower quadrant. Her leukocyte count is 12,800/mm3. An enzyme immunoassay is positive for glutamate dehydrogenase antigen and toxins A and B. Which of the following is the mechanism of action of the most appropriate pharmacotherapy for this patient's condition?
- A. Blocking of protein synthesis at 50S ribosomal subunit
- B. Inhibition of cell wall peptidoglycan formation (Correct Answer)
- C. Inhibition of RNA polymerase sigma subunit
- D. Inhibition of bacterial topoisomerases II and IV
- E. Generation of toxic free radical metabolites
Vancomycin and other glycopeptides Explanation: ***Inhibition of cell wall peptidoglycan formation***
- The patient presents with **foul-smelling, watery diarrhea**, recent **clindamycin use**, fever, **leukocytosis**, and positive **_C. difficile_ toxins A and B**, indicating **_C. difficile_ infection (CDI)**.
- According to **current IDSA/SHEA guidelines (2021)**, **oral vancomycin** is the **first-line therapy** for CDI, regardless of severity.
- While the patient has a history of adverse reactions to vancomycin in the past, this was likely with **IV vancomycin**. **Oral vancomycin** has **negligible systemic absorption** (<1%) and rarely causes systemic allergic reactions, making it safe to use even in patients with IV vancomycin allergy.
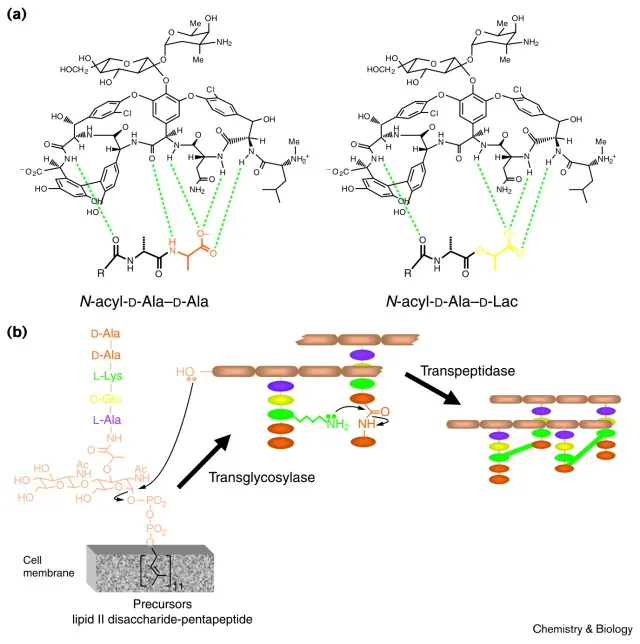
- **Vancomycin** acts by **inhibiting cell wall peptidoglycan formation**, binding to the D-Ala-D-Ala terminus of peptidoglycan precursors and preventing cross-linking.
*Generation of toxic free radical metabolites*
- This describes the mechanism of action of **metronidazole**, which was historically used as first-line therapy for mild-to-moderate CDI.
- However, **metronidazole is no longer recommended as first-line therapy** due to lower cure rates and higher recurrence rates compared to vancomycin.
- Metronidazole is now reserved for situations where vancomycin and fidaxomicin are unavailable.
*Blocking of protein synthesis at 50S ribosomal subunit*
- This mechanism describes drugs like **clindamycin**, **macrolides**, and **linezolid**.
- **Clindamycin** was the precipitating factor for this patient's CDI and should not be used for treatment.
*Inhibition of RNA polymerase sigma subunit*
- This describes the mechanism of action of **fidaxomicin**, which is also a first-line option for CDI with potentially lower recurrence rates than vancomycin.
- **Rifaximin** also inhibits bacterial RNA polymerase but is not typically used for initial CDI treatment.
- Fidaxomicin could be considered if oral vancomycin were truly contraindicated, but given the minimal systemic absorption of oral vancomycin, this is unlikely to be necessary.
*Inhibition of bacterial topoisomerases II and IV*
- This is the mechanism of action of **fluoroquinolones** (e.g., ciprofloxacin, levofloxacin).
- Fluoroquinolones are associated with an **increased risk of _C. difficile_ infection** and are contraindicated in its treatment.
