Proteasome inhibitors US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Proteasome inhibitors. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Proteasome inhibitors US Medical PG Question 1: A research team develops a new monoclonal antibody checkpoint inhibitor for advanced melanoma that has shown promise in animal studies as well as high efficacy and low toxicity in early phase human clinical trials. The research team would now like to compare this drug to existing standard of care immunotherapy for advanced melanoma. The research team decides to conduct a non-randomized study where the novel drug will be offered to patients who are deemed to be at risk for toxicity with the current standard of care immunotherapy, while patients without such risk factors will receive the standard treatment. Which of the following best describes the level of evidence that this study can offer?
- A. Level 1
- B. Level 3 (Correct Answer)
- C. Level 5
- D. Level 4
- E. Level 2
Proteasome inhibitors Explanation: ***Level 3***
- A **non-randomized controlled trial** like the one described, where patient assignment to treatment groups is based on specific characteristics (risk of toxicity), falls into Level 3 evidence.
- This level typically includes **non-randomized controlled trials** and **well-designed cohort studies** with comparison groups, which are prone to selection bias and confounding.
- The study compares two treatments but lacks randomization, making it Level 3 evidence.
*Level 1*
- Level 1 evidence is the **highest level of evidence**, derived from **systematic reviews and meta-analyses** of multiple well-designed randomized controlled trials or large, high-quality randomized controlled trials.
- The described study is explicitly stated as non-randomized, ruling out Level 1.
*Level 2*
- Level 2 evidence involves at least one **well-designed randomized controlled trial** (RCT) or **systematic reviews** of randomized trials.
- The current study is *non-randomized*, which means it cannot be classified as Level 2 evidence, as randomization is a key criterion for this level.
*Level 4*
- Level 4 evidence includes **case series**, **case-control studies**, and **poorly designed cohort or case-control studies**.
- While the study is non-randomized, it is a controlled comparative trial rather than a case series or retrospective case-control study, placing it at Level 3.
*Level 5*
- Level 5 evidence is the **lowest level of evidence**, typically consisting of **expert opinion** without explicit critical appraisal, or based on physiology, bench research, or animal studies.
- While the drug was initially tested in animal studies, the current human comparative study offers a higher level of evidence than expert opinion or preclinical data.
Proteasome inhibitors US Medical PG Question 2: A 62-year-old retired professor comes to the clinic with the complaints of back pain and increasing fatigue over the last 4 months. For the past week, his back pain seems to have worsened. It radiates to his legs and is burning in nature, 6/10 in intensity. There is no associated tingling sensation. He has lost 4.0 kg (8.8 lb) in the past 2 months. There is no history of trauma. He has hypertension which is well controlled with medications. Physical examination is normal. Laboratory studies show normocytic normochromic anemia. Serum calcium is 12.2 mg/dL and Serum total proteins is 8.8 gm/dL. A serum protein electrophoresis shows a monoclonal spike. X-ray of the spine shows osteolytic lesions over L2–L5 and right femur. A bone marrow biopsy reveals plasmacytosis. Which of the following is the most preferred treatment option?
- A. Renal dialysis
- B. Palliative care
- C. Chemotherapy and autologous stem cell transplant (Correct Answer)
- D. Bisphosphonates
- E. Chemotherapy alone
Proteasome inhibitors Explanation: ***Chemotherapy and autologous stem cell transplant***
- This patient presents with classic features of **multiple myeloma**, including bone pain with osteolytic lesions, hypercalcemia, normocytic anemia, elevated total protein with a monoclonal spike, and plasmacytosis in the bone marrow.
- In a relatively healthy patient with newly diagnosed multiple myeloma who is fit for intensive therapy (as suggested by the absence of significant comorbidities beyond controlled hypertension), **chemotherapy followed by autologous stem cell transplant (ASCT)** is the preferred treatment to achieve deeper and more durable remission.
