Congenital defects

On this page
🧬 The Congenital Blueprint: Decoding Nature's Developmental Mysteries
Congenital defects arise when development goes awry during precise embryological windows, creating recognizable patterns that span organ systems and demand systematic clinical reasoning. You'll learn to decode the timing and mechanisms behind these malformations, recognize constellation patterns that guide diagnosis, and master the differential frameworks that separate genetic syndromes from teratogenic exposures. This lesson builds your ability to integrate embryology with clinical presentation, equipping you to make evidence-based treatment decisions and achieve rapid pattern recognition at the bedside.
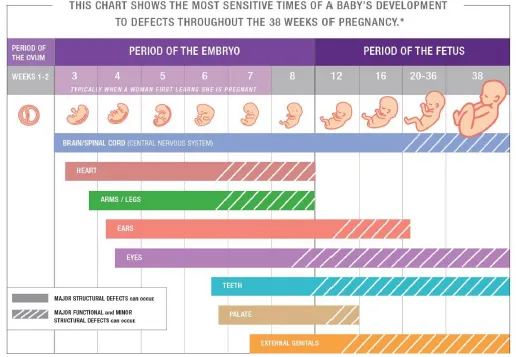
📌 Remember: TORCH-S infections cause congenital defects - Toxoplasmosis, Other (syphilis, varicella), Rubella, Cytomegalovirus, Herpes simplex, Streptococcus agalactiae. Each has specific timing windows: rubella causes cardiac defects if exposure occurs during weeks 2-8, while CMV affects CNS throughout pregnancy.
The embryological foundation reveals why certain defects cluster together. Neural tube closure occurs during days 18-28 post-conception, explaining why folic acid supplementation must begin pre-conceptionally. Cardiac development spans weeks 3-8, creating vulnerability windows for specific lesions.
- Critical Periods by System
- Cardiac: weeks 3-8 (septation, valve formation)
- Neural: weeks 2-4 (tube closure, brain development)
- Neural tube defects: 1 in 1000 births
- Anencephaly: 75% mortality rate
- Limb: weeks 4-8 (bud formation, digit separation)
- Palatal: weeks 6-12 (fusion processes)
⭐ Clinical Pearl: The "all or nothing" principle applies to exposures during weeks 0-2: severe teratogens either cause embryonic death or have no effect. After week 2, organ-specific malformations become possible with dose-dependent severity.
| System | Critical Period | Common Defects | Incidence | Key Features |
|---|---|---|---|---|
| Cardiac | Weeks 3-8 | VSD, ASD, TOF | 8 per 1000 | Cyanosis, murmurs |
| Neural | Weeks 2-4 | Spina bifida, anencephaly | 1 per 1000 | AFP elevation |
| GI | Weeks 4-10 | TEF, omphalocele | 3 per 1000 | Feeding issues |
| GU | Weeks 4-12 | Hypospadias, renal agenesis | 4 per 1000 | Oligohydramnios |
| Skeletal | Weeks 4-8 | Limb defects, clubfoot | 2 per 1000 | Asymmetry |
Understanding teratogenic mechanisms reveals three fundamental pathways: genetic mutations (15-25% of cases), chromosomal abnormalities (5-10%), and environmental exposures (5-10%). The remaining 60-75% represent complex multifactorial inheritance patterns where multiple genes interact with environmental triggers.
Connect these foundational principles through embryological timing to understand how specific defects emerge during critical developmental windows.
🧬 The Congenital Blueprint: Decoding Nature's Developmental Mysteries
⚡ Embryological Timing: The Critical Windows of Vulnerability
📌 Remember: CARDIAC timing - Conotruncal (weeks 6-8), Atrial septation (week 5), Right ventricle (week 7), Ductus arteriosus (week 6), Interventricular septum (week 7), Aortic arch (weeks 4-6), Coronary arteries (week 7). Each structure's timing explains specific defect patterns.
