Antibiotic resistance mechanisms US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Antibiotic resistance mechanisms. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Antibiotic resistance mechanisms US Medical PG Question 1: If the genetic material were isolated and injected into the cytoplasm of a human cell, which of the following would produce viable, infectious virions?
- A. Lassa fever virus
- B. Rabies virus
- C. Rhinovirus (Correct Answer)
- D. Mumps virus
- E. Influenza virus
Antibiotic resistance mechanisms Explanation: ***Rhinovirus***
- **Rhinovirus** is a **positive-sense single-stranded RNA virus**. Its genetic material can directly serve as mRNA in the host cell cytoplasm, leading to immediate protein synthesis and viral replication without needing DNA intermediates or a nuclear phase.
- This direct translation allows for the production of viable, infectious virions upon cytoplasmic injection of the genetic material.
*Lassa fever virus*
- **Lassa fever virus** is an **ambisense RNA virus** and requires an RNA-dependent RNA polymerase (RdRp) to transcribe its genome into mRNA.
- This RdRp is packaged within the virion, meaning the injected genetic material alone is not sufficient to initiate replication without the viral proteins.
*Rabies virus*
- **Rabies virus** is a **negative-sense single-stranded RNA virus**. Its genome cannot directly act as mRNA.
- It requires a virion-associated **RNA-dependent RNA polymerase (RdRp)** to transcribe its negative-sense RNA into positive-sense mRNA, which is essential for protein synthesis.
*Mumps virus*
- **Mumps virus** is a **negative-sense single-stranded RNA virus** and, like rabies virus, cannot directly translate its genome into proteins.
- It also requires its own **virion-associated RNA-dependent RNA polymerase** to synthesize mRNA from its negative-sense genome.
*Influenza virus*