*Renal dialysis*
- While **renal impairment** can occur in multiple myeloma due to myeloma kidney, it is not described in this patient, and **dialysis** is a supportive measure for end-stage kidney disease, not the primary treatment for the underlying malignancy.
- The patient's symptoms are primarily related to bone involvement and systemic effects of myeloma, not severe renal failure.
*Palliative care*
- **Palliative care** focuses on symptom relief and quality of life, which is essential at any stage of a serious illness, but it is not the initial primary therapeutic intervention for a newly diagnosed, symptomatic, and treatable cancer like multiple myeloma in a patient who could benefit from curative or remission-inducing therapy.
- The goal at this stage is disease control and prolonging survival.
*Bisphosphonates*
- **Bisphosphonates** (e.g., zoledronic acid) are an important adjunctive therapy in multiple myeloma to manage and prevent **skeletal-related events** by inhibiting osteoclast activity, but they do not treat the underlying plasma cell malignancy itself.
- They would be used in conjunction with chemotherapy, not as a standalone primary treatment.
*Chemotherapy alone*
- While **chemotherapy** (often a combination of proteasome inhibitors, immunomodulatory drugs, and dexamethasone) is central to treating multiple myeloma, **chemotherapy alone** without subsequent ASCT is typically reserved for patients who are not candidates for transplantation due to age, comorbidities, or frailty.
- For transplant-eligible patients, ASCT after induction chemotherapy significantly improves progression-free survival and overall survival compared to chemotherapy alone.
Proteasome inhibitors US Medical PG Question 3: A 65-year-old man comes to the physician because of a 1-month history of progressive back pain. He has also had a 5-kg (11-lb) weight loss over the past 3 months. His only medications are a daily multivitamin and ibuprofen, which he takes daily for the back pain. Physical examination shows tenderness to palpation over the lower spine and the left iliac crest. His hemoglobin concentration is 9.3 g/dL, his serum calcium concentration is 12 mg/dL, and his serum creatinine concentration is 2.1 mg/dL. A bone marrow biopsy shows 21% plasma cells. A diagnosis of multiple myeloma is established. In preparation for an autologous hematopoietic stem cell transplantation, the patient receives a myeloablative treatment regimen that includes busulfan. Which of the following drugs acts via a similar mechanism of action to busulfan?
- A. Etoposide
- B. Vemurafenib
- C. Vincristine
- D. Cytarabine
- E. Lomustine (Correct Answer)
Proteasome inhibitors Explanation: ***Lomustine***
- Both **busulfan** and **lomustine** are **alkylating agents**. They act by transferring **alkyl groups** to DNA, leading to cross-linking of DNA strands and inhibition of DNA synthesis and function.
- This **DNA damage** results in cell cycle arrest and apoptosis, particularly in rapidly dividing cells like cancer cells.
*Etoposide*
- **Etoposide** is a **topoisomerase II inhibitor** that prevents DNA relegation after strand breaks, leading to DNA damage and cell death.
- While it also targets DNA, its mechanism is distinct from the alkylation process of busulfan.
*Vemurafenib*
- **Vemurafenib** is a **BRAF kinase inhibitor** used in melanoma treatment. It specifically targets the **BRAF V600E mutation**.
- Its mechanism involves blocking signal transduction pathways critical for cell proliferation, rather than directly damaging DNA.
*Vincristine*
- **Vincristine** is a **vinca alkaloid** that acts as a **microtubule inhibitor**, preventing the formation of the **mitotic spindle** during cell division.
- This leads to metaphase arrest and apoptosis, a mechanism fundamentally different from DNA alkylation.
*Cytarabine*
- **Cytarabine** is an **antimetabolite**, specifically a **pyrimidine analog**, that inhibits **DNA polymerase**.
- It gets incorporated into DNA, leading to chain termination and inhibition of DNA synthesis and repair, making its action different from direct DNA alkylation.