Vancomycin and other glycopeptides US Medical PG Question 2: A 54-year-old man comes to the physician for a follow-up examination. One week ago, he was treated in the emergency department for chest pain, palpitations, and dyspnea. As part of his regimen, he was started on a medication that irreversibly inhibits the synthesis of thromboxane A2 and prostaglandins. Which of the following is the most likely adverse effect of this medication?
- A. Tinnitus
- B. Gout attack
- C. Chronic rhinosinusitis
- D. Acute interstitial nephritis
- E. Gastrointestinal hemorrhage (Correct Answer)
Vancomycin and other glycopeptides Explanation: ***Gastrointestinal hemorrhage***
- The medication described, which **irreversibly inhibits thromboxane A2 and prostaglandins**, is **aspirin**. Aspirin's inhibition of **prostaglandin synthesis** in the stomach reduces the protective mucous barrier, leading to an increased risk of **gastric ulcers** and **hemorrhage**.
- **Thromboxane A2 inhibition** by aspirin also impairs platelet aggregation, thereby increasing the risk of bleeding, including **gastrointestinal hemorrhage**.
- This is the **most common serious adverse effect** of chronic aspirin therapy, occurring in approximately 2-4% of patients on long-term low-dose aspirin for cardiovascular prophylaxis.
*Tinnitus*
- **Tinnitus** is a known adverse effect of **salicylate toxicity**, which usually occurs with higher doses of aspirin (>3-4 g/day). While possible, it's **uncommon with standard prophylactic doses** (81-325 mg/day) used for cardiovascular events.
- The question describes a regimen for a cardiac patient, implying a therapeutic dose rather than an overdose scenario.
*Gout attack*
- Aspirin's effect on **uric acid excretion** is dose-dependent: low doses (<1-2 g/day) can **decrease uric acid excretion**, potentially precipitating a gout attack, while high doses increase excretion.
- However, this effect is **less common** than GI complications, and aspirin is generally avoided in patients with known gout due to this complex effect and the availability of safer alternatives.
*Chronic rhinosinusitis*
- **Chronic rhinosinusitis** is not a direct adverse effect of aspirin. However, **aspirin-exacerbated respiratory disease (AERD)**, a condition involving asthma, nasal polyps, and chronic rhinosinusitis, can be triggered by aspirin in susceptible individuals.
- This is a **rare, specific syndrome** affecting approximately 7% of adults with asthma, not a general adverse effect for all patients on aspirin.
*Acute interstitial nephritis*
- **Acute interstitial nephritis** is more commonly associated with **non-steroidal anti-inflammatory drugs (NSAIDs)**, which also inhibit prostaglandin synthesis, but their effect on cyclooxygenase (COX) enzymes is typically reversible, unlike aspirin.
- While NSAIDs can cause AIN by acting as haptens and triggering an immune response, aspirin is **less frequently implicated** in this specific renal pathology compared to other NSAIDs.
Vancomycin and other glycopeptides US Medical PG Question 3: Six days after undergoing an elective hip replacement surgery, a 79-year-old man develops dysuria, flank pain, and fever. His temperature is 38.5°C (101.3°F). Examination shows marked tenderness in the right costovertebral area. Treatment with an antibiotic is begun, but his symptoms do not improve. Further evaluation shows that the causal organism produces an enzyme that inactivates the antibiotic via phosphorylation. An agent from which of the following classes of antibiotics was most likely administered?
- A. Macrolides
- B. Tetracyclines
- C. Aminoglycosides (Correct Answer)
- D. Glycopeptides
- E. Fluoroquinolones
Vancomycin and other glycopeptides Explanation: ***Aminoglycosides***
- **Aminoglycosides** are commonly inactivated by bacterial enzymes through **phosphorylation**, acetylation, or adenylation, leading to resistance.
- The patient's lack of improvement despite antibiotic treatment and the mechanism of inactivation point towards this class of antibiotics.
*Macrolides*
- **Macrolide resistance** typically involves mechanisms such as modification of the ribosomal binding site (e.g., methylation), drug efflux pumps, or enzymatic inactivation by esterases, not phosphorylation.
- While macrolides can treat various infections, their inactivation mechanism is different from what is described.
*Tetracyclines*
- **Tetracycline resistance** is primarily mediated by bacterial efflux pumps that actively transport the antibiotic out of the cell, or by ribosomal protection proteins that interfere with drug binding.