- **Influenza virus** is a **negative-sense segmented RNA virus**. Its replication cycle involves the nucleus, where its RNA genome is transcribed into mRNA.
- This process requires the viral **RNA-dependent RNA polymerase**, which is brought into the cell by the virion, and interaction with host nuclear machinery.
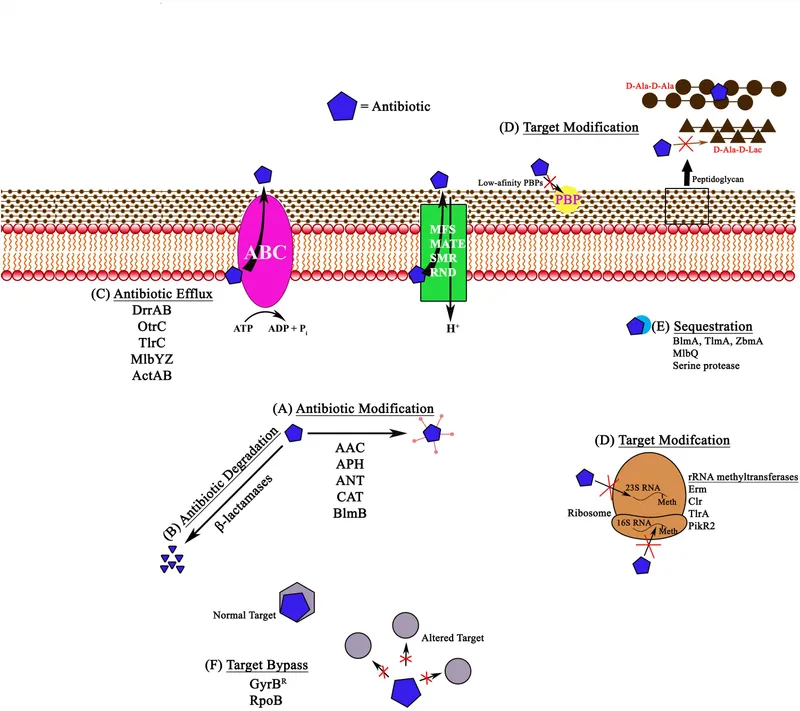
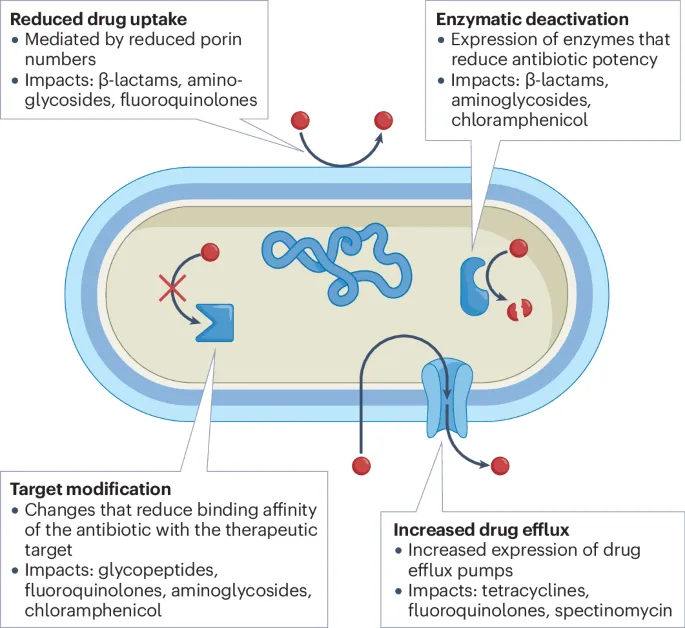
Antibiotic resistance mechanisms US Medical PG Question 2: A team of intensivists working in a private intensive care unit (ICU) observe that the clinical efficacy of vancomycin is low, and proven nosocomial infections have increased progressively over the past year. A clinical microbiologist is invited to conduct a bacteriological audit of the ICU. He analyzes the microbiological reports of all patients treated with vancomycin over the last 2 years and takes relevant samples from the ICU for culture and antibiotic sensitivity analysis. The audit concludes that there is an increased incidence of vancomycin-resistant Enterococcus fecalis infections. Which of the following mechanisms best explains the changes that took place in the bacteria?
- A. Decreased number of porins in the bacterial cell wall leading to decreased intracellular entry of the antibiotic
- B. Production of an enzyme that hydrolyzes the antibiotic
- C. Protection of the antibiotic-binding site by Qnr protein
- D. Increased expression of efflux pumps which extrude the antibiotic from the bacterial cell
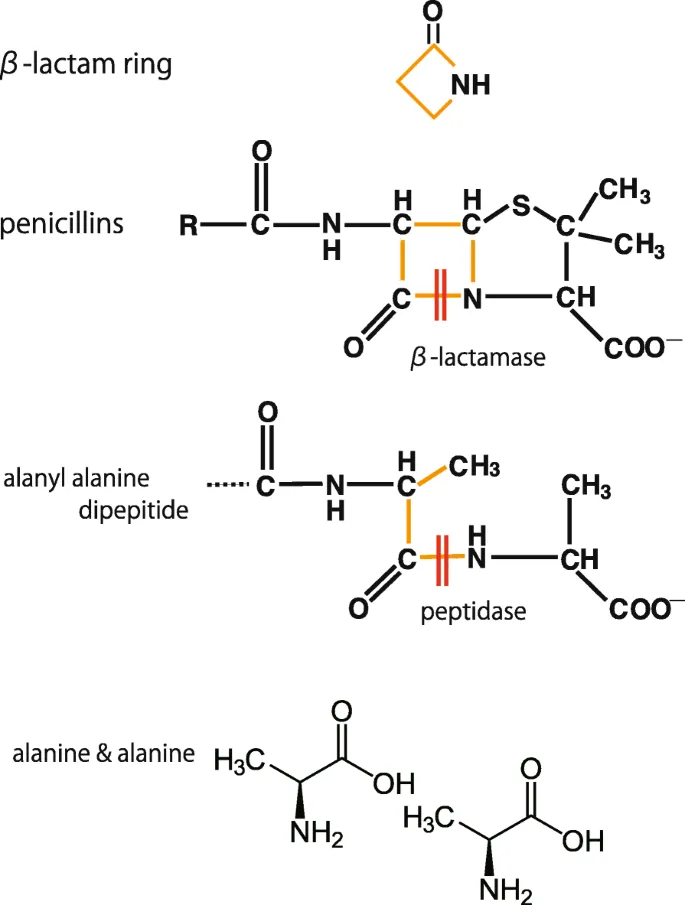
- E. Replacement of the terminal D-Ala in the cell wall peptidoglycan by D-lactate (Correct Answer)
Antibiotic resistance mechanisms Explanation: ***Replacement of the terminal D-ala in the cell wall peptidoglycan by D-lactate***
- **Vancomycin** exerts its antibacterial effect by binding to the **D-Ala-D-Ala** terminus of the peptidoglycan precursor in the bacterial cell wall, preventing its incorporation.