Proteasome inhibitors US Medical PG Question 4: A 43-year-old man with HIV infection comes to the physician because of a 2-week history of progressive diarrhea and a 3-kg (6.6-lb) weight loss. During this period, he has had 3–4 episodes of watery stools daily, with multiple instances of blood in the stool. He is currently receiving antiretroviral therapy with zidovudine, lamivudine, and dolutegravir. Physical examination shows pallor and dry mucous membranes. A colonoscopy shows multiple linear ulcers. Polymerase chain reaction of a stool sample is positive for cytomegalovirus. Treatment with valganciclovir is begun. Adding this drug to his current medication regimen puts this patient at greatest risk for which of the following adverse effects?
- A. Hepatic steatosis
- B. Abnormal dreams
- C. Pancytopenia (Correct Answer)
- D. Orthostatic dysregulation
- E. Hyperglycemia
Proteasome inhibitors Explanation: ***Pancytopenia***
- **Valganciclovir** is a known cause of **bone marrow suppression**, leading to **pancytopenia** (low red blood cells, white blood cells, and platelets).
- The patient is also on **zidovudine**, an antiretroviral that can cause **myelosuppression**, thus the combined use significantly increases the risk of pancytopenia.
*Hepatic steatosis*
- **Hepatic steatosis** (fatty liver) is a rare but known adverse effect of some nucleoside reverse transcriptase inhibitors (NRTIs), particularly older ones.
- While lamivudine is an NRTI, **valganciclovir** is not primarily associated with hepatic steatosis, and the combination does not specifically heighten this risk more than other options.
*Abnormal dreams*
- **Abnormal dreams** are a common side effect associated with certain antiretroviral drugs, particularly the non-nucleoside reverse transcriptase inhibitor **efavirenz**.
- This patient is on dolutegravir (an integrase inhibitor), zidovudine, and lamivudine, none of which are primarily known for causing abnormal dreams as a prominent side effect, and valganciclovir does not contribute to this.
*Orthostatic dysregulation*
- **Orthostatic dysregulation** (orthostatic hypotension) can be a side effect of various medications, but it is not a prominent adverse effect of either **valganciclovir** or the patient's current antiretroviral regimen.
- While dehydration from diarrhea can cause it, the medication itself does not directly increase this risk in particular.
*Hyperglycemia*
- **Hyperglycemia** can be a side effect of certain antiretroviral drugs, particularly some **protease inhibitors** and older NRTIs.
- However, the patient's current regimen (zidovudine, lamivudine, dolutegravir) and **valganciclovir** are not strongly associated with hyperglycemia as a primary adverse effect compared to other options.
Proteasome inhibitors US Medical PG Question 5: A 62-year-old woman presents to her oncologist to discuss the chemotherapy options for her newly diagnosed breast cancer. During the meeting, they discuss a drug that inhibits the breakdown of mitotic spindles in cells. Her oncologist explains that this will be more toxic to cancer cells because those cells are dividing more rapidly. Which of the following side effects is closely associated with the use of this chemotherapeutic agent?
- A. Photosensitivity
- B. Peripheral neuropathy (Correct Answer)
- C. Paralytic ileus
- D. Hemorrhagic cystitis
- E. Pulmonary fibrosis
Proteasome inhibitors Explanation: ***Peripheral neuropathy***
- Drugs that inhibit the breakdown of **mitotic spindles** are **microtubule-targeting agents** (e.g., **taxanes** like paclitaxel/docetaxel, **vinca alkaloids** like vincristine/vinblastine).
- These agents interfere with **microtubule function** in neurons, leading to **axonal damage** and **peripheral neuropathy**.
- This is the **most characteristic and common dose-limiting toxicity** of microtubule inhibitors, affecting sensory and motor nerves (numbness, tingling, weakness in extremities).
*Photosensitivity*
- **Photosensitivity** is a common adverse effect associated with certain chemotherapeutic agents like **fluorouracil** (5-FU) or **methotrexate**, but is not linked to microtubule inhibitors.