Neural tube closure follows a precise zipper-like mechanism starting at day 18 and completing by day 28. Failure at different sites produces distinct defects: cranial closure defects cause anencephaly (75% lethal), while caudal defects produce spina bifida with variable neurological impact.
- Neural Tube Closure Sequence
- Day 18: Initial closure at cervical level
- Day 20: Cranial neuropore begins closing
- Failure → anencephaly, encephalocele
- Incidence: 1 in 4,000 births
- Day 24: Cranial closure complete
- Day 28: Caudal neuropore closes
- Failure → spina bifida, lipomyelomeningocele
- L4-S1 most common location (85% of cases)
⭐ Clinical Pearl: Folic acid reduces neural tube defects by 50-70% when started pre-conceptionally. The mechanism involves one-carbon metabolism essential for DNA synthesis during rapid cell division. Standard dose: 400 mcg daily; high-risk patients need 4-5 mg daily.
Cardiac development involves complex looping, septation, and valve formation processes. The primitive heart tube begins beating at day 22, with rightward looping occurring during week 4. Septation defects arise from failures in endocardial cushion development or neural crest cell migration.
| Cardiac Structure | Development Week | Defect if Disrupted | Clinical Significance |
|---|---|---|---|
| Atrial septum | Week 5 | ASD (secundum) | 25% spontaneous closure |
| Ventricular septum | Week 7 | VSD | 90% muscular close spontaneously |
| Aortic arch | Weeks 4-6 | Coarctation, hypoplasia | HTN in upper extremities |
| Truncal septation | Weeks 6-8 | TOF, TGA | Cyanosis from birth |
| Valve formation | Weeks 5-9 | Stenosis, atresia | CHF in neonatal period |
Limb development follows proximal-to-distal and anterior-to-posterior gradients controlled by HOX genes and signaling centers. The apical ectodermal ridge (AER) maintains distal growth, while the zone of polarizing activity (ZPA) determines digit identity. Disruption during weeks 4-8 produces reduction defects or polydactyly.
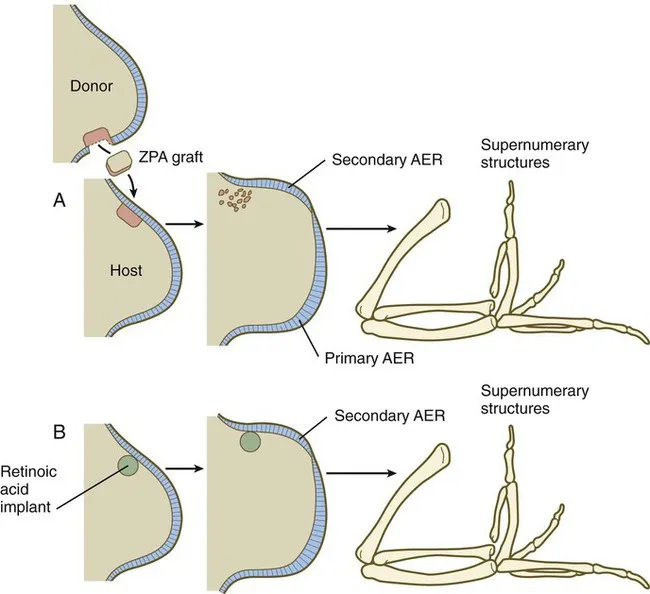
Connect these timing principles through pattern recognition to understand how developmental windows create predictable defect constellations.