- In **vancomycin-resistant Enterococcus (VRE)**, the D-Ala-D-Ala is replaced by **D-Ala-D-Lac**, which significantly reduces vancomycin's binding affinity, leading to resistance.
*Decreased number of porins in the bacterial cell wall leading to decreased intracellular entry of the antibiotic*
- This mechanism primarily affects **Gram-negative bacteria**, where porins are crucial for antibiotic entry through the outer membrane.
- **Enterococcus faecalis** is a **Gram-positive bacterium** and does not rely on porins in the same way for vancomycin uptake.
*Production of an enzyme that hydrolyzes the antibiotic*
- This mechanism is characteristic of resistance to **beta-lactam antibiotics** (e.g., penicillinases, cephalosporinases).
- Vancomycin is not a beta-lactam, and its resistance mechanism in Enterococcus does not typically involve enzymatic hydrolysis.
*Protection of the antibiotic-binding site by Qnr protein*
- **Qnr proteins** are associated with **quinolone resistance**, specifically by protecting DNA gyrase and topoisomerase IV from quinolone inhibition.
- This mechanism is irrelevant to vancomycin, which targets the bacterial cell wall.
*Increased expression of efflux pumps which extrude the antibiotic from the bacterial cell*
- Efflux pumps are a common mechanism of antibiotic resistance against a wide range of antibiotics, including **tetracyclines, macrolides, and fluoroquinolones**.
- While efflux pumps can contribute to some forms of resistance, they are not the primary or best-explained mechanism for **high-level vancomycin resistance in Enterococcus**.
Antibiotic resistance mechanisms US Medical PG Question 3: An 18-year old college freshman presents to his university clinic because he has not been feeling well for the past two weeks. He has had a persistent headache, occasional cough, and chills without rigors. The patient’s vital signs are normal and physical exam is unremarkable. His radiograph shows patchy interstitial lung infiltrates and he is diagnosed with atypical pneumonia. The patient is prescribed azithromycin and takes his medication as instructed. Despite adherence to his drug regimen, he returns to the clinic one week later because his symptoms have not improved. The organism responsible for this infection is likely resistant to azithromycin through which mechanism?
- A. Mutation in topoisomerase II
- B. Methylation of ribosomal binding site
- C. Presence of a beta-lactamase
- D. Decreased binding to RNA polymerase
- E. Insertion of drug efflux pumps (Correct Answer)
Antibiotic resistance mechanisms Explanation: ***Insertion of drug efflux pumps***
- **Azithromycin** is a macrolide antibiotic that inhibits bacterial protein synthesis by binding to the **50S ribosomal subunit**.
- In **Mycoplasma pneumoniae** (the most common cause of atypical pneumonia in young adults), the **most common** mechanism of macrolide resistance is through **efflux pumps**, particularly the **mef genes**.
- These efflux pumps actively transport macrolides out of the bacterial cell, reducing intracellular drug concentration and conferring resistance.
- This mechanism is responsible for the majority of macrolide-resistant *M. pneumoniae* isolates worldwide.
*Methylation of ribosomal binding site*
- **Methylation** of the ribosomal binding site (specifically the **23S rRNA** via erm genes) does prevent azithromycin from binding effectively.
- While this is a valid macrolide resistance mechanism seen in organisms like *Streptococcus pneumoniae* and *Streptococcus pyogenes*, it is **less common** in *Mycoplasma pneumoniae*.
- Efflux pumps (mef) are the predominant mechanism in *M. pneumoniae* resistant strains.
*Mutation in topoisomerase II*
- **Topoisomerase II** (DNA gyrase) is the target of **fluoroquinolone antibiotics**, not macrolides.
- Mutations in this enzyme lead to resistance against fluoroquinolones, such as **ciprofloxacin**.
