Gene therapy updates US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Gene therapy updates. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Gene therapy updates US Medical PG Question 1: A 10-year-old boy presents to the emergency department with his parents. The boy complains of fever, neck stiffness, and drowsiness for the last several days. His past medical history is noncontributory. The boy was born at 39 weeks gestation via spontaneous vaginal delivery. He is up to date on all vaccines and is meeting all developmental milestones. There were no sick contacts at home or at school. The family did not travel out of the area recently. His heart rate is 100/min, respiratory rate is 22/min, blood pressure is 105/65 mm Hg, and temperature is 40.5ºC (104.9°F). On physical examination, he appears unwell and confused. His heart rate is elevated with a regular rhythm and his lungs are clear to auscultation bilaterally. During the examination, he experiences a right-sided focal seizure, which is controlled with lorazepam. A head CT reveals bilateral asymmetrical hypodensities of the temporal region. A lumbar puncture is performed and reveals the following:
WBC count 25/mm3
Cell predominance lymphocytes
Protein elevated
The patient is started on a medication to treat the underlying cause of his symptoms. What is the mechanism of action of this medication?
- A. Fusion inhibition
- B. Nucleoside reverse transcriptase inhibition
- C. Inhibition of DNA polymerase (Correct Answer)
- D. Binding with ergosterol in the cell membrane
- E. Cell wall synthesis inhibition
Gene therapy updates Explanation: ***Inhibition of DNA polymerase***
- The patient's symptoms (fever, neck stiffness, drowsiness, focal seizure, temporal lobe hypodensities) and CSF findings (lymphocytic pleocytosis, elevated protein) are highly suggestive of **herpes simplex encephalitis (HSE)**.
- The primary treatment for HSE is **acyclovir**, which works by inhibiting **viral DNA polymerase**.
*Fusion inhibition*
- This mechanism is characteristic of **antiviral drugs used for HIV**, such as enfuvirtide, which block the entry of the virus into host cells by preventing fusion of the viral and cellular membranes.
- This mechanism is not relevant to the treatment of herpes simplex virus.
*Nucleoside reverse transcriptase inhibition*
- This mechanism is also primarily associated with **antiretroviral drugs for HIV** (e.g., zidovudine).
- These drugs inhibit the enzyme **reverse transcriptase**, which HIV uses to convert its RNA into DNA.
*Binding with ergosterol in the cell membrane*
- This is the mechanism of action for certain **antifungal medications**, such as **amphotericin B** and **nystatin**, which bind to ergosterol in fungal cell membranes, leading to cell lysis.
- This mechanism is not applicable to antiviral treatment for HSE.
*Cell wall synthesis inhibition*
- This mechanism describes the action of many **antibacterial agents** (e.g., penicillins, cephalosporins) that interfere with the formation of the bacterial cell wall.
- This is not relevant to viral infections like HSE.
Gene therapy updates US Medical PG Question 2: An HIV-positive 48-year-old man comes to the emergency department because of a 3-month history of recurrent, painful mouth ulcers. This time, the pain is so severe that the patient cannot eat. He has a history of a seizure disorder but currently does not take any medications. He appears very ill. His temperature is 39.0°C (102.2°F). Physical examination shows numerous vesicular ulcerations on the lips and sloughing of the gums, buccal mucosa, and hard palate. Genetic analysis of the pathogen isolated from the lesions shows a mutation in a gene encoding viral phosphotransferases. Which of the following drugs is the most appropriate treatment?
- A. Acyclovir
- B. Famciclovir
- C. Cidofovir
- D. Ganciclovir
- E. Foscarnet (Correct Answer)
Gene therapy updates Explanation: ***Foscarnet***
- The presence of **recurrent, painful vesicular ulcerations** in an HIV-positive patient, especially with **gingivostomatitis-like symptoms** (sloughing gums, buccal mucosa), points to a severe **herpes simplex virus (HSV) infection**, likely resistant to nucleoside analogues given the **phosphotransferase mutation**.
- **Foscarnet** is a pyrophosphate analog that directly inhibits viral DNA polymerase without requiring phosphorylation by viral thymidine kinase, making it effective against **acyclovir-resistant HSV** strains, which often develop resistance via mutations in viral phosphotransferases or thymidine kinase.