- It involves an increased sensitivity to UV light, often manifesting as a rash or exaggerated sunburn.
*Paralytic ileus*
- **Paralytic ileus** can occur with **vinca alkaloids** (especially vincristine) due to autonomic neuropathy affecting the **enteric nervous system**.
- However, this is **less common** than peripheral neuropathy and occurs more specifically with vincristine rather than taxanes.
- **Peripheral neuropathy** is the more pervasive, dose-limiting, and universally characteristic side effect across all microtubule inhibitors.
*Hemorrhagic cystitis*
- **Hemorrhagic cystitis** is a classic side effect of **alkylating agents** like **cyclophosphamide** and **ifosfamide**, which produce the toxic metabolite **acrolein**.
- It is prevented/managed with **mesna**, which inactivates acrolein.
- Not associated with microtubule inhibitors.
*Pulmonary fibrosis*
- **Pulmonary fibrosis** is a known side effect of certain chemotherapeutic drugs, most notably **bleomycin** and **busulfan**.
- This adverse effect is not associated with agents that target **mitotic spindle breakdown**.
Proteasome inhibitors US Medical PG Question 6: A 47-year-old woman presents to the physician with complaints of fatigue accompanied by symmetric pain, swelling, and stiffness in her wrists, fingers, knees, and other joints. She describes the stiffness as being particularly severe upon awakening, but gradually improves as she moves throughout her day. Her physician initially suggests that she take NSAIDs. However, after a few months of minimal symptomatic improvement, she is prescribed an immunosuppressive drug that has a mechanism of preventing IL-2 transcription. What is the main toxicity that the patient must be aware of with this particular class of drugs?
- A. Pancytopenia
- B. Osteoporosis
- C. Hepatotoxicity
- D. Nephrotoxicity (Correct Answer)
- E. Hyperglycemia
Proteasome inhibitors Explanation: ***Nephrotoxicity***
- The drug described, which prevents **IL-2 transcription**, is likely a **calcineurin inhibitor** like cyclosporine or tacrolimus, often used in autoimmune diseases.
- **Nephrotoxicity** (kidney damage) is a major dose-limiting toxicity of calcineurin inhibitors, causing both acute and chronic kidney injury.
*Pancytopenia*
- While some immunosuppressants can cause **pancytopenia** (e.g., azathioprine, methotrexate), it is not the classic or primary toxicity associated with calcineurin inhibitors.
- Calcineurin inhibitors primarily affect **renal function** and can cause other side effects like hypertension or neurotoxicity.
*Osteoporosis*
- **Osteoporosis** is a known side effect of long-term glucocorticoid use, but not typically a primary toxicity of calcineurin inhibitors.
- Glucocorticoids reduce bone formation and increase bone resorption, leading to bone density loss.
*Hepatotoxicity*
- **Hepatotoxicity** (liver damage) can occur with various immunosuppressants, such as methotrexate, but it is not the most prominent or defining toxicity for calcineurin inhibitors.
- While cyclosporine can cause some liver enzyme elevation, **nephrotoxicity** is far more common and severe.
*Hyperglycemia*
- **Hyperglycemia** can be a side effect of some immunosuppressants, particularly **glucocorticoids** and **tacrolimus** (another calcineurin inhibitor).
- However, for the class of drugs that prevent IL-2 transcription (calcineurin inhibitors), **nephrotoxicity** remains the most significant and common major toxicity to be aware of.
Proteasome inhibitors US Medical PG Question 7: A 64-year-old woman comes to the physician because of a 7-month history of abdominal discomfort, fatigue, and a 6.8-kg (15-lb) weight loss. Physical examination shows generalized pallor and splenomegaly. Laboratory studies show anemia with pronounced leukocytosis and thrombocytosis. Cytogenetic analysis shows a BCR-ABL fusion gene. A drug with which of the following mechanisms of action is most appropriate for this patient?