*Presence of a beta-lactamase*
- **Beta-lactamase enzymes** inactivate **beta-lactam antibiotics** (e.g., penicillin, cephalosporins) by hydrolyzing their beta-lactam ring.
- Additionally, *Mycoplasma pneumoniae* **lacks a cell wall**, making it inherently resistant to all beta-lactam antibiotics regardless of beta-lactamase production.
*Decreased binding to RNA polymerase*
- **RNA polymerase** is the target for antibiotics like **rifampin**, which inhibits bacterial transcription.
- Decreased binding to RNA polymerase would lead to rifampin resistance, not azithromycin resistance.
Antibiotic resistance mechanisms US Medical PG Question 4: A 77-year-old woman is brought to the emergency department from her nursing home because she was found down overnight. On presentation she was found to be delirious and was unable to answer questions. Chart review shows that she is allergic to cephalosporins. Her temperature is 102.2°F (39°C), blood pressure is 105/52 mmHg, pulse is 94/min, and respirations are 23/min. Physical exam reveals a productive cough. A metabolic panel is obtained with the following results:
Serum:
Na+: 135 mEq/L
Cl-: 95 mEq/L
K+: 4 mEq/L
HCO3-: 19 mEq/L
BUN: 40 mg/dL
Creatinine: 2.5 mg/dL
Glucose: 150 mg/dL
Based on these findings two different drugs are started empirically. Gram stain on a blood sample is performed showing the presence of gram-positive organisms on all samples. One of the drugs is subsequently stopped. The drug that was most likely stopped has which of the following characteristics?
- A. Resistance conveyed through acetylation
- B. Associated with red man syndrome
- C. Single-ringed ß-lactam structure (Correct Answer)
- D. Causes discolored teeth in children
- E. Accumulates inside bacteria via O2-dependent uptake
Antibiotic resistance mechanisms Explanation: ***Single-ringed ß-lactam structure***
- The patient presents with **sepsis** due to **pneumonia** likely caused by **gram-positive organisms**. Given a cephalosporin allergy, **aztreonam** (a monobactam) would be an initial empirical antibiotic choice to cover gram-negative bacteria, alongside a drug for gram-positive coverage (like vancomycin).
- Since the **blood cultures** confirmed **gram-positive organisms**, the drug covering gram-negative bacteria (aztreonam) would be stopped. Aztreonam is characterized by its **single-ringed β-lactam structure**.
*Resistance conveyed through acetylation*
- This mechanism of resistance is typical of **aminoglycosides** (e.g., gentamicin) and **chloramphenicol**.
- Aminoglycosides were unlikely to be one of the empirically started drugs, as they are often used in combination with β-lactams, and this patient has a cephalosporin allergy.
*Associated with red man syndrome*
- **Red man syndrome** is a common adverse effect associated with **vancomycin** administration, especially with rapid infusion.
- Vancomycin would likely be continued, as it effectively targets gram-positive organisms, including **MRSA**, and is a suitable alternative given the cephalosporin allergy.
*Causes discolored teeth in children*
- This is a characteristic side effect of **tetracyclines** (e.g., doxycycline), which are contraindicated in young children and pregnant women due to their effects on bone and teeth development.
- Tetracyclines are not typically first-line empiric therapy for severe pneumonia or sepsis, especially in an elderly patient.
*Accumulates inside bacteria via O2-dependent uptake*
- This describes the mechanism of uptake for **aminoglycosides**. Their entry into bacteria is an **energy-dependent process** requiring oxygen.
- As mentioned, aminoglycosides are less likely to be the initial drug stopped in this scenario, as they target gram-negative bacteria.
Antibiotic resistance mechanisms US Medical PG Question 5: A 64-year-old female with type 2 diabetes mellitus comes to the physician because of a 1-week history of painful red swelling on her left thigh. Examination shows a 3- x 4-cm, tender, fluctuant mass. Incision and drainage of the abscess are performed. Culture of the abscess fluid grows gram-positive, coagulase-positive cocci that are resistant to oxacillin. Which of the following best describes the mechanism of resistance of the causal organism to oxacillin?
- A. Degradation of the antibiotic
- B. Decreased uptake of the antibiotic
- C. Decreased activation of the antibiotic
- D. Altered target of the antibiotic (Correct Answer)
- E. Acetylation of the antibiotic
Antibiotic resistance mechanisms Explanation: ***Altered target of the antibiotic***
- The organism described (gram-positive, coagulase-positive cocci, oxacillin-resistant) is **methicillin-resistant *Staphylococcus aureus* (MRSA)**.
