Renal pathology

On this page
🔬 The Renal Pathology Battlefield: Where Kidneys Fight for Survival
Your kidneys filter 180 liters daily, but when disease strikes these silent sentinels, the entire body pays the price. You'll master how glomerular barriers break down, tubules fail, and systemic diseases leave telltale renal signatures. Through pattern recognition and diagnostic reasoning, you'll learn to decode biopsy findings, distinguish look-alike syndromes, and deploy targeted therapies that can mean the difference between dialysis and recovery.
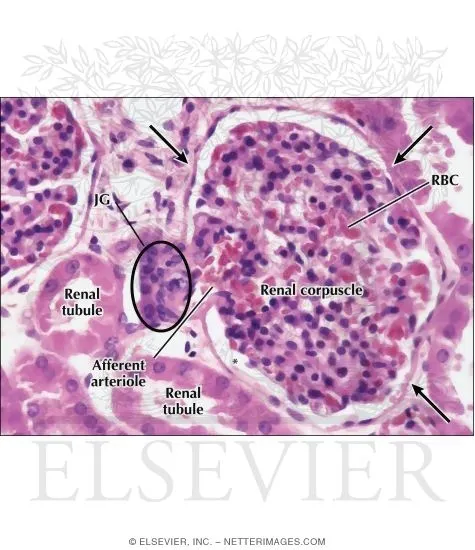
The kidney's 1.2 million nephrons each function as independent filtration units, processing 180 liters of filtrate daily while maintaining precise electrolyte balance. When pathology strikes, understanding which anatomical compartment suffers damage - glomerular, tubular, interstitial, or vascular - determines both clinical presentation and therapeutic approach.
📌 Remember: GLINT - Glomerular (proteinuria), Loop/tubular (electrolyte disorders), Interstitial (inflammation), Nephrovascular (hypertension), Tumors (masses). Each compartment produces distinct clinical signatures with specific laboratory patterns.
- Glomerular Diseases (15-20% of ESRD cases)