*Acyclovir*
- **Acyclovir** is a nucleoside analog that requires phosphorylation by viral thymidine kinase (a phosphotransferase) to become active.
- A **mutation in viral phosphotransferases** would render the virus resistant to acyclovir, making it an ineffective treatment.
*Famciclovir*
- **Famciclovir** is a prodrug of penciclovir, which is also a nucleoside analog that requires phosphorylation by viral thymidine kinase for activation.
- Similar to acyclovir, a **mutation in viral phosphotransferases** would lead to resistance and make famciclovir ineffective.
*Cidofovir*
- **Cidofovir** is a nucleotide analog that does not require phosphorylation by viral enzymes for its initial activation.
- While it can be effective against some resistant strains, **foscarnet is generally preferred** for severe, resistant HSV infections as cidofovir is primarily used for **CMV retinitis** and is associated with significant nephrotoxicity.
*Ganciclovir*
- **Ganciclovir** is a nucleoside analog primarily used for **CMV infections**, and it also requires phosphorylation by viral kinases for activation.
- It is not the first-line treatment for HSV, and the **phosphotransferase mutation** would likely confer resistance to ganciclovir as well.
Gene therapy updates US Medical PG Question 3: A 65-year-old man comes to the physician because of a 1-month history of progressive back pain. He has also had a 5-kg (11-lb) weight loss over the past 3 months. His only medications are a daily multivitamin and ibuprofen, which he takes daily for the back pain. Physical examination shows tenderness to palpation over the lower spine and the left iliac crest. His hemoglobin concentration is 9.3 g/dL, his serum calcium concentration is 12 mg/dL, and his serum creatinine concentration is 2.1 mg/dL. A bone marrow biopsy shows 21% plasma cells. A diagnosis of multiple myeloma is established. In preparation for an autologous hematopoietic stem cell transplantation, the patient receives a myeloablative treatment regimen that includes busulfan. Which of the following drugs acts via a similar mechanism of action to busulfan?
- A. Etoposide
- B. Vemurafenib
- C. Vincristine
- D. Cytarabine
- E. Lomustine (Correct Answer)
Gene therapy updates Explanation: ***Lomustine***
- Both **busulfan** and **lomustine** are **alkylating agents**. They act by transferring **alkyl groups** to DNA, leading to cross-linking of DNA strands and inhibition of DNA synthesis and function.
- This **DNA damage** results in cell cycle arrest and apoptosis, particularly in rapidly dividing cells like cancer cells.
*Etoposide*
- **Etoposide** is a **topoisomerase II inhibitor** that prevents DNA relegation after strand breaks, leading to DNA damage and cell death.
- While it also targets DNA, its mechanism is distinct from the alkylation process of busulfan.
*Vemurafenib*
- **Vemurafenib** is a **BRAF kinase inhibitor** used in melanoma treatment. It specifically targets the **BRAF V600E mutation**.
- Its mechanism involves blocking signal transduction pathways critical for cell proliferation, rather than directly damaging DNA.
*Vincristine*
- **Vincristine** is a **vinca alkaloid** that acts as a **microtubule inhibitor**, preventing the formation of the **mitotic spindle** during cell division.
- This leads to metaphase arrest and apoptosis, a mechanism fundamentally different from DNA alkylation.
*Cytarabine*
- **Cytarabine** is an **antimetabolite**, specifically a **pyrimidine analog**, that inhibits **DNA polymerase**.
- It gets incorporated into DNA, leading to chain termination and inhibition of DNA synthesis and repair, making its action different from direct DNA alkylation.
Gene therapy updates US Medical PG Question 4: A clinical trial is being run with patients that have a genetic condition characterized by abnormal hemoglobin that can undergo polymerization when exposed to hypoxia, acidosis, or dehydration. This process of polymerization is responsible for the distortion of the red blood cell (RBC) that acquires a crescent shape and the hemolysis of RBCs. Researchers are studying the mechanisms of the complications commonly observed in these patients such as stroke, aplastic crisis, and auto-splenectomy. What kind of mutation leads to the development of the disease?