- MRSA achieves oxacillin (and other beta-lactam) resistance by acquiring the ***mecA* gene**, which encodes for a **modified penicillin-binding protein (PBP2a)** with reduced affinity for beta-lactam antibiotics.
*Degradation of the antibiotic*
- This mechanism, primarily through the production of **beta-lactamase enzymes**, can degrade beta-lactam antibiotics.
- While *Staphylococcus aureus* can produce beta-lactamases, oxacillin (a **penicillinase-resistant penicillin**) is specifically engineered to be stable against these enzymes.
*Decreased uptake of the antibiotic*
- Reduced permeability of the bacterial cell wall can lead to decreased uptake, a mechanism more commonly associated with **gram-negative bacteria** due to their outer membrane.
- This is not the primary mechanism of resistance for MRSA to oxacillin.
*Decreased activation of the antibiotic*
- Some antibiotics are prodrugs that require activation by bacterial enzymes, and resistance can arise from mutations affecting this activation.
- Oxacillin is active in its administered form and does not require bacterial activation.
*Acetylation of the antibiotic*
- **Enzymatic modification**, such as acetylation, adenylylation, or phosphorylation, is a common mechanism of resistance, particularly against **aminoglycoside antibiotics**.
- This specific mechanism is not responsible for oxacillin resistance in MRSA.
Antibiotic resistance mechanisms US Medical PG Question 6: A 7-year-old boy is brought to the clinic by his parents due to right ear pain. For the past few days, the patient's parents say he has had a low-grade fever, a runny nose, and has been frequently pulling on his right ear. Past medical history is significant for a similar episode one month ago for which he has prescribed a 10-day course of amoxicillin. He is up-to-date on all vaccinations and is doing well at school. His temperature is 38.5°C (101.3°F), blood pressure is 106/75 mm Hg, pulse is 101/min, and respiratory rate is 20/min. Findings on otoscopic examination are shown in the image. The patient is treated with amoxicillin with clavulanic acid. Which of the following best describes the benefit of adding clavulanic acid to amoxicillin?
- A. Tachyphylactic effect
- B. Permissive effect
- C. Additive effect
- D. Inhibitor effect
- E. Synergistic effect (Correct Answer)
Antibiotic resistance mechanisms Explanation: ***Synergistic effect***
- **Clavulanic acid** is a **beta-lactamase inhibitor** that augments the efficacy of **amoxicillin**, a beta-lactam antibiotic, against resistant bacteria.
- This combination results in a therapeutic effect that is greater than the sum of their individual effects, meaning clavulanic acid **protects amoxicillin from degradation** by bacterial beta-lactamases, allowing amoxicillin to exert its antimicrobial action effectively.
*Tachyphylactic effect*
- Refers to a **rapidly diminishing response** to successive doses of a drug, making it less effective over time due to receptor desensitization or depletion of mediators.
- It does not describe the interaction between amoxicillin and clavulanic acid, where the latter enhances the former's activity.
*Permissive effect*
- Describes a situation where one hormone or drug **allows another to exert its full effect**, often by upregulating receptors or acting as a co-factor, without directly having the primary effect itself.
- Clavulanic acid's role is more direct; it actively inhibits bacterial enzymes, protecting amoxicillin, rather than simply "permitting" its action.
*Additive effect*
- Occurs when the combined effect of two drugs is **equal to the sum of their individual effects**.
- In this case, clavulanic acid does not directly contribute to the antibacterial killing but rather enhances amoxicillin's ability to kill, making the combined effect **greater than additive**.
*Inhibitor effect*
- While clavulanic acid *is* an **inhibitor** (of beta-lactamase), saying it has an "inhibitor effect" on amoxicillin would be incorrect.
- The "inhibitor effect" refers to its action on bacterial enzymes, which then leads to a synergistic outcome with amoxicillin.
Antibiotic resistance mechanisms US Medical PG Question 7: You are treating a neonate with meningitis using ampicillin and a second antibiotic, X, that is known to cause ototoxicity. What is the mechanism of antibiotic X?
