Genetic disorders

On this page
🧬 The Genetic Blueprint: Decoding Hereditary Disorders
Genetic disorders reveal how single molecular missteps can cascade through entire biological systems, transforming the blueprint of life into clinical disease. You'll master the mechanisms linking DNA mutations to patient presentations, learning to recognize inheritance patterns that unlock diagnoses, interpret molecular tests that confirm suspicions, and apply emerging therapies that target root causes rather than symptoms. This journey from gene to bedside will sharpen your clinical reasoning while building fluency in the language of precision medicine that's reshaping how we understand and treat disease across every specialty.
📌 Remember: GENETIC - Genetic testing, Etiology assessment, Newborn screening, Ethical counseling, Treatment planning, Inheritance patterns, Clinical phenotyping
Genetic disorders affect approximately 6-8% of all births globally, with >7,000 recognized single-gene disorders documented in medical literature. These conditions span every medical specialty, from the 1 in 2,500 incidence of cystic fibrosis in Caucasian populations to the 1 in 700 occurrence of Down syndrome across all ethnicities.
⭐ Clinical Pearl: 80% of genetic disorders manifest before age 5 years, but 25% remain undiagnosed until adulthood due to variable expressivity and incomplete penetrance patterns.
-
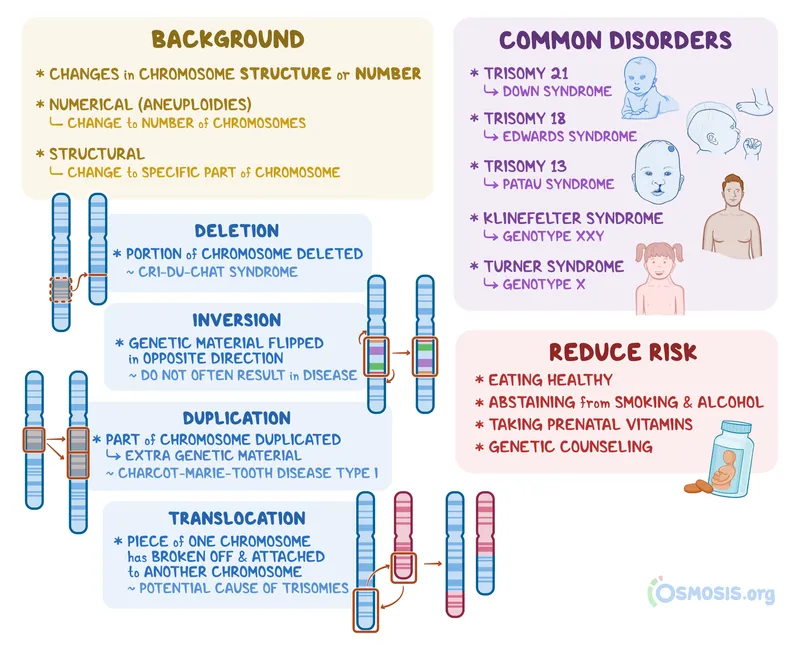
Chromosomal Disorders (6% of genetic conditions)
- Numerical abnormalities: Trisomies, monosomies
- Structural abnormalities: Deletions, duplications, translocations
- Down syndrome: 1 in 700 births
- Turner syndrome: 1 in 2,500 female births
- 22q11.2 deletion: 1 in 4,000 births
-
Single-Gene Disorders (80% of genetic conditions)
- Autosomal dominant: 50% transmission risk
- Autosomal recessive: 25% affected offspring risk
- X-linked: 50% risk for male offspring
- Duchenne muscular dystrophy: 1 in 3,500 males
- Fragile X syndrome: 1 in 4,000 males
| Disorder Category | Prevalence | Age at Diagnosis | Inheritance Pattern | Screening Available | Treatment Options |
|---|---|---|---|---|---|
| Chromosomal | 1 in 150 births | Birth-2 years | Sporadic (90%) | Prenatal/Newborn | Supportive |
| Single-gene | 1 in 200 births | Variable | Mendelian | Targeted | Gene therapy emerging |
| Multifactorial | 1 in 33 births | Birth-adulthood | Complex | Limited | Preventive |
| Mitochondrial | 1 in 5,000 births | Childhood-adult | Maternal | Specialized | Symptomatic |
| Imprinting | 1 in 15,000 births | Infancy | Parent-specific | Molecular | Supportive |
The diagnostic yield of genetic testing has revolutionized from <5% with traditional karyotyping to >40% with whole exome sequencing in patients with suspected genetic disorders. This transformation enables targeted therapies, family planning guidance, and prevention strategies that fundamentally alter patient outcomes and healthcare delivery approaches.