- A. Silent mutation
- B. Splice site
- C. Missense mutation (Correct Answer)
- D. Nonsense mutation
- E. Frameshift mutation
Gene therapy updates Explanation: ***Missense mutation***
- A missense mutation results in a **single nucleotide substitution** that changes the codon to code for a different amino acid, altering the protein.
- In **sickle cell disease**, a missense mutation in the beta-globin gene (GAG to GTG) leads to the substitution of **glutamic acid for valine**, causing abnormal hemoglobin (HbS) that polymerizes under deoxygenated conditions.
*Silent mutation*
- A silent mutation is a **point mutation** that results in a new codon that still codes for the **same amino acid**, meaning there is no change in the protein sequence.
- Therefore, it would not lead to an **abnormal hemoglobin** protein or the described disease phenotype.
*Splice site*
- A splice site mutation occurs at the **splice junctions** of introns and exons, leading to errors in mRNA processing.
- This can result in **incorrect protein synthesis** due to exon skipping or intron retention, but it typically does not cause the specific amino acid substitution seen in sickle cell disease.
*Nonsense mutation*
- A nonsense mutation is a point mutation that results in a **premature stop codon**, leading to a **truncated, non-functional protein**.
- While this can cause severe disease, it would typically lead to a complete absence or severe deficiency of functional hemoglobin rather than a structurally altered hemoglobin like HbS.
*Frameshift mutation*
- A frameshift mutation involves the **insertion or deletion of nucleotides** (not in multiples of three), which shifts the reading frame of the mRNA.
- This typically leads to a completely **altered amino acid sequence** downstream of the mutation and usually results in a premature stop codon, leading to a non-functional protein rather than a specific single amino acid substitution.
Gene therapy updates US Medical PG Question 5: A 72-year-old man presents to the primary care clinic for evaluation of progressive fatigue and weight loss. His past medical history is significant for hypercholesterolemia, type 2 diabetes mellitus, aortic stenosis, and chronic renal insufficiency. He endorses being well-rested after waking from sleep but fatiguing rapidly during the day. In addition, he states that he has lost 15lbs over the previous month. His temperature is 98.3°F (36.8°C), pulse is 100/min, blood pressure is 110/85 mmHg, respirations are 16/min, and oxygen saturation is 96% on room air. Physical exam is notable for conjunctival pallor and scattered areas of ecchymoses. His laboratory results are shown below:
Serum:
Na+: 140 mEq/L
K+: 4.0 mEq/L
Cl-: 101 mEq/L
HCO3-: 22 mEq/L
BUN: 30 mg/dL
Glucose: 160 mg/dL
Creatinine: 1.9 mg/dL
Leukocyte count: 1,100/mm^3
Absolute neutrophil count 920/mm^3
Hemoglobin 8.4 g/dL
Platelet count: 45,000/mm^3
Mean corpuscular hemoglobin concentration: 34%
Red blood cell distribution width: 12.0%
Mean corpuscular volume: 92 µm^3
Lactate dehydrogenase: 456 IU/L
Haptoglobin 120 mg/dL
Fibrinogen 214 mg/dL
A bone marrow biopsy is performed which shows cells that are CD19+, CD20+, CD11c+, and stain with acid phosphatase 5 and tartrate-resistant. Which of the following is the next best step in the treatment of his disorder?
- A. Cyclophosphamide
- B. Hydroxyurea
- C. Cladribine (Correct Answer)
- D. Filgrastim
- E. Doxorubicin
Gene therapy updates Explanation: ***Cladribine***
- The patient's blood work (pancytopenia: **leukopenia**, **anemia**, **thrombocytopenia**) along with bone marrow biopsy findings (**CD19+, CD20+, CD11c+, tartrate-resistant acid phosphatase-positive** cells) are highly indicative of **hairy cell leukemia**.
- **Cladribine** is a purine analog, which is considered the most effective first-line treatment for hairy cell leukemia, often leading to long-lasting remissions.
*Cyclophosphamide*
- This is an **alkylating agent** used in various cancers and autoimmune conditions, but it is not the most effective or preferred first-line treatment for hairy cell leukemia.