⚡ Embryological Timing: The Critical Windows of Vulnerability
🎯 Pattern Recognition: The Defect Constellation Map
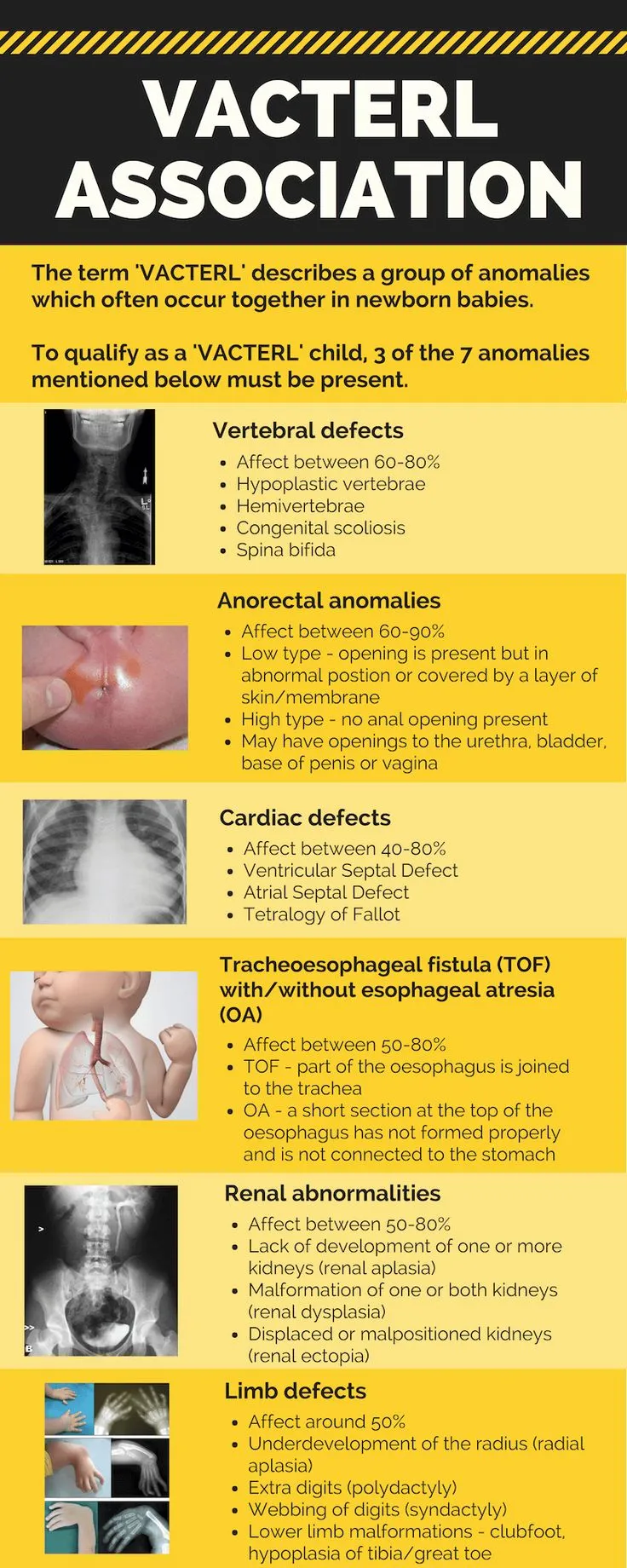
- VACTERL Association Pattern Recognition
- Vertebral defects (70% of cases)
- Hemivertebrae, fusion anomalies
- Scoliosis develops in 60%
- Anal atresia (55% of cases)
- High vs low lesions determine surgical approach
- Cardiac defects (75% of cases)
- VSD most common (40% of cardiac cases)
- Tracheoesophageal fistula (70% of cases)
- Type C (distal TEF) in 85%
- Renal anomalies (60% of cases)
- Unilateral agenesis, dysplasia
- Limb defects (70% of cases)
- Radial ray defects, thumb hypoplasia
- Vertebral defects (70% of cases)
📌 Remember: CHARGE syndrome features - Coloboma, Heart defects, Atresia choanae, Retarded growth, Genital hypoplasia, Ear anomalies. CHD7 gene mutations cause 90% of cases, with cardiac defects in 75-85% requiring surgical intervention.
Chromosomal syndromes create recognizable facial gestalt patterns combined with specific organ defects. Down syndrome (trisomy 21) affects 1 in 700 births, with cardiac defects in 40-50% of cases, predominantly endocardial cushion defects and VSDs.