🧬 The Genetic Blueprint: Decoding Hereditary Disorders
🔬 Molecular Mechanisms: The Cellular Command Center
📌 Remember: PATHWAYS - Protein dysfunction, Altered gene expression, Transcription errors, Haploinsufficiency, Wrong protein folding, Abnormal cell signaling, Yield reduction, Splicing defects
Loss-of-Function Mechanisms account for 70% of genetic disorders, where mutations reduce or eliminate normal protein activity. Cystic fibrosis exemplifies this pattern, where >2,000 different CFTR mutations cause variable degrees of chloride channel dysfunction, correlating with disease severity from pancreatic insufficiency (85% of patients) to preserved pancreatic function (15% of patients).
-
Haploinsufficiency Disorders (25% of dominant conditions)
- Single functional gene copy insufficient for normal function
- 22q11.2 deletion syndrome: >40 genes deleted
- TBX1 gene: Critical for cardiac and pharyngeal development
- COMT gene: Affects dopamine metabolism and behavior
- Penetrance: >90% for major features
-
Dominant Negative Effects (15% of dominant conditions)
- Mutant protein interferes with normal protein function
- Marfan syndrome: Abnormal fibrillin-1 disrupts connective tissue
- Aortic root dilatation: >80% of patients by age 20
- Lens dislocation: 60% of patients
⭐ Clinical Pearl: Gain-of-function mutations cause <5% of genetic disorders but often present with severe, early-onset phenotypes requiring immediate intervention, such as hyperinsulinemic hypoglycemia or long QT syndrome.
| Mechanism | Frequency | Example Disorder | Protein Effect | Clinical Onset | Inheritance |
|---|---|---|---|---|---|
| Loss of function | 70% | Cystic fibrosis | Reduced/absent activity | Variable | Recessive |
| Haploinsufficiency | 20% | 22q11.2 deletion | 50% protein reduction | Early | Dominant |
| Dominant negative | 8% | Marfan syndrome | Interferes with normal | Progressive | Dominant |
| Gain of function | 2% | Huntington disease | Toxic new property | Adult-onset | Dominant |
Epigenetic Mechanisms increasingly explain 10-15% of genetic disorders through DNA methylation changes, histone modifications, or imprinting defects. Prader-Willi and Angelman syndromes demonstrate how identical 15q11-q13 deletions cause completely different phenotypes depending on parental origin, highlighting the critical role of genomic imprinting in human development and disease pathogenesis.