- Cyclophosphamide is associated with significant side effects and would likely be reserved for other hematological malignancies.
*Hydroxyurea*
- **Hydroxyurea** is a ribonucleotide reductase inhibitor primarily used in myeloproliferative disorders like **chronic myeloid leukemia** or **polycythemia vera** to reduce cell counts.
- While it can lower white blood cell counts, it is not curative and not the standard primary therapy for hairy cell leukemia.
*Filgrastim*
- **Filgrastim** is a **granulocyte colony-stimulating factor (G-CSF)** used to stimulate neutrophil production, often to counter neutropenia caused by chemotherapy.
- It would not be used to treat hairy cell leukemia itself, and in some cases, can even paradoxically induce leukocytosis, which may not be desired in a condition characterized by abnormal white blood cells.
*Doxorubicin*
- **Doxorubicin** is an **anthracycline antibiotic** used in the treatment of many cancers (e.g., lymphomas, breast cancer, sarcomas) but not hairy cell leukemia.
- Its mechanism of action involves DNA intercalation and inhibition of topoisomerase II, which is not the primary target for hairy cell leukemia therapy.
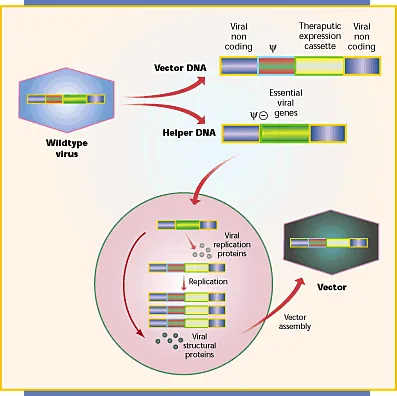
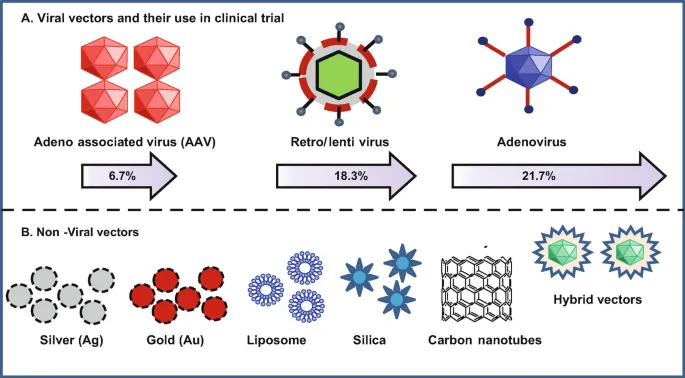
Gene therapy updates US Medical PG Question 6: A 9-year-old boy is brought to the emergency department by his parents after a 2-day history of fever, productive cough, and severe dyspnea. The parents report that the boy had no health problems at birth but developed respiratory problems as an infant that have continued throughout his life, including recurrent pulmonary infections. Vital signs include: temperature of 37.5ºC (99.5ºF), pulse of 105/min, respiratory rate of 34/min, and SpO2 of 87%. Physical examination shows digital clubbing and cyanosis. Chest X-rays show hyperinflation of the lungs and chronic interstitial changes. The boy’s FEV1/FVC ratio is decreased, and his FRC is increased. The resident reviewing his case is studying new gene therapies for this boy’s condition that will reintroduce the gene for which this boy is defective. An important component of this therapy is identifying a vector for the selective introduction of the replacement gene into the human body. Which of the following would be the best vector to provide gene therapy for this boy’s respiratory symptoms?
- A. Human immunodeficiency virus-1
- B. Adenovirus (Correct Answer)
- C. Rabies virus
- D. Rhinovirus
- E. Coxsackie A virus
Gene therapy updates Explanation: ***Adenovirus***
- Adenoviruses are the **most suitable vector for respiratory gene therapy** due to their high efficiency in gene delivery to respiratory epithelial cells and their ability to infect both dividing and non-dividing cells.
- The clinical presentation (recurrent pulmonary infections, dyspnea, hyperinflation, digital clubbing, and cyanosis) is characteristic of **cystic fibrosis**, which results from a defect in the *CFTR* gene—a prime target for gene therapy via respiratory delivery.