- **Enzymatic inactivation via phosphorylation** is not a characteristic resistance mechanism for tetracyclines.
*Glycopeptides*
- **Glycopeptide resistance**, particularly to vancomycin, is mainly associated with alterations in the cell wall precursor target (e.g., D-Ala-D-Lac modification), which prevents the antibiotic from binding.
- This mechanism is distinct from enzymatic phosphorylation of the antibiotic molecule itself.
*Fluoroquinolones*
- **Fluoroquinolone resistance** primarily arises from mutations in the genes encoding bacterial DNA gyrase and topoisomerase IV, or via efflux pumps.
- There is no significant mechanism of resistance involving direct enzymatic phosphorylation of fluoroquinolone drugs.
Vancomycin and other glycopeptides US Medical PG Question 4: A 64-year-old female with type 2 diabetes mellitus comes to the physician because of a 1-week history of painful red swelling on her left thigh. Examination shows a 3- x 4-cm, tender, fluctuant mass. Incision and drainage of the abscess are performed. Culture of the abscess fluid grows gram-positive, coagulase-positive cocci that are resistant to oxacillin. Which of the following best describes the mechanism of resistance of the causal organism to oxacillin?
- A. Degradation of the antibiotic
- B. Decreased uptake of the antibiotic
- C. Decreased activation of the antibiotic
- D. Altered target of the antibiotic (Correct Answer)
- E. Acetylation of the antibiotic
Vancomycin and other glycopeptides Explanation: ***Altered target of the antibiotic***
- The organism described (gram-positive, coagulase-positive cocci, oxacillin-resistant) is **methicillin-resistant *Staphylococcus aureus* (MRSA)**.
- MRSA achieves oxacillin (and other beta-lactam) resistance by acquiring the ***mecA* gene**, which encodes for a **modified penicillin-binding protein (PBP2a)** with reduced affinity for beta-lactam antibiotics.
*Degradation of the antibiotic*
- This mechanism, primarily through the production of **beta-lactamase enzymes**, can degrade beta-lactam antibiotics.
- While *Staphylococcus aureus* can produce beta-lactamases, oxacillin (a **penicillinase-resistant penicillin**) is specifically engineered to be stable against these enzymes.
*Decreased uptake of the antibiotic*
- Reduced permeability of the bacterial cell wall can lead to decreased uptake, a mechanism more commonly associated with **gram-negative bacteria** due to their outer membrane.
- This is not the primary mechanism of resistance for MRSA to oxacillin.
*Decreased activation of the antibiotic*
- Some antibiotics are prodrugs that require activation by bacterial enzymes, and resistance can arise from mutations affecting this activation.
- Oxacillin is active in its administered form and does not require bacterial activation.
*Acetylation of the antibiotic*
- **Enzymatic modification**, such as acetylation, adenylylation, or phosphorylation, is a common mechanism of resistance, particularly against **aminoglycoside antibiotics**.
- This specific mechanism is not responsible for oxacillin resistance in MRSA.
Vancomycin and other glycopeptides US Medical PG Question 5: An experimental drug, ES 62, is being studied. It prohibits the growth of vancomycin-resistant Staphylococcus aureus. It is highly lipid-soluble. The experimental design is dependent on a certain plasma concentration of the drug. The target plasma concentration is 100 mmol/dL. Which of the following factors is most important for calculating the appropriate loading dose?
- A. Volume of distribution (Correct Answer)
- B. Half-life of the drug
- C. Therapeutic index
- D. Clearance of the drug
- E. Rate of administration
Vancomycin and other glycopeptides Explanation: **Volume of distribution**
- The **loading dose** is primarily determined by the desired **plasma concentration** and the **volume of distribution (Vd)**, as it reflects how extensively a drug is distributed in the body.
- The formula for loading dose is: Loading Dose = (Target Plasma Concentration × Vd).
*Half-life of the drug*
- The **half-life** is crucial for determining the **dosing interval** and the time it takes to reach **steady-state concentrations**, not the initial loading dose.
- It reflects the rate at which the drug is eliminated from the body.
*Therapeutic index*
- The **therapeutic index** is a measure of a drug's relative safety, indicating the ratio between the **toxic dose** and the **effective dose**.
- While important for drug safety, it does not directly determine the magnitude of the loading dose itself.