- A. Ribonucleotide reductase inhibitor
- B. Monoclonal anti-HER-2 antibody
- C. Topoisomerase II inhibitor
- D. Monoclonal anti-CD20 antibody
- E. Tyrosine kinase inhibitor (Correct Answer)
Proteasome inhibitors Explanation: ***Tyrosine kinase inhibitor***
- The patient's symptoms (abdominal discomfort, fatigue, weight loss, pallor, splenomegaly), laboratory findings (**anemia with pronounced leukocytosis and thrombocytosis**), and the presence of a **BCR-ABL fusion gene** are highly characteristic of **Chronic Myeloid Leukemia (CML)**.
- The **BCR-ABL fusion gene** encodes a constitutively active **tyrosine kinase**, which is the hallmark of CML and the primary therapeutic target for **tyrosine kinase inhibitors (TKIs)** like imatinib.
*Ribonucleotide reductase inhibitor*
- **Ribonucleotide reductase inhibitors** (e.g., hydroxyurea) block DNA synthesis and are used in myeloproliferative disorders to reduce cell counts, but they are not specific to the **BCR-ABL fusion gene** and are not the most appropriate first-line targeted therapy for CML.
- While they can control symptoms, they do not target the underlying molecular defect in CML as effectively as TKIs.
*Monoclonal anti-HER-2 antibody*
- **Monoclonal anti-HER-2 antibodies** (e.g., trastuzumab) are used to treat **HER-2 positive breast cancer** and some gastric cancers.
- They are not relevant to the treatment of CML, which is characterized by the **BCR-ABL fusion gene**.
*Topoisomerase II inhibitor*
- **Topoisomerase II inhibitors** (e.g., etoposide, doxorubicin) prevent DNA unwinding and replication, leading to cell death, and are used in various hematologic malignancies and solid tumors.
- These drugs are broad-spectrum chemotherapeutic agents not specifically targeted to the **BCR-ABL fusion protein** in CML and are not first-line therapy for this condition.
*Monoclonal anti-CD20 antibody*
- **Monoclonal anti-CD20 antibodies** (e.g., rituximab) target the CD20 protein on B lymphocytes and are primarily used to treat **B-cell non-Hodgkin lymphoma** and some autoimmune diseases.
- They have no role in the direct treatment of CML, which is a myeloid malignancy.
Proteasome inhibitors US Medical PG Question 8: Parkinson’s disease is a progressive neurodegenerative disease. It is characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta and the formation of cellular inclusions called Lewy bodies. These are composed of α-synuclein that has been bound to ubiquitin. In healthy individuals, α-synuclein bound to ubiquitin would be degraded by which of the following?
- A. Peroxisome
- B. Lysosome
- C. Proteasome (Correct Answer)
- D. Ribosome
- E. Vesicle
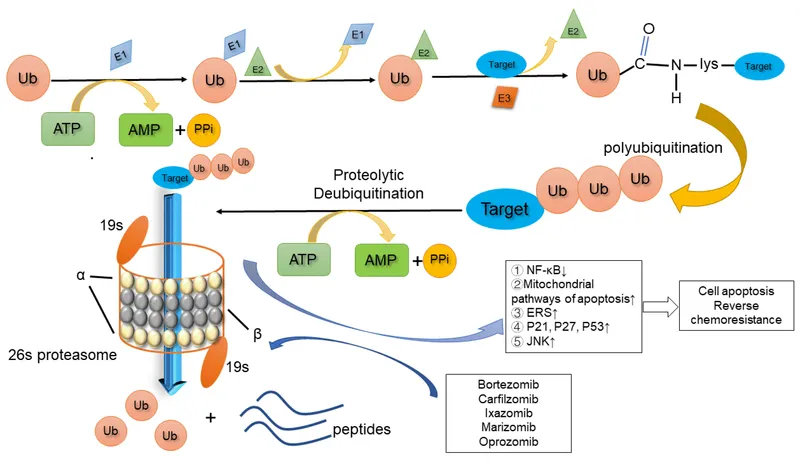
Proteasome inhibitors Explanation: ***Proteasome***
- The **ubiquitin-proteasome system** is the primary pathway for degrading misfolded or damaged proteins, such as **α-synuclein** bound to **ubiquitin**, within the cell.