⭐ Clinical Pearl: 22q11.2 deletion syndrome (DiGeorge) presents with cardiac defects (74%), palatal abnormalities (69%), learning difficulties (90%), and immune deficiency (77%). Conotruncal heart defects (TOF, truncus arteriosus) are characteristic.
Sequence vs syndrome distinction guides diagnostic approach. Potter sequence results from oligohydramnios of any cause, producing characteristic facies, limb deformities, and pulmonary hypoplasia. The underlying cause (renal agenesis, PROM, etc.) determines recurrence risk.
| Pattern Type | Definition | Example | Recurrence Risk |
|---|---|---|---|
| Syndrome | Multiple anomalies, single cause | Down syndrome | 1% (translocation 10-15%) |
| Sequence | Single defect → cascade | Potter sequence | Depends on primary cause |
| Association | Non-random clustering | VACTERL | 1% empiric risk |
| Complex | Multiple developmental fields | Holoprosencephaly | 6% (isolated), 25% (familial) |
| Deformation | Mechanical forces | Clubfoot | 2-5% |
💡 Master This: The "face predicts the brain" principle in holoprosencephaly: normal face suggests lobar type with better prognosis, while severe midline facial defects indicate alobar type with profound disability or lethality.
Laterality defects (heterotaxy) disrupt left-right axis determination, causing complex cardiac defects with abnormal visceral situs. Right isomerism associates with asplenia and cyanotic heart disease, while left isomerism links to polysplenia and heart block.
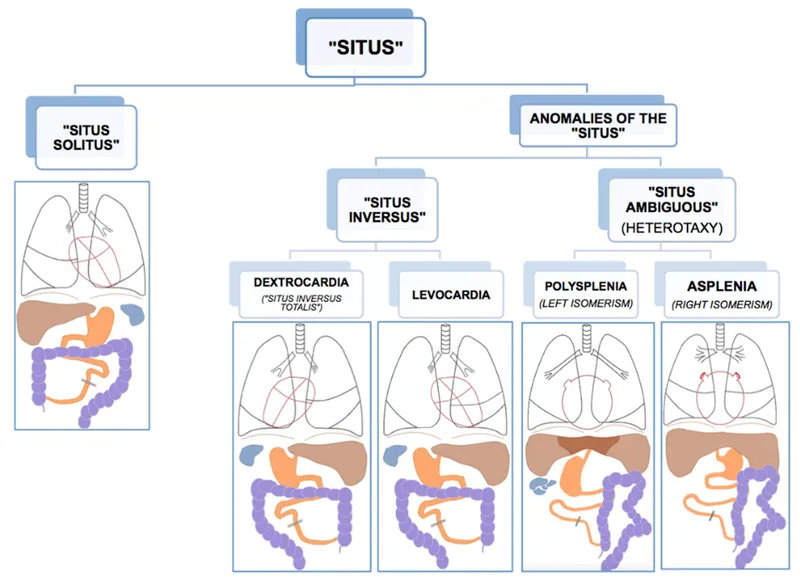
Connect these recognition patterns through systematic analysis to build differential frameworks for complex presentations.
🎯 Pattern Recognition: The Defect Constellation Map
🔬 Systematic Analysis: The Differential Architecture
- Major Anomaly Systematic Analysis
- Life-threatening: Require immediate intervention
- Diaphragmatic hernia: 40-60% mortality
- Hypoplastic left heart: 95% mortality without surgery
- Anencephaly: 100% lethal
- Functional impairment: Affect quality of life
- Cleft palate: Speech, feeding difficulties
- Spina bifida: Neurological deficits (80% have hydrocephalus)
- Congenital heart disease: Exercise intolerance, cyanosis
- Cosmetic significance: Require reconstruction
- Cleft lip: Facial asymmetry, dental problems
- Limb reduction defects: Functional limitations
- Life-threatening: Require immediate intervention
📌 Remember: 3-2-1 Rule for multiple anomalies - 3+ minor anomalies suggest underlying syndrome (90% likelihood), 2 minor + 1 major anomaly warrants genetic evaluation (70% syndrome risk), 1 isolated major anomaly has <10% syndrome association.