🔬 Molecular Mechanisms: The Cellular Command Center
🎯 Pattern Recognition: The Clinical Detective Framework
📌 Remember: RECOGNIZE - Recurrent infections, Early developmental delays, Congenital anomalies, Organ system clustering, Growth abnormalities, Neurological features, Intellectual disability, Zone-specific dysmorphism, Endocrine dysfunction
"When You See X, Think Y" Clinical Correlations:
-
Webbed neck + short stature + cardiac defects → Turner syndrome (1 in 2,500 females)
- Coarctation of aorta: 15% of patients
- Bicuspid aortic valve: 50% of patients
- Renal anomalies: 40% of patients
-
Intellectual disability + large ears + macroorchidism → Fragile X syndrome (1 in 4,000 males)
- Autism spectrum features: 60% of affected males
- Seizures: 20% of patients
- Mitral valve prolapse: 80% of adult males
-
Hypotonia + feeding difficulties + hyperphagia transition → Prader-Willi syndrome (1 in 15,000 births)
- Phase 1 (0-2 years): Severe hypotonia, feeding difficulties
- Phase 2 (2-8 years): Weight gain without hyperphagia
- Phase 3 (8+ years): Hyperphagia and obesity
Systematic Phenotyping Approach:
-
Craniofacial Assessment (>90% diagnostic yield in syndromic patients)
- Measure head circumference: <3rd or >97th percentile significant
- Document eye spacing: Hypertelorism vs. hypotelorism
- Assess ear position: Low-set ears <2 SD below normal
- Down syndrome: Upslanting palpebral fissures (90%)
- Turner syndrome: Webbed neck (25%), low hairline (80%)
-
Growth Pattern Analysis (85% sensitivity for genetic disorders)
- Plot serial measurements on syndrome-specific growth charts
- Calculate growth velocity: <25th percentile concerning
- Assess proportionality: Head-to-height ratios
- Achondroplasia: Disproportionate short stature
- Russell-Silver syndrome: Asymmetric growth restriction
⭐ Clinical Pearl: Three or more minor anomalies in a patient indicates >90% likelihood of an associated major malformation, warranting comprehensive genetic evaluation and imaging studies.
| Syndrome | Key Facial Features | Growth Pattern | Cardiac Involvement | Developmental Delay |
|---|---|---|---|---|
| Down syndrome | Upslanting eyes, flat nasal bridge | Normal birth weight, slow growth | 50% CHD | Mild-moderate ID |
| Turner syndrome | Webbed neck, low hairline | Short stature, normal head | 25% coarctation | Normal-mild LD |
| 22q11.2 deletion | Long face, prominent nose | Normal growth | 75% conotruncal defects | Mild ID, psychiatric |
| Fragile X | Large ears, prominent jaw | Macrocephaly, tall stature | 80% MVP in adults | Moderate ID, autism |
| Prader-Willi | Almond eyes, thin upper lip | Obesity after age 2 | Rare | Mild-moderate ID |
Red Flag Combinations requiring immediate genetic consultation include unexplained intellectual disability plus ≥2 major anomalies, multiple miscarriages with structural defects, or progressive neurodegeneration with metabolic decompensation - patterns suggesting specific genetic etiologies with time-sensitive treatment implications.
🎯 Pattern Recognition: The Clinical Detective Framework
🔍 Diagnostic Precision: The Molecular Microscope
📌 Remember: TESTING - Targeted gene panels, Exome sequencing, Single gene analysis, Trisome screening, Imprinting studies, Newborn screening, Genome sequencing
Testing Hierarchy Based on Clinical Presentation:
-
Intellectual Disability/Autism Spectrum Disorder
- First-tier: Chromosomal microarray (15-20% diagnostic yield)
- Second-tier: Fragile X testing (2-6% yield in males)
- Third-tier: Whole exome sequencing (25-30% additional yield)
- Combined diagnostic rate: 40-50% in unexplained cases
- Turnaround time: 2-6 weeks for urgent cases
-
Multiple Congenital Anomalies
- First-tier: Chromosomal microarray + karyotype (20-25% yield)
- Second-tier: Targeted gene panels (15-20% additional yield)
- Third-tier: Whole exome/genome sequencing (30-35% yield)
- Rapid trio analysis: 48-72 hours for critically ill neonates
Technology-Specific Capabilities and Limitations:
-
Chromosomal Microarray Analysis (Gold standard for copy number variants)
- Detection limit: >25-50 kb deletions/duplications
- Diagnostic yield: 15-20% in developmental disorders
- Cannot detect: Balanced translocations, point mutations, methylation
- 22q11.2 deletion: >99% detection sensitivity
- Williams-Beuren syndrome: >95% detection rate
-
Whole Exome Sequencing (Protein-coding regions, ~1.5% of genome)
- Coverage: >20x depth for >95% of target regions
- Diagnostic yield: 25-30% in undiagnosed patients
- Limitations: Intronic variants, structural variants, repeat expansions
- Turnaround time: 4-6 weeks standard, 1-2 weeks rapid
- Cost: $800-1,500 per sample
⭐ Clinical Pearl: Trio analysis (proband + both parents) increases diagnostic yield by 15-20% compared to singleton testing by enabling de novo variant identification and inheritance pattern confirmation.