- The proteasome specifically recognizes and breaks down proteins that have been tagged with multiple copies of the small protein **ubiquitin**.
*Peroxisome*
- Peroxisomes are involved in **fatty acid metabolism**, detoxification of reactive oxygen species, and other metabolic processes.
- They do not play a primary role in the degradation of ubiquitinated proteins.
*Lysosome*
- Lysosomes contain hydrolytic enzymes and are primarily responsible for the degradation of **extracellular material**, organelles, and certain intracellular proteins through **autophagy**.
- While they can degrade some ubiquitinated proteins, the proteasome is the dominant pathway for the specific degradation of misfolded cytoplasmic proteins.
*Ribosome*
- Ribosomes are responsible for **protein synthesis** (translation) based on mRNA templates.
- They are not involved in the degradation of proteins.
*Vesicle*
- Vesicles are small, membrane-bound sacs involved in transporting substances within the cell or releasing them outside the cell.
- They are primarily involved in storage and transport, not the enzymatic degradation of ubiquitinated proteins.
Proteasome inhibitors US Medical PG Question 9: A 67-year-old man comes to the physician for a follow-up examination after he was diagnosed with mantle cell lymphoma. The physician recommends a chemotherapeutic regimen containing bortezomib. Which of the following best describes the effect of this drug?
- A. Crosslinking of purine bases
- B. Preventing the relaxation of DNA supercoils
- C. Inhibition of tyrosine kinase receptors
- D. Accumulation of ubiquitinated proteins (Correct Answer)
- E. Stabilization of tubulin polymers
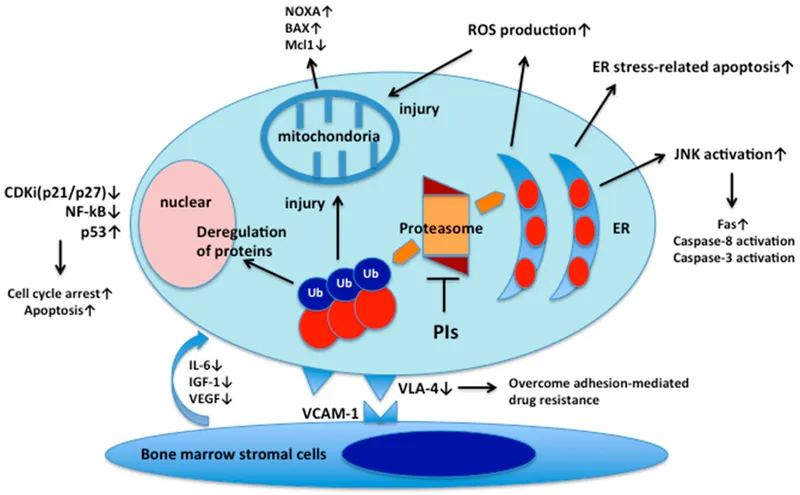
Proteasome inhibitors Explanation: ***Accumulation of ubiquitinated proteins***
- **Bortezomib** is a **proteasome inhibitor**, specifically targeting the 26S proteasome, which is responsible for degrading ubiquitinated proteins.
- Its inhibition leads to the accumulation of various **ubiquitinated proteins**, including pro-apoptotic factors, ultimately inducing **apoptosis** in cancer cells.
*Crosslinking of purine bases*
- This mechanism is characteristic of **alkylating agents** such as cyclophosphamide or cisplatin, which form covalent bonds with DNA, preventing replication and transcription.
- **Bortezomib** does not directly crosslink DNA bases; its primary action is on protein degradation pathways.