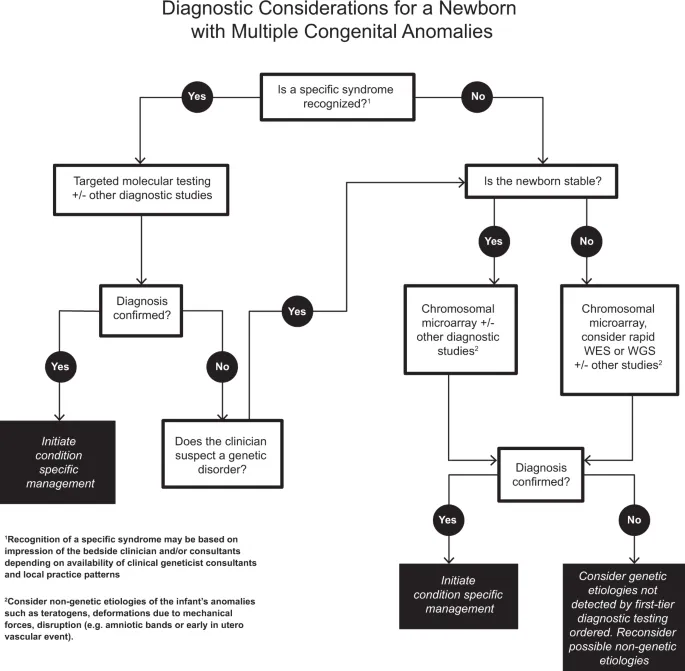
Chromosomal vs single-gene discrimination relies on specific pattern recognition. Chromosomal disorders typically present with multiple system involvement, intellectual disability, and growth retardation, while single-gene defects often show organ-specific or metabolic patterns.
⭐ Clinical Pearl: Microdeletion syndromes bridge chromosomal and single-gene patterns. 22q11.2 deletion affects 1 in 4,000 births, presenting with variable expressivity: cardiac defects (74%), palatal anomalies (69%), immune deficiency (77%), psychiatric disorders (25% develop schizophrenia).
Environmental vs genetic causation analysis requires detailed exposure history and family pedigree analysis. Teratogenic exposures typically produce specific patterns during defined gestational windows, while genetic causes show familial clustering or consanguinity.
| Discriminator | Environmental | Genetic | Mixed/Unknown |
|---|---|---|---|
| Family history | Negative | Positive (25% recurrence) | Variable |
| Exposure timing | Critical period | Any time | Variable |
| Pattern specificity | Exposure-specific | Gene-specific | Non-specific |
| Recurrence risk | Low (<5%) | High (25-50%) | Moderate (2-10%) |
| Associated features | Exposure-related | Syndrome-specific | Variable |
💡 Master This: Amniotic band sequence exemplifies disruption: asymmetric, random defects including limb amputations, facial clefts, and body wall defects. Recurrence risk is <1% because the amniotic rupture is typically sporadic.
Isolated vs syndromic determination affects genetic counseling and surveillance protocols. Isolated defects have lower recurrence risk (2-5%) and limited surveillance needs, while syndromic presentations require comprehensive evaluation and long-term monitoring.
- Syndromic Red Flags
- Multiple system involvement (≥2 unrelated systems)
- Growth parameters: <3rd percentile height/weight
- Microcephaly: <2 SD below mean
- Macrocephaly: >2 SD above mean
- Developmental delay: >25% delay in ≥2 domains
- Dysmorphic features: ≥3 minor anomalies
- Facial asymmetry, ear malformations
- Digital anomalies, skin findings
Connect these analytical frameworks through evidence-based treatment algorithms to guide optimal management decisions.
🔬 Systematic Analysis: The Differential Architecture
⚕️ Treatment Algorithms: The Evidence-Based Decision Matrix
Surgical timing decisions balance anatomical readiness, physiological stability, and developmental windows. Emergency repairs address life-threatening conditions, while elective procedures optimize timing for best outcomes and minimal complications.