| Testing Method | Detection Capability | Diagnostic Yield | Turnaround Time | Cost Range | Best Use Case |
|---|---|---|---|---|---|
| Karyotype | Large chromosomal changes | 3-5% | 1-2 weeks | $200-400 | Suspected aneuploidy |
| CMA | CNVs >25kb | 15-20% | 1-2 weeks | $400-800 | ID/MCA first-tier |
| Gene panels | Known disease genes | 10-25% | 2-4 weeks | $300-1000 | Specific phenotypes |
| Exome sequencing | Coding variants | 25-30% | 4-6 weeks | $800-1500 | Undiagnosed cases |
| Genome sequencing | All variant types | 35-40% | 6-8 weeks | $1500-3000 | Exome-negative |
Prenatal Testing Evolution has transformed from invasive procedures with 0.1-0.3% miscarriage risk to cell-free DNA screening with >99% sensitivity for common trisomies and <0.1% false positive rate, enabling earlier detection at 9-10 weeks gestation with simple blood draw from maternal circulation.
🔍 Diagnostic Precision: The Molecular Microscope
⚖️ Treatment Algorithms: The Therapeutic Revolution
📌 Remember: THERAPY - Targeted treatments, Hormone replacement, Enzyme therapy, Rehabilitation, Anticipatory guidance, Prevention strategies, Yearly monitoring
Evidence-Based Treatment Protocols:
-
Turner Syndrome Management (Comprehensive care improves outcomes >80%)
- Growth hormone therapy: Start at 4-6 years, gain 5-10 cm final height
- Estrogen replacement: Begin at 12-14 years for pubertal development
- Cardiac monitoring: Echo every 5 years, MRI if aortic dilatation
- Oxandrolone addition: 2-4 cm additional height gain
- Fertility preservation: Ovarian tissue cryopreservation in 5-10%
-
22q11.2 Deletion Syndrome (Multidisciplinary approach essential)
- Calcium supplementation: 1-2 grams daily for hypocalcemia
- Immunology evaluation: 75% have immune deficiency
- Psychiatric screening: 25% develop schizophrenia by age 25
- Speech therapy: >90% require intervention
- Cardiac surgery: 75% need intervention in first year
-
Duchenne Muscular Dystrophy (Corticosteroids extend ambulation 2-3 years)
- Deflazacort: 0.9 mg/kg/day preferred over prednisone
- Cardiac monitoring: ACE inhibitors when EF <55%
- Respiratory support: BiPAP when FVC <50% predicted
- Eteplirsen (exon 51 skipping): 13% of patients eligible
- Gene therapy trials: Micro-dystrophin showing promising results
Emerging Therapeutic Modalities:
-
Gene Therapy Successes (FDA-approved treatments)
- Luxturna (RPE65-associated blindness): >90% vision improvement
- Zolgensma (spinal muscular atrophy): 100% survival at 2 years
- Hemgenix (hemophilia B): >95% reduction in bleeding episodes
- Cost: $2-3.5 million per treatment
- Durability: >5 years sustained benefit demonstrated
-
Antisense Oligonucleotides (Precision targeting)
- Spinraza (SMA): >80% achieve motor milestones
- Eteplirsen (DMD): Modest dystrophin restoration
- Milasen (individual patient): Personalized antisense therapy
- Development time: 10-12 months for personalized treatments
- Cost: $100,000-500,000 annually
⭐ Clinical Pearl: Early intervention in genetic disorders provides exponentially greater benefits - growth hormone therapy started before age 6 in Turner syndrome yields 2-3x greater height gain compared to later initiation.