*Clearance of the drug*
- **Clearance** is the rate at which the drug is removed from the body and is a primary determinant of the **maintenance dose** required to sustain a desired plasma concentration.
- It does not directly calculate the initial loading dose needed to achieve an immediate target concentration.
*Rate of administration*
- The **rate of administration** (e.g., infusion rate) primarily influences how quickly the drug reaches its target concentration, but not the total quantity of drug needed for the initial loading dose.
- It affects the kinetics of how the loading dose achieves the target concentration, rather than defining the dose amount.
Vancomycin and other glycopeptides US Medical PG Question 6: A 23-year-old woman on prednisone for lupus presents to her primary care physician because she experiences a burning sensation with urination. She has also been urinating more frequently than normal. The patient denies fever, chills, nausea/vomiting, abdominal or back pain, or other changes with urination. Her vital signs and physical exam are unremarkable, and her urine analysis is positive for leukocyte esterase and nitrites. The patient receives a diagnosis and is then prescribed an antimicrobial that acts by inhibiting DNA gyrase. Which adverse effect should the patient be counseled about?
- A. Facial redness/flushing
- B. Tendon rupture (Correct Answer)
- C. Rhabdomyolysis
- D. Hemolytic anemia
- E. Leukopenia
Vancomycin and other glycopeptides Explanation: ***Tendon rupture***
- The patient's symptoms (dysuria, frequent urination, positive leukocyte esterase, and nitrites) are consistent with a **urinary tract infection (UTI)**. The antimicrobial that inhibits **DNA gyrase** is a **fluoroquinolone**, and a well-known adverse effect of fluoroquinolones is **tendon rupture**.
- Risk factors for tendon rupture with fluoroquinolones include older age, corticosteroid use, and renal insufficiency, all of which are pertinent to this patient on **prednisone** for lupus.
*Facial redness/flushing*
- This is an adverse effect more commonly associated with drugs like **niacin** or calcium channel blockers, not fluoroquinolones.
- It is generally not a recognized side effect of antibiotics used to treat UTIs.
*Rhabdomyolysis*
- This serious condition involves the breakdown of muscle tissue and is associated with various drugs (e.g., statins, street drugs) and conditions (e.g., trauma, extreme exertion), but not typically fluoroquinolones.
- While muscle pain can occur with fluoroquinolones, severe rhabdomyolysis is rare.
*Hemolytic anemia*
- Certain antibiotics, like sulfonamides or penicillin, can rarely cause drug-induced hemolytic anemia, particularly in patients with **G6PD deficiency**.
- Fluoroquinolones are not commonly associated with hemolytic anemia.
*Leukopenia*
- While some antibiotics can cause bone marrow suppression leading to leukopenia (e.g., chloramphenicol, trimethoprim-sulfamethoxazole), this is not a common or significant adverse effect of fluoroquinolones.
- The patient's underlying lupus and prednisone use might contribute to immune dysregulation, but leukopenia is not the primary concern with fluoroquinolone use.
Vancomycin and other glycopeptides US Medical PG Question 7: You are treating a neonate with meningitis using ampicillin and a second antibiotic, X, that is known to cause ototoxicity. What is the mechanism of antibiotic X?
- A. It binds the 50S ribosomal subunit and inhibits formation of the initiation complex
- B. It binds the 30S ribosomal subunit and inhibits formation of the initiation complex (Correct Answer)
- C. It binds the 30S ribosomal subunit and reversibly inhibits translocation
- D. It binds the 50S ribosomal subunit and inhibits peptidyltransferase
- E. It binds the 50S ribosomal subunit and reversibly inhibits translocation
Vancomycin and other glycopeptides Explanation: ***It binds the 30s ribosomal subunit and inhibits formation of the initiation complex***
- The second antibiotic, X, is likely an **aminoglycoside**, such as **gentamicin** or **amikacin**, which are commonly used in combination with ampicillin for neonatal meningitis and are known to cause ototoxicity.
- Aminoglycosides exert their bactericidal effect by **irreversibly binding to the 30S ribosomal subunit**, thereby **inhibiting the formation of the initiation complex** and leading to misreading of mRNA.
*It binds the 50S ribosomal subunit and inhibits formation of the initiation complex*
- This mechanism is characteristic of **linezolid**, which targets the 50S ribosomal subunit to prevent the formation of the initiation complex.