- Adenoviral vectors were extensively studied in CF gene therapy trials due to their **excellent tropism for airway epithelium**.
*Incorrect: Human immunodeficiency virus-1*
- While HIV-1-derived lentiviruses can transduce non-dividing cells and integrate into the host genome, they are **less efficient in delivering genes to respiratory epithelial cells** compared to adenoviruses.
- Concerns regarding **potential insertional mutagenesis** and immune responses make them less ideal for respiratory gene therapy.
*Incorrect: Rabies virus*
- Rabies virus has strong **neurotropism**, meaning it primarily targets the nervous system, making it unsuitable for direct delivery to lung epithelial cells.
- Its use would likely lead to **severe neurological side effects** without effectively treating the underlying lung pathology.
*Incorrect: Rhinovirus*
- Rhinoviruses typically cause **mild, self-limiting infections of the upper respiratory tract** and are not optimized for stable gene transfer to the lower respiratory tract.
- They lack the capacity for **long-term gene expression** required for conditions like cystic fibrosis.
*Incorrect: Coxsackie A virus*
- Coxsackie A viruses are associated with diseases such as **hand, foot, and mouth disease** and cause acute, transient infections.
- They are **not efficient gene delivery vectors** for the respiratory system and could cause unwanted inflammatory responses in the lungs.
Gene therapy updates US Medical PG Question 7: A parent presents to her pediatrician requesting information about immunizations for her newborn. The pediatrician explains about basic principles of immunization, types of vaccines, possible adverse effects, and the immunization schedule. Regarding how immunizations work, the pediatrician explains that there are mainly 2 types of vaccines. The first type of vaccine provides stronger and more lasting immunity as it induces both cellular and humoral immune responses. The second type of vaccine produces mainly a humoral response only, and its overall efficacy is less as compared to the first type. Which of the following vaccines belongs to the first type of vaccine that the pediatrician is talking about?
- A. Hepatitis A vaccine
- B. Polio vaccine (Salk)
- C. Yellow fever vaccine (Correct Answer)
- D. Rabies vaccine
- E. Hepatitis B vaccine
Gene therapy updates Explanation: ***Yellow fever vaccine***
- The Yellow fever vaccine is a **live-attenuated vaccine**, which mimics natural infection and effectively stimulates both **cellular and humoral immune responses**, leading to strong and long-lasting immunity.
- Live-attenuated vaccines contain a weakened form of the pathogen, allowing for replication within the host and robust immune system activation.
*Hepatitis A vaccine*
- The Hepatitis A vaccine is an **inactivated vaccine**, which primarily induces a **humoral (antibody-mediated) immune response**.
- Inactivated vaccines generally do not stimulate a strong cellular immune response and often require booster doses to maintain protective immunity.
*Polio vaccine (Salk)*
- The Salk polio vaccine is an **inactivated polio vaccine (IPV)**, meaning it contains killed viral particles.
- As an inactivated vaccine, it mainly elicits a **humoral immune response** producing circulating antibodies but less mucosal or cellular immunity.
*Rabies vaccine*
- The Rabies vaccine is an **inactivated vaccine** given after exposure or for pre-exposure prophylaxis.
- It primarily induces a **humoral antibody response** rather than a strong cellular immune response.
*Hepatitis B vaccine*
- The Hepatitis B vaccine is a **recombinant vaccine**, containing only a portion of the viral antigen (HBsAg).
- This type of vaccine primarily stimulates a **humoral immune response** leading to antibody production, which is effective but does not typically induce a strong cellular response like live vaccines.
Gene therapy updates US Medical PG Question 8: A 3-year-old girl presents with delayed growth, anemia, and jaundice. Her mother reports no personal history of blood clots, but states that the patient's grandmother has been treated for pulmonary embolism and multiple episodes of unexplained pain in the past. The patient's prenatal history is significant for preeclampsia, preterm birth, and a neonatal intensive care unit (NICU) stay of 6 weeks. The vital signs include: temperature 36.7°C (98.0°F), blood pressure 102/54 mm Hg, heart rate 111/min, and respiratory rate 23/min. On physical examination, the pulses are bounding, the complexion is pale, but breath sounds remain clear. Oxygen saturation was initially 81% on room air, with a new oxygen requirement of 4 L by nasal cannula. Upon further examination, her physician notices that her fingers appear inflamed. A peripheral blood smear demonstrates sickle-shaped red blood cells (RBCs). What is the most appropriate treatment for this patient?