| Disorder | Primary Treatment | Success Rate | Monitoring Required | Cost/Year | Emerging Options |
|---|---|---|---|---|---|
| Turner syndrome | Growth hormone + estrogen | 85% improved outcomes | Cardiac, renal, hearing | $30,000-50,000 | Fertility preservation |
| 22q11.2 deletion | Multidisciplinary care | 80% prevent complications | Immune, cardiac, psych | $20,000-40,000 | Thymus transplant |
| Duchenne MD | Corticosteroids | 70% extend ambulation | Cardiac, respiratory | $40,000-60,000 | Gene therapy trials |
| Fragile X | Behavioral intervention | 60% functional improvement | Development, behavior | $15,000-30,000 | Targeted medications |
| Prader-Willi | Growth hormone + diet | 75% prevent severe obesity | Growth, behavior | $35,000-55,000 | Setmelanotide |
Precision Medicine Integration enables genotype-phenotype correlation for individualized treatment plans - CFTR modulator therapy in cystic fibrosis demonstrates >90% efficacy in specific mutations, while pharmacogenomic testing optimizes medication selection and dosing in >60% of genetic conditions requiring chronic therapy.
⚖️ Treatment Algorithms: The Therapeutic Revolution
🔗 Multi-System Integration: The Genetic Network
📌 Remember: SYSTEMS - Syndromic associations, Yearly surveillance, Specialist coordination, Transition planning, Emergency protocols, Multidisciplinary care, Screening guidelines
Cross-System Manifestation Patterns:
-
Connective Tissue Disorders (Marfan, Ehlers-Danlos syndromes)
- Cardiovascular: Aortic root dilatation (>80% Marfan patients)
- Ophthalmologic: Lens dislocation (60% Marfan patients)
- Orthopedic: Joint hypermobility, scoliosis (>70% patients)
- Pulmonary: Spontaneous pneumothorax (5-10% annually)
- Aortic dissection risk: 1-2% per year if root >5cm
- Pregnancy complications: 10x increased risk aortic dissection
-
Chromosomal Disorders (Multi-system involvement universal)
- Down syndrome: Cardiac (50%), GI (12%), endocrine (15%), hematologic (10%)
- Turner syndrome: Cardiac (25%), renal (40%), endocrine (>90%), auditory (>50%)
- 22q11.2 deletion: Cardiac (75%), immune (75%), endocrine (50%), psychiatric (60%)
Integrated Care Model Implementation:
-
Medical Home Coordination (Primary care as central hub)
- Genetic counselor: Initial and ongoing family education
- Subspecialists: Condition-specific expertise and monitoring
- Allied health: Developmental, nutritional, therapeutic support
- Care coordination reduces emergency visits by 40-60%
- Improves quality of life scores by 25-35%
-
Transition to Adult Care (Critical period ages 16-25)
- 50% of genetic disorder patients lost to follow-up during transition
- Adult providers often unfamiliar with pediatric genetic conditions
- Structured transition programs improve continuity by >80%
- Transition readiness assessment at age 14
- Joint pediatric-adult visits ages 16-18
- Adult provider education on condition-specific needs
⭐ Clinical Pearl: Genetic disorders require lifelong surveillance - Turner syndrome patients need cardiac imaging every 5 years, Down syndrome patients require thyroid screening annually, and 22q11.2 deletion patients need psychiatric monitoring throughout adolescence and early adulthood.