📌 Remember: EMERGENCY surgical priorities - Esophageal atresia (24-48 hours), Myelomeningocele (24-72 hours), Encephalocele (immediate), Rupture risk (omphalocele >5cm), Gastroschisis (6-12 hours), Extrophy (48-72 hours), Necrotizing enterocolitis (immediate), Congenital diaphragmatic hernia (after stabilization), Yanked bowel (volvulus - immediate).
Cardiac defect management follows physiology-based algorithms. Cyanotic lesions require early intervention to prevent irreversible pulmonary vascular disease, while acyanotic defects allow selective timing based on symptoms and growth patterns.
- Cardiac Surgery Timing Protocols
- Immediate (<24 hours)
- Hypoplastic left heart: Norwood stage 1
- Transposition: Balloon atrial septostomy
- Total anomalous pulmonary venous return: Obstructed type
- Early (<6 months)
- Tetralogy of Fallot: Symptomatic or severe cyanosis
- Truncus arteriosus: Before pulmonary vascular disease
- Large VSD: CHF or failure to thrive
- Elective (6 months - 2 years)
- ASD: Significant shunt (Qp:Qs >1.5)
- Coarctation: Gradient >20 mmHg
- Pulmonary stenosis: Gradient >50 mmHg
- Immediate (<24 hours)
⭐ Clinical Pearl: Pulmonary vascular resistance drops to adult levels by 6-8 weeks, creating the optimal window for left-to-right shunt repairs. Delays beyond 2 years risk irreversible pulmonary hypertension (Eisenmenger syndrome).
Neural tube defect management prioritizes neurological preservation and infection prevention. Myelomeningocele requires closure within 72 hours to minimize infection risk and neurological deterioration. Hydrocephalus develops in 80-90%, requiring ventriculoperitoneal shunt placement.
| Defect Type | Timing | Success Rate | Complications | Long-term Outcomes |
|---|---|---|---|---|
| Myelomeningocele | <72 hours | 95% closure | 15% infection | 85% ambulatory (L4-L5) |
| Encephalocele | Immediate | 90% survival | 20% hydrocephalus | Variable cognitive |
| Craniosynostosis | 6-12 months | 95% success | 5% re-stenosis | Normal development |
| Chiari II | Symptomatic | 80% improvement | 10% mortality | Stable function |
| Tethered cord | Progressive symptoms | 85% stabilization | 5% neurological loss | Prevents deterioration |
💡 Master This: Gross classification for esophageal atresia guides surgical approach: Type A (8% - pure atresia), Type B (1% - proximal TEF), Type C (85% - distal TEF), Type D (1% - double TEF), Type E (4% - H-type TEF). Type C has best outcomes with >95% survival.
Multidisciplinary care coordination optimizes long-term outcomes through specialized teams. Spina bifida clinics integrate neurosurgery, urology, orthopedics, and developmental pediatrics to address complex needs and prevent complications.
Connect these treatment principles through advanced integration concepts to understand how multiple systems interact in complex presentations.
⚕️ Treatment Algorithms: The Evidence-Based Decision Matrix
🌐 Advanced Integration: The Multi-System Orchestration
Developmental field interactions explain why seemingly unrelated defects cluster together. The neural crest contributes to cardiac, craniofacial, and enteric nervous system development, explaining why 22q11.2 deletion syndrome produces conotruncal heart defects, palatal abnormalities, and Hirschsprung disease in the same patient.
- Neural Crest Migration Pathways
- Cranial neural crest (weeks 4-6)
- Facial bones, dental structures
- Cranial nerves V, VII, IX, X
- Defects: Treacher Collins, CHARGE syndrome
- Cardiac neural crest (weeks 3-5)
- Aortic arch arteries, outflow tract septation
- Parasympathetic innervation
- Defects: DiGeorge sequence, conotruncal anomalies
- Trunk neural crest (weeks 4-7)
- Sympathetic ganglia, adrenal medulla
- Enteric nervous system
- Defects: Hirschsprung disease, neuroblastoma
- Cranial neural crest (weeks 4-6)
📌 Remember: CATCH-22 features of 22q11.2 deletion - Cardiac defects (74%), Abnormal facies (90%), Thymic hypoplasia (77%), Cleft palate (69%), Hypocalcemia (50%), 22q11.2 deletion. TBX1 gene haploinsufficiency affects neural crest migration.