- A. It binds the 50S ribosomal subunit and inhibits formation of the initiation complex
- B. It binds the 30S ribosomal subunit and inhibits formation of the initiation complex (Correct Answer)
- C. It binds the 30S ribosomal subunit and reversibly inhibits translocation
- D. It binds the 50S ribosomal subunit and inhibits peptidyltransferase
- E. It binds the 50S ribosomal subunit and reversibly inhibits translocation
Antibiotic resistance mechanisms Explanation: ***It binds the 30s ribosomal subunit and inhibits formation of the initiation complex***
- The second antibiotic, X, is likely an **aminoglycoside**, such as **gentamicin** or **amikacin**, which are commonly used in combination with ampicillin for neonatal meningitis and are known to cause ototoxicity.
- Aminoglycosides exert their bactericidal effect by **irreversibly binding to the 30S ribosomal subunit**, thereby **inhibiting the formation of the initiation complex** and leading to misreading of mRNA.
*It binds the 50S ribosomal subunit and inhibits formation of the initiation complex*
- This mechanism is characteristic of **linezolid**, which targets the 50S ribosomal subunit to prevent the formation of the initiation complex.
- While linezolid can cause side effects, **ototoxicity** is less commonly associated with it compared to aminoglycosides, and it is not a primary drug for neonatal meningitis alongside ampicillin.
*It binds the 50S ribosomal subunit and inhibits peptidyltransferase*
- This is the mechanism of action for **chloramphenicol**, which inhibits **peptidyltransferase** activity on the 50S ribosomal subunit, preventing peptide bond formation.
- Although chloramphenicol can cause **ototoxicity** and **aplastic anemia**, its use in neonates is limited due to the risk of **Gray Baby Syndrome**.
*It binds the 30s ribosomal subunit and reversibly inhibits translocation*
- This describes the mechanism of action of **tetracyclines**, which reversibly bind to the 30S ribosomal subunit and prevent the attachment of aminoacyl-tRNA, thereby inhibiting protein synthesis.
- Tetracyclines are **contraindicated in neonates** due to their potential to cause **tooth discoloration** and **bone growth inhibition**, and ototoxicity is not their primary adverse effect.
*It binds the 50s ribosomal subunit and reversibly inhibits translocation*
- This mechanism of reversibly inhibiting translocation by binding to the 50S ribosomal subunit is characteristic of **macrolides** (e.g., erythromycin, azithromycin) and **clindamycin**.
- While some macrolides can cause **transient ototoxicity**, they are not typically the second antibiotic of choice for neonatal meningitis in combination with ampicillin, and clindamycin's side effect profile is different.
Antibiotic resistance mechanisms US Medical PG Question 8: A 61-year-old woman who recently emigrated from India comes to the physician because of a 2-month history of fever, fatigue, night sweats, and a productive cough. She has had a 5-kg (11-lb) weight loss during this period. She has a history of type 2 diabetes mellitus and poorly controlled asthma. She has had multiple asthma exacerbations in the past year that were treated with glucocorticoids. An x-ray of the chest shows a cavitary lesion of the posterior apical segment of the left upper lobe with consolidation of the surrounding parenchyma. The pathogen identified on sputum culture is found to be resistant to multiple drugs, including streptomycin. Which of the following mechanisms is most likely involved in bacterial resistance to this drug?
- A. Alteration in the sequence of gyrA genes
- B. Upregulation of arabinosyl transferase production
- C. Upregulation of mycolic acid synthesis
- D. Alteration in 30S ribosomal subunit (Correct Answer)
- E. Inhibition of bacterial synthesis of RNA
Antibiotic resistance mechanisms Explanation: ***Alteration in 30S ribosomal subunit***
- Streptomycin is an **aminoglycoside antibiotic** that acts by binding to the **16S rRNA of the 30S ribosomal subunit**, which interferes with bacterial protein synthesis.
- **Resistance to streptomycin** most commonly arises from mutations in the genes encoding ribosomal proteins (e.g., *rpsL*) or the 16S rRNA that alter the drug's binding site on the 30S ribosomal subunit, preventing its inhibitory effect.
*Alteration in the sequence of gyrA genes*
- Mutations in the *gyrA* gene typically confer resistance to **fluoroquinolone antibiotics**, such as ciprofloxacin and levofloxacin.