- A. Intravenous immunoglobulin
- B. Corticosteroids
- C. Epoetin
- D. Hydroxyurea (Correct Answer)
- E. Darbepoetin
Gene therapy updates Explanation: ***Hydroxyurea***
- This patient presents with symptoms highly suggestive of **sickle cell disease (SCD)**, including **delayed growth, anemia, jaundice, oxygen desaturation**, and **sickle-shaped red blood cells** on peripheral smear. **Hydroxyurea** is a first-line treatment for SCD in children and adults to reduce the frequency of **pain crises, acute chest syndrome**, and the need for transfusions.
- Hydroxyurea works by increasing the production of **fetal hemoglobin (HbF)**, which inhibits the sickling of red blood cells and improves their oxygen-carrying capacity. It also has anti-inflammatory properties, which can help manage SCD complications.
*Intravenous immunoglobulin*
- **Intravenous immunoglobulin (IVIG)** is primarily used in conditions involving immune dysfunction, such as **Kawasaki disease**, **immune thrombocytopenia**, or certain primary immunodeficiencies.
- There is no indication of an **immune-mediated disorder** or infection requiring IVIG in this patient's presentation of sickle cell crisis.
*Corticosteroids*
- **Corticosteroids** are potent anti-inflammatory and immunosuppressive agents often used in conditions like asthma, autoimmune disorders, or allergic reactions.
- While inflammation plays a role in sickle cell crises, corticosteroids are generally not a primary treatment for the underlying disease mechanism and can have significant side effects with long-term use.
*Epoetin*
- **Epoetin** is a recombinant form of **erythropoietin**, a hormone that stimulates red blood cell production. It is used to treat anemia associated with chronic kidney disease or chemotherapy.
- In sickle cell disease, the anemia is due to increased red blood cell destruction (hemolysis) and dysfunctional red blood cells, rather than *insufficient erythropoietin production*, making epoetin generally ineffective.
*Darbepoetin*
- **Darbepoetin** is a longer-acting form of **epoetin**, primarily used to treat anemia in chronic kidney disease, chemotherapy-induced anemia, or certain myelodysplastic syndromes.
- Similar to epoetin, darbepoetin is not a primary treatment for the **hemolytic anemia** characteristic of sickle cell disease, where the problem lies with abnormal hemoglobin and cell shape rather than erythropoietin deficiency.
Gene therapy updates US Medical PG Question 9: A 14-month-old African American boy is brought to the emergency department because of fever, lethargy, and lack of appetite for 6 days. The patient’s mother says he fell off the changing table 10 days ago and landed on his left side, which she says has been tender since then. His vital signs include: temperature 38.0°C (100.4°F), blood pressure 85/41 mm Hg, pulse 132/min. Physical examination reveals conjunctival pallor and reduced range of motion at the left hip. C-reactive protein (CRP) is raised. A magnetic resonance imaging (MRI) scan shows signs of infection in the medullary canal of the left femoral bone and surrounding soft tissues. Blood cultures are positive for Salmonella. Which of the following would most likely confirm the underlying diagnosis in this patient?
- A. Hemoglobin electrophoresis (Correct Answer)
- B. Antinuclear antibodies
- C. Iron studies
- D. Peripheral blood smear
- E. Full blood count
Gene therapy updates Explanation: ***Hemoglobin electrophoresis***
- The presence of **Salmonella osteomyelitis** in an African American child should raise suspicion for **sickle cell disease**, as these patients are particularly susceptible to Salmonella infections due to splenic dysfunction.
- **Hemoglobin electrophoresis** is the definitive test to diagnose sickle cell disease by identifying abnormal hemoglobin S.