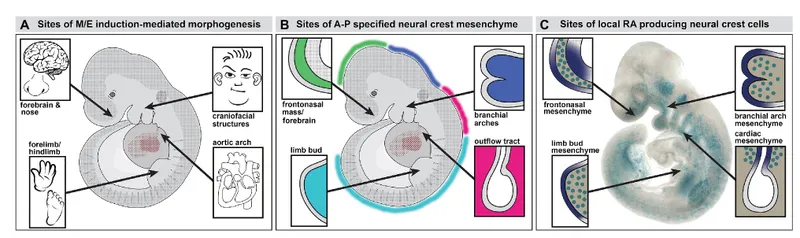
Hemodynamic interactions in complex cardiac defects create physiological interdependencies where surgical interventions must consider systemic effects. Single ventricle physiology requires staged palliation that balances systemic and pulmonary circulation through calculated resistance modifications.
⭐ Clinical Pearl: Fontan circulation creates non-pulsatile pulmonary flow with elevated systemic venous pressure (12-15 mmHg). Long-term complications include protein-losing enteropathy (5-10%), arrhythmias (50% by age 20), and thromboembolism (20% lifetime risk).
Genetic modifier effects explain phenotypic variability within the same syndrome. CHARGE syndrome patients with CHD7 mutations show variable expressivity: some have life-threatening cardiac defects while others have mild features. Modifier genes and environmental factors influence penetrance and severity.
| Syndrome | Primary Gene | Penetrance | Variable Features | Modifier Factors |
|---|---|---|---|---|
| CHARGE | CHD7 | 90% | Cardiac (75-85%), choanal atresia (65%) | Genetic background |
| 22q11.2 deletion | TBX1 | >95% | Cardiac (74%), palatal (69%) | Deletion size |
| Noonan | PTPN11 | 85% | Cardiac (80%), short stature (70%) | Mutation type |
| Marfan | FBN1 | >90% | Aortic dilation (70-80%), lens dislocation (60%) | Mutation location |
| Williams | ELN deletion | >95% | Supravalvar AS (75%), hypercalcemia (15%) | Deletion boundaries |
💡 Master This: Fetal programming concept explains how intrauterine environment influences adult disease risk. Low birth weight (<2500g) associates with increased risk of cardiovascular disease (2-fold), diabetes (3-fold), and hypertension (1.5-fold) in adulthood through epigenetic mechanisms.
Stem cell niche disruption affects regenerative capacity and repair mechanisms. Congenital defects often involve stem cell populations, explaining why some tissues show limited regenerative potential while others demonstrate remarkable plasticity. Understanding these mechanisms guides tissue engineering approaches.
- Regenerative Potential by Tissue Type
- High regenerative capacity
- Liver: 75% regeneration possible
- Bone: Complete healing with proper alignment
- Skin: Excellent wound healing capacity
- Moderate regenerative capacity
- Cardiac muscle: Limited cardiomyocyte renewal (1%/year)
- Renal tissue: Compensatory hypertrophy
- Lung: Alveolar regeneration possible
- Limited regenerative capacity
- Central nervous system: Minimal neuron replacement
- Retina: No photoreceptor regeneration
- Inner ear: No hair cell replacement
- High regenerative capacity
Connect these integration principles through rapid mastery frameworks to build comprehensive clinical competence.
🌐 Advanced Integration: The Multi-System Orchestration
🎯 Clinical Mastery Arsenal: The Rapid Recognition Toolkit
The 5-Minute Congenital Assessment Protocol provides structured evaluation for any suspected congenital defect presentation. This systematic approach ensures comprehensive evaluation while prioritizing life-threatening conditions.