- While linezolid can cause side effects, **ototoxicity** is less commonly associated with it compared to aminoglycosides, and it is not a primary drug for neonatal meningitis alongside ampicillin.
*It binds the 50S ribosomal subunit and inhibits peptidyltransferase*
- This is the mechanism of action for **chloramphenicol**, which inhibits **peptidyltransferase** activity on the 50S ribosomal subunit, preventing peptide bond formation.
- Although chloramphenicol can cause **ototoxicity** and **aplastic anemia**, its use in neonates is limited due to the risk of **Gray Baby Syndrome**.
*It binds the 30s ribosomal subunit and reversibly inhibits translocation*
- This describes the mechanism of action of **tetracyclines**, which reversibly bind to the 30S ribosomal subunit and prevent the attachment of aminoacyl-tRNA, thereby inhibiting protein synthesis.
- Tetracyclines are **contraindicated in neonates** due to their potential to cause **tooth discoloration** and **bone growth inhibition**, and ototoxicity is not their primary adverse effect.
*It binds the 50s ribosomal subunit and reversibly inhibits translocation*
- This mechanism of reversibly inhibiting translocation by binding to the 50S ribosomal subunit is characteristic of **macrolides** (e.g., erythromycin, azithromycin) and **clindamycin**.
- While some macrolides can cause **transient ototoxicity**, they are not typically the second antibiotic of choice for neonatal meningitis in combination with ampicillin, and clindamycin's side effect profile is different.
Vancomycin and other glycopeptides US Medical PG Question 8: A 54-year-old man presents with fever, abdominal pain, nausea, and bloody diarrhea. He says that his symptoms started 36 hours ago and have not improved. Past medical history is significant for a left-leg abscess secondary to an injury he sustained from a fall 4 days ago while walking his dog. He has been taking clindamycin for this infection. In addition, he has long-standing gastroesophageal reflux disease, managed with omeprazole. His vital signs include: temperature 38.5°C (101.3°F), respiratory rate 19/min, heart rate 90/min, and blood pressure 110/70 mm Hg. Which of the following is the best course of treatment for this patient’s most likely diagnosis?
- A. Tetracycline
- B. Ciprofloxacin
- C. Trimethoprim-sulfamethoxazole
- D. Erythromycin
- E. Vancomycin (Correct Answer)
Vancomycin and other glycopeptides Explanation: ***Vancomycin***
- The patient's history of recent **clindamycin** use for an abscess, development of **fever, abdominal pain, nausea, and bloody diarrhea**, and use of **omeprazole** (a risk factor), strongly suggests **_Clostridioides difficile_ infection (CDI)**.
- **Oral vancomycin** is a first-line treatment for **severe non-fulminant CDI**, which this patient's symptoms (fever, bloody diarrhea) are consistent with.
*Tetracycline*
- **Tetracycline** is typically used for bacterial infections like **chlamydia, Lyme disease, and rickettsial infections**; it is not effective against _C. difficile_.
- It works by **inhibiting bacterial protein synthesis** but does not target the cell wall of _C. difficile_.
*Ciprofloxacin*
- **Ciprofloxacin**, a fluoroquinolone, is generally **contraindicated in CDI** as it can be a risk factor for developing the infection or exacerbate it due to disruption of gut flora.
- While effective against many gram-negative bacteria, it has **no significant activity against _C. difficile_**.
*Trimethoprim-sulfamethoxazole*
- **Trimethoprim-sulfamethoxazole** is a combination antibiotic used for various bacterial infections, including **UTIs and some respiratory infections**.
- It is **not effective against _C. difficile_** and is not recommended for its treatment.
*Erythromycin*
- **Erythromycin**, a macrolide, is effective against a range of bacterial infections including **atypical pneumonia and skin infections**.
- It has **no role in the treatment of _C. difficile_ infection** and its use could potentially further disrupt the gut microbiome.