*Preventing the relaxation of DNA supercoils*
- This describes the mechanism of **topoisomerase inhibitors**, such as etoposide (topoisomerase II) or irinotecan (topoisomerase I), which block DNA replication and repair.
- Bortezomib has a distinct mechanism involving proteasome inhibition, not direct interaction with DNA or topoisomerases.
*Inhibition of tyrosine kinase receptors*
- This is the action of **tyrosine kinase inhibitors**, a class of drugs like imatinib or gefitinib, that target specific signaling pathways involved in cell growth and proliferation.
- Bortezomib's anti-cancer effects are mediated through protein degradation pathways, not by inhibiting receptor tyrosine kinases.
*Stabilization of tubulin polymers*
- This mechanism is characteristic of **taxanes** (e.g., paclitaxel), which hyperstabilize microtubules, interfering with cell division.
- **Bortezomib** does not affect microtubule dynamics; its action is focused on the proteasomal degradation system.
Proteasome inhibitors US Medical PG Question 10: A 69-year-old African American man is brought to the emergency department with sudden onset lower limb paralysis and back pain. He has had generalized bone pain for 2 months. He has no history of severe illnesses. He takes ibuprofen for pain. On examination, he is pale. The vital signs include: temperature 37.1°C (98.8°F), pulse 68/min, respiratory rate 16/min, and blood pressure 155/90 mm Hg. The neurologic examination shows paraparesis. The 8th thoracic vertebra is tender to palpation. An X-ray of the thoracic vertebrae confirms a compression fracture at the same level. The laboratory studies show the following:
Laboratory test
Hemoglobin 9 g/dL
Mean corpuscular volume 95 μm3
Leukocyte count 5,000/mm3
Platelet count 240,000/mm3
ESR 85 mm/hour
Serum
Na+ 135 mEq/L
K+ 4.2 mEq/L
Cl− 113 mEq/L
HCO3− 20 mEq/L
Ca+ 11.5 mg/dL
Albumin 4 g/dL
Urea nitrogen 18 mg/dL
Creatinine 1.2 mg/dL
Serum electrophoresis shows a monoclonal protein level of 38 g/L. To reduce the likelihood of fracture recurrence, it is most appropriate to administer which of the following?
- A. Calcitonin
- B. Calcitriol
- C. Pamidronate (Correct Answer)
- D. Fluoride
- E. Testosterone
Proteasome inhibitors Explanation: ***Pamidronate***
- The patient's presentation with **bone pain**, **hypercalcemia**, **anemia**, **elevated ESR**, **renal insufficiency**, and a **monoclonal protein** in serum electrophoresis is highly suggestive of **multiple myeloma**.
- **Bisphosphonates** like pamidronate are crucial in managing multiple myeloma by inhibiting osteoclast activity, reducing bone resorption, and thereby decreasing the risk of **pathological fractures** and managing **hypercalcemia**.
*Calcitonin*
- **Calcitonin** primarily works to lower serum calcium levels quickly but has a less sustained effect on bone remodeling compared to bisphosphonates.
- While it can be used for acute hypercalcemia, its role in preventing long-term fracture recurrence in multiple myeloma is limited.
*Calcitriol*
- **Calcitriol**, the active form of **vitamin D**, promotes calcium absorption from the gut and bone mineralization.
- Administering calcitriol in a patient with pre-existing hypercalcemia due to multiple myeloma would worsen the condition.
*Fluoride*
- **Fluoride** can increase bone density by affecting hydroxyapatite crystal formation.
- However, high doses of fluoride can lead to **fluorosis** and paradoxically increase bone fragility, making it unsuitable for preventing fractures in multiple myeloma.
*Testosterone*
- **Testosterone** is an anabolic steroid that can improve bone density in individuals with **hypogonadism**.
- It is not indicated for preventing fractures in the context of multiple myeloma, where bone destruction is driven by osteoclast activation due to plasma cell proliferation.
More Proteasome inhibitors US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.