*Antinuclear antibodies*
- **Antinuclear antibodies (ANA)** are primarily used to screen for **autoimmune diseases** such as systemic lupus erythematosus, which is not indicated by the patient's presentation.
- While autoimmune conditions can affect bone, the specific context of Salmonella osteomyelitis points to a different underlying predisposition.
*Iron studies*
- **Iron studies** measure iron levels, total iron-binding capacity, and ferritin, primarily used to diagnose **anemia** or **hemochromatosis**.
- Although the patient has conjunctival pallor, suggesting anemia, iron studies would not explain the susceptibility to Salmonella osteomyelitis.
*Peripheral blood smear*
- A **peripheral blood smear** can show general abnormalities in red blood cells, white blood cells, and platelets, including **sickled cells**.
- While it might provide clues, an abnormal smear alone is not **diagnostic for sickle cell disease** and would require further confirmation with hemoglobin electrophoresis.
*Full blood count*
- A **full blood count (FBC)** provides information on red blood cell count, hemoglobin, hematocrit, white blood cell count, and platelet count.
- It would confirm **anemia** (consistent with conjunctival pallor) and possibly **leukocytosis** (due to infection), but it does not diagnose the **underlying cause** of susceptibility to Salmonella osteomyelitis.
Gene therapy updates US Medical PG Question 10: A 7-year-old boy is brought to the emergency department because of a 3-day history of generalized fatigue, myalgia, and fever. He has sickle cell disease. His current medications include hydroxyurea and folic acid. He appears ill. His temperature is 39.2°C (102.6°F), pulse is 103/min, and respirations are 28/min. Examination shows pale conjunctivae. The lungs are clear to auscultation. The abdomen is soft and nontender. Neurologic examination shows no focal findings, His hemoglobin concentration is 10.3 g/dL and leukocyte count is 14,100/mm3. Intravenous fluid is administered and blood cultures are obtained. Which of the following is the most appropriate next step in treatment?
- A. Clindamycin
- B. Prednisone
- C. Vancomycin
- D. Ceftriaxone (Correct Answer)
- E. Levofloxacin
Gene therapy updates Explanation: ***Ceftriaxone***
- This patient presents with **fever** and **sickle cell disease**, placing him at high risk for bacterial infections, especially from **encapsulated organisms** like *Streptococcus pneumoniae* and *Haemophilus influenzae*. **Ceftriaxone** is a broad-spectrum third-generation cephalosporin that provides excellent coverage against these common pathogens.
- Due to the high risk of **sepsis** and rapid progression of infection in sickle cell patients, empiric, prompt administration of **intravenous antibiotics** is crucial, even before culture results are available.
*Clindamycin*
- **Clindamycin** is primarily effective against **anaerobic bacteria** and some gram-positive organisms, including methicillin-sensitive *Staphylococcus aureus* (MSSA).
- It does not provide adequate coverage against the most common and life-threatening pathogens in febrile sickle cell patients, such as encapsulated bacteria.
*Prednisone*
- **Prednisone** is a corticosteroid used for its **anti-inflammatory** and immunosuppressive effects. It is not indicated for the initial management of fever and suspected bacterial infection.
- Administering corticosteroids in a patient with suspected infection without appropriate antibiotic coverage could worsen the infection.
*Vancomycin*
- **Vancomycin** is a powerful antibiotic primarily used to cover **multi-drug resistant gram-positive bacteria**, especially **MRSA** and drug-resistant *S. pneumoniae*.
- While it covers gram-positive organisms well, it does **not cover gram-negative bacteria** such as *H. influenzae* or *Salmonella* species, which are important pathogens in sickle cell patients. **Ceftriaxone** provides broader coverage including both gram-positive and gram-negative encapsulated organisms, making it the preferred empiric choice.
*Levofloxacin*
- **Levofloxacin** is a fluoroquinolone that provides broad-spectrum coverage, including against atypical organisms and some gram-negatives and gram-positives.
- However, **fluoroquinolones** are generally avoided in children due to potential adverse effects on cartilage development, and it is not the first-line empiric choice for severe bacterial infections in this age group, especially when **cephalosporins** are highly effective and safer.
More Gene therapy updates US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.