Vancomycin and other glycopeptides US Medical PG Question 9: A 49-year-old man presents to the emergency department with acute onset of pain and redness of the skin of his lower leg for the past 3 days. He has had type 2 diabetes mellitus for the past 12 years, but he is not compliant with his medications. He has smoked 10–15 cigarettes per day for the past 20 years. His temperature is 38°C (100.4°F), pulse is 95/min, and blood pressure is 110/70 mm Hg. On physical examination, the pretibial area is erythematous, edematous, and tender. He is diagnosed with acute cellulitis, and intravenous ceftazidime sodium is started. On the 5th day of antibiotic therapy, the patient complains of severe watery diarrhea, fever, and abdominal tenderness without rigidity. Complete blood count is ordered for the patient and shows 14,000 white blood cells/mm3. Which of the following is the best initial therapy for this patient?
- A. Intravenous vancomycin
- B. Oral ciprofloxacin
- C. Fecal microbiota transplantation
- D. Oral vancomycin (Correct Answer)
- E. Oral metronidazole
Vancomycin and other glycopeptides Explanation: ***Oral vancomycin***
- The patient exhibits classic symptoms of **Clostridioides difficile infection (CDI)**: watery diarrhea, fever, abdominal tenderness, and leukocytosis following antibiotic use (ceftazidime). Oral vancomycin is the **first-line therapy** for severe CDI.
- Oral vancomycin achieves high intraluminal concentrations, effectively targeting C. difficile in the colon with minimal systemic absorption.
*Intravenous vancomycin*
- Intravenous vancomycin has **poor penetration** into the gastrointestinal tract and is therefore ineffective for treating C. difficile infection.
- It is primarily used for systemic infections caused by **methicillin-resistant Staphylococcus aureus (MRSA)**.
*Oral ciprofloxacin*
- **Fluoroquinolones** like ciprofloxacin are associated with an increased risk of developing C. difficile infection due to their broad-spectrum activity.
- They are not effective treatments for C. difficile and can potentially worsen the condition or select for resistant strains.
*Fecal microbiota transplantation*
- **Fecal microbiota transplantation (FMT)** is a highly effective treatment for recurrent C. difficile infection, but it is typically reserved for patients who have failed multiple courses of standard antibiotic therapy.
- It is not considered the initial therapy for acute, uncomplicated C. difficile infection.
*Oral metronidazole*
- **Oral metronidazole** was historically used for C. difficile infection but is **no longer recommended** as first-line therapy per current **2021 IDSA/SHEA guidelines** due to inferior clinical outcomes compared to vancomycin or fidaxomicin.
- Given the patient's fever and leukocytosis indicating severe infection, vancomycin is the preferred initial treatment.
Vancomycin and other glycopeptides US Medical PG Question 10: A 65-year-old female patient with a past medical history of diabetes mellitus and an allergy to penicillin develops an infected abscess positive for MRSA on the third day of her hospital stay. She is started on an IV infusion of vancomycin at a dose of 1000 mg every 12 hours. Vancomycin is eliminated by first-order kinetics and has a half life of 6 hours. The volume of distribution of vancomycin is 0.5 L/kg. Assuming no loading dose is given, how long will it take for the drug to reach 94% of its plasma steady state concentration?
- A. 30 hours
- B. 12 hours
- C. 6 hours
- D. 18 hours
- E. 24 hours (Correct Answer)
Vancomycin and other glycopeptides Explanation: ***24 hours***
- For a drug eliminated by **first-order kinetics**, it takes approximately **4 half-lives** to reach **93.75%** of steady state concentration, which is conventionally rounded to **94%**.
- Since the half-life of vancomycin is **6 hours**, reaching 94% of steady state requires: 4 × 6 hours = **24 hours**.
- This follows the pharmacokinetic principle that each half-life brings the drug closer to steady state: 1 t½ = 50%, 2 t½ = 75%, 3 t½ = 87.5%, 4 t½ = 93.75%.
*30 hours*
- This duration represents **five half-lives** (5 × 6 hours), at which point approximately **96.875%** (often rounded to 97%) of steady state would be reached.
- This exceeds the 94% target specified in the question.
*18 hours*
- This duration represents **three half-lives** (3 × 6 hours), at which point approximately **87.5%** of steady state concentration would be reached.
- This falls short of the 94% target.
*12 hours*
- This duration represents **two half-lives** (2 × 6 hours), at which point approximately **75%** of steady state concentration would be reached.
- This is insufficient time to reach 94% of plasma steady state concentration.
*6 hours*
- This duration represents **one half-life**, at which point approximately **50%** of steady state concentration would be reached.
- This is far too short to achieve near-steady state levels.
More Vancomycin and other glycopeptides US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.