- Fluoroquinolones target **DNA gyrase (topoisomerase II)**, which is encoded by *gyrA*, not the ribosomes.
*Upregulation of arabinosyl transferase production*
- **Arabinogalactan**, a major component of the mycobacterial cell wall, is synthesized by **arabinosyl transferases** (e.g., EmbB).
- Resistance to **ethambutol** is often associated with mutations or upregulation of these enzymes, leading to increased synthesis of the arabinogalactan layer.
*Upregulation of mycolic acid synthesis*
- **Mycolic acid** is a crucial component of the mycobacterial cell wall, and its synthesis is inhibited by drugs like **isoniazid**.
- Upregulation of mycolic acid synthesis or mutations in genes related to its production (e.g., *kasA*) can lead to **isoniazid resistance**, but not directly to streptomycin resistance.
*Inhibition of bacterial synthesis of RNA*
- **Rifampin** is an antibiotic that inhibits bacterial RNA synthesis by binding to the **DNA-dependent RNA polymerase**.
- While resistance to rifampin often involves mutations in the *rpoB* gene, this mechanism is specific to rifampin and not streptomycin.
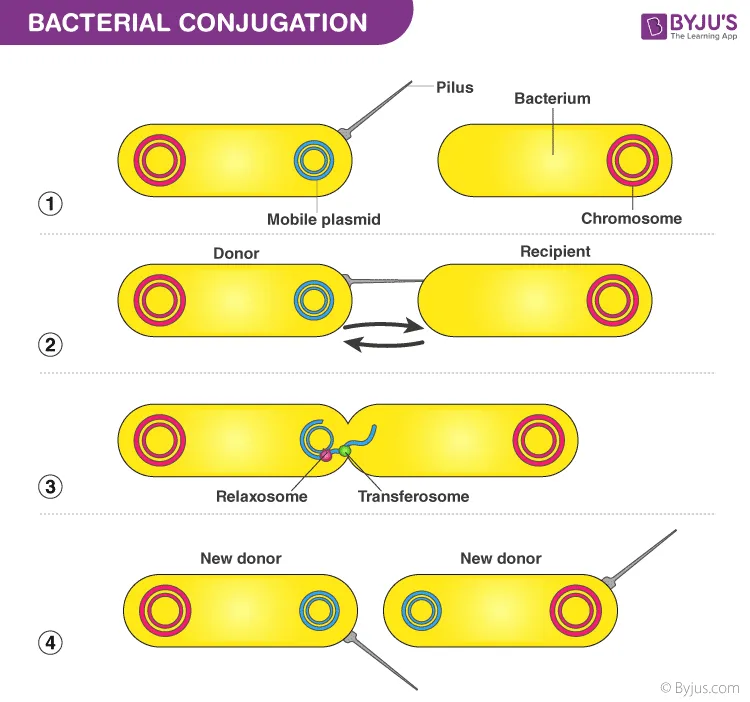
Antibiotic resistance mechanisms US Medical PG Question 9: An investigator studying mechanisms of acquired antibiotic resistance in bacteria conducts a study using isolated strains of Escherichia coli and Staphylococcus aureus. The E. coli strain harbors plasmid pRK212.1, which conveys resistance to kanamycin. The S. aureus strain is susceptible to kanamycin. Both bacterial strains are mixed in a liquid growth medium containing deoxyribonuclease. After incubation for 2 days and subsequent transfer to a solid medium, the S. aureus colonies show no lysis in response to the application of kanamycin. Analysis of chromosomal DNA from the kanamycin-resistant S. aureus strain does not reveal the kanamycin-resistance gene. Which of the following mechanisms is most likely responsible for this finding?
- A. Transformation
- B. Conjugation (Correct Answer)
- C. Transduction
- D. Transposition
- E. Secretion
Antibiotic resistance mechanisms Explanation: ***Conjugation***
- The presence of **deoxyribonuclease (DNase)** in the growth medium inhibits **transformation**, ruling out the uptake of naked DNA. The transfer of the kanamycin resistance gene from a plasmid in *E. coli* to *S. aureus* in the presence of DNase strongly points to **cell-to-cell contact** via conjugation.