📌 Remember: ABCDE congenital emergency assessment - Airway (choanal atresia, micrognathia), Breathing (diaphragmatic hernia, lung hypoplasia), Circulation (ductal-dependent lesions), Disability (neural tube defects), Exposure (gastroschisis, omphalocele). Address in priority order with immediate interventions.
- Rapid Assessment Framework
- Growth parameters (<2 minutes)
- Weight: <10th percentile suggests syndrome
- Length: <3rd percentile indicates skeletal dysplasia
- Head circumference: >97th or <3rd percentile abnormal
- Dysmorphology scan (<3 minutes)
- Facial gestalt: Recognizable pattern?
- Digital examination: Polydactyly, syndactyly, clinodactyly
- Skin findings: Café-au-lait spots, hypopigmentation
- System-specific red flags (<2 minutes)
- Cardiac: Murmur, cyanosis, poor feeding
- Neurological: Hypotonia, seizures, developmental delay
- Renal: Oligohydramnios, Potter facies
- Growth parameters (<2 minutes)
| Assessment Component | Time Allocation | Key Findings | Action Threshold |
|---|---|---|---|
| Vital signs | 30 seconds | O2 sat <95%, HR >180 | Immediate intervention |
| Growth parameters | 60 seconds | <3rd percentile any parameter | Genetic consultation |
| Dysmorphic features | 90 seconds | ≥3 minor anomalies | Syndrome evaluation |
| System examination | 120 seconds | Major anomaly identified | Specialist referral |
| Family history | 60 seconds | Consanguinity, recurrence | Genetic counseling |
Essential Numbers Arsenal provides instant access to critical thresholds and normal values essential for rapid decision-making in congenital defect management.
💡 Master This: Recurrence risk memory matrix - Chromosomal: 1% (balanced translocation 10-15%), Single gene: 25% (AR), 50% (AD), Multifactorial: 2-5%, Teratogenic: <1% (if exposure avoided), Unknown: 3-4% empiric risk.
Pattern Recognition Drill Framework builds instant recognition of high-yield congenital defect presentations through systematic exposure to classic patterns.
- Cardiac Pattern Drills
- Cyanotic newborn + decreased pulmonary markings = Tetralogy of Fallot
- CHF + continuous murmur = PDA or arteriovenous malformation
- Differential cyanosis = Coarctation with right-to-left ductal shunt
- Cardiomegaly + poor feeding = Large left-to-right shunt
Clinical Decision Support Tools provide evidence-based guidance for complex management decisions, integrating multiple variables into actionable recommendations.
| Clinical Scenario | Decision Factors | Recommendation | Evidence Level |
|---|---|---|---|
| VSD >5mm at 6 months | CHF symptoms, FTT | Surgical closure | Class I |
| ASD with Qp:Qs >1.5 | Age >4 years, symptoms | Device closure | Class I |
| Coarctation with gradient >20 mmHg | HTN, LV dysfunction | Balloon angioplasty | Class IIa |
| TOF with severe cyanosis | Hypercyanotic spells | Complete repair | Class I |
| MMC diagnosed prenatally | Gestational age >26 weeks | Fetal surgery consideration | Class IIb |
🎯 Clinical Mastery Arsenal: The Rapid Recognition Toolkit
Practice Questions: Congenital defects
Test your understanding with these related questions
A 1-year-old girl born to a 40-year-old woman is undergoing an examination by a pediatric resident in the hospital. The pregnancy was uneventful and there were no complications during the delivery. The physical examination reveals midface hypoplasia with a flat nasal bridge and upslanting palpebral fissures. She has a small mouth and chest auscultation reveals a blowing holosystolic murmur that is heard best along the sternal border. The family history is unremarkable. A karyotype analysis is ordered because the resident suspects a numerical chromosomal disorder. Which of the following phenomena leads to the infant’s condition?