Emergency Protocol Development:
-
Metabolic Disorders (Acute decompensation protocols)
- Urea cycle defects: Immediate protein restriction, IV glucose
- Organic acidemias: Carnitine supplementation, bicarbonate for acidosis
- Glycogen storage diseases: Frequent feeding, cornstarch supplementation
- Emergency letter: Specific management instructions for ED staff
- Medical alert bracelet: Critical information for first responders
-
Cardiac Genetic Conditions (Sudden death prevention)
- Long QT syndrome: Beta-blockers, activity restrictions
- Hypertrophic cardiomyopathy: ICD placement, competitive sports restriction
- Marfan syndrome: Aortic monitoring, blood pressure control
- Family screening: 50% risk for first-degree relatives
- Genetic testing: >95% sensitivity for known pathogenic variants
| System | Surveillance Frequency | Key Monitoring | Specialist Involvement | Emergency Considerations |
|---|---|---|---|---|
| Cardiac | Annual-5 years | Echo, ECG, BP | Cardiology, genetics | Arrhythmia, dissection |
| Endocrine | 6 months-2 years | Growth, hormones | Endocrinology | Adrenal crisis, hypoglycemia |
| Neurologic | 6 months-1 year | Development, seizures | Neurology, genetics | Status epilepticus, regression |
| Renal | Annual-2 years | Function, imaging | Nephrology | Acute kidney injury |
| Ophthalmologic | 6 months-2 years | Vision, structure | Ophthalmology | Acute vision loss |
Research Integration enables natural history studies, treatment trials, and outcome registries that continuously improve care standards - patient participation in research networks provides access to cutting-edge therapies while contributing to evidence-based management guidelines for future patients with similar conditions.
🔗 Multi-System Integration: The Genetic Network
🎯 Clinical Mastery Arsenal: The Genetic Medicine Toolkit
📌 Remember: MASTERY - Molecular diagnosis, Anticipatory guidance, Surveillance protocols, Treatment algorithms, Emergency planning, Risk assessment, Yield optimization
Essential Clinical Arsenal:
-
Rapid Recognition Tools (Pattern-based diagnosis)
- Face2Gene AI: >90% accuracy for common syndromes
- Growth chart overlays: Syndrome-specific percentiles
- Developmental milestone checklists: Age-appropriate expectations
- Phenotyping apps: Real-time syndrome suggestions
- Dysmorphology databases: >8,000 documented syndromes
-
Testing Decision Trees (Cost-effective diagnostic pathways)
- Intellectual disability: CMA → Fragile X → Exome (40-50% yield)
- Multiple anomalies: CMA + Karyotype → Gene panels → Exome (45-55% yield)
- Metabolic symptoms: Biochemical → Targeted panels → Exome (60-70% yield)
Quick Reference Protocols:
| Clinical Scenario | First-Line Test | Expected Yield | Turnaround | Next Step if Negative |
|---|---|---|---|---|
| Unexplained ID | Chromosomal microarray | 15-20% | 1-2 weeks | Fragile X testing |
| Multiple birth defects | CMA + karyotype | 20-25% | 1-2 weeks | Targeted gene panels |
| Suspected metabolic | Biochemical studies | 30-40% | 3-7 days | Metabolic gene panel |
| Family history positive | Single gene testing | 50-95% | 1-3 weeks | Cascade family testing |
| Dysmorphic features | Clinical genetics consult | Variable | 2-4 weeks | Syndrome-specific testing |
💡 Master This: Genetic medicine success requires integration of molecular diagnostics, clinical expertise, and family-centered care - the highest-yield approach combines systematic phenotyping, evidence-based testing, and comprehensive counseling to optimize outcomes for patients and families affected by genetic disorders.
🎯 Clinical Mastery Arsenal: The Genetic Medicine Toolkit
Practice Questions: Genetic disorders
Test your understanding with these related questions
A 25-year-old man with a genetic disorder presents for genetic counseling because he is concerned about the risk that any children he has will have the same disease as himself. Specifically, since childhood he has had difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy. He has also had diarrhea and malabsorption requiring enzyme replacement therapy. If his wife comes from a population where 1 in 10,000 people are affected by this same disorder, which of the following best represents the likelihood a child would be affected as well?