- The resistance gene is found on a **plasmid** in *E. coli* and is transferred to *S. aureus*, resulting in kanamycin resistance without integrating into the *S. aureus* chromosome, which is characteristic of conjugative plasmid transfer.
- **Key experimental clue**: DNase destroys free DNA in the medium, so the only way for genetic material to transfer is through **direct cell-to-cell contact**, which is the hallmark of conjugation.
*Transformation*
- This process involves the uptake of **naked DNA** from the environment by a bacterial cell, which would have been prevented by the presence of **deoxyribonuclease** in the medium.
- Transformation typically results in the integration of the foreign DNA into the host cell's **chromosome** or stable maintenance as a plasmid, but DNase would degrade any free DNA before uptake could occur.
*Transduction*
- **Transduction** involves the transfer of genetic material via a **bacteriophage**. The scenario does not describe the presence of any phage particles, nor is there mention of viral vectors.
- The resistance gene originates from a **plasmid** in *E. coli*, and transduction would require a phage capable of infecting both species, which is not mentioned in the experimental design.
*Transposition*
- **Transposition** is the movement of a segment of DNA from one location to another within the **same cell** (e.g., between a plasmid and chromosome). It does not explain the transfer of genetic material **between** two different bacterial cells.
- While a **transposon** might carry the kanamycin resistance gene on the plasmid, transposition itself is not the mechanism for **inter-species transfer** observed in this experiment.
*Secretion*
- **Secretion** refers to the active release of molecules (proteins, enzymes, toxins) from a cell. It is not a mechanism for the direct transfer of **genetic material** (like a plasmid or gene) from one bacterium to another.
- Genetic material is transferred through conjugation, transformation, or transduction, not by secretion pathways.
Antibiotic resistance mechanisms US Medical PG Question 10: A 15-year-old boy presents with his father to the urgent care with 5 days of frequent diarrhea, occasionally with streaks of blood mixed in. Stool cultures are pending, but preliminary stool samples demonstrate fecal leukocytes and erythrocytes. His vital signs are as follows: blood pressure is 126/83 mm Hg, heart rate is 97/min, and respiratory rate is 15/min. He is started on outpatient therapy for presumed Shigella infection. Which of the following is the most appropriate therapy?
- A. Oral doxycycline
- B. Oral vancomycin
- C. Oral TMP-SMX
- D. Oral azithromycin (Correct Answer)
- E. Oral ciprofloxacin
Antibiotic resistance mechanisms Explanation: ***Oral azithromycin***
- **Azithromycin** is the **first-line empiric treatment** for suspected **Shigella infection** based on current CDC and WHO guidelines, particularly in pediatric and adolescent patients.
- The presence of **fecal leukocytes and erythrocytes** indicates an invasive bacterial infection, which warrants antibiotic therapy to shorten the course of illness and reduce transmission risks.
- Azithromycin has excellent efficacy against Shigella with relatively low resistance rates compared to older agents, and it is well-tolerated in adolescents.
*Oral TMP-SMX*
- **TMP-SMX (trimethoprim-sulfamethoxazole)** was historically first-line for Shigella, but **widespread resistance** (often >50% globally) has made it no longer recommended for empiric therapy.
- It may still be used if culture and susceptibility testing confirm sensitivity, but should not be chosen empirically.
*Oral ciprofloxacin*
- **Ciprofloxacin**, a fluoroquinolone, is highly effective against **Shigella** and is first-line in adults.
- However, its use in **pediatric patients under 18 years** is generally limited due to potential adverse effects on **cartilage development** and risk of tendinopathy.
- In a 15-year-old, while approaching adult age, azithromycin remains preferred unless there are specific contraindications.
*Oral doxycycline*
- **Doxycycline** has limited activity against **Shigella** and is not considered appropriate empiric therapy for this infection.
- It is more commonly used for atypical pathogens, certain sexually transmitted infections, or specific tick-borne diseases.
*Oral vancomycin*
- **Oral vancomycin** is primarily used to treat **Clostridioides difficile infection** (CDI) and is completely ineffective against **Shigella**.
- Vancomycin acts only on gram-positive bacteria and does not penetrate the systemic circulation when given orally, making it unsuitable for gram-negative enteric infections.
More Antibiotic resistance mechanisms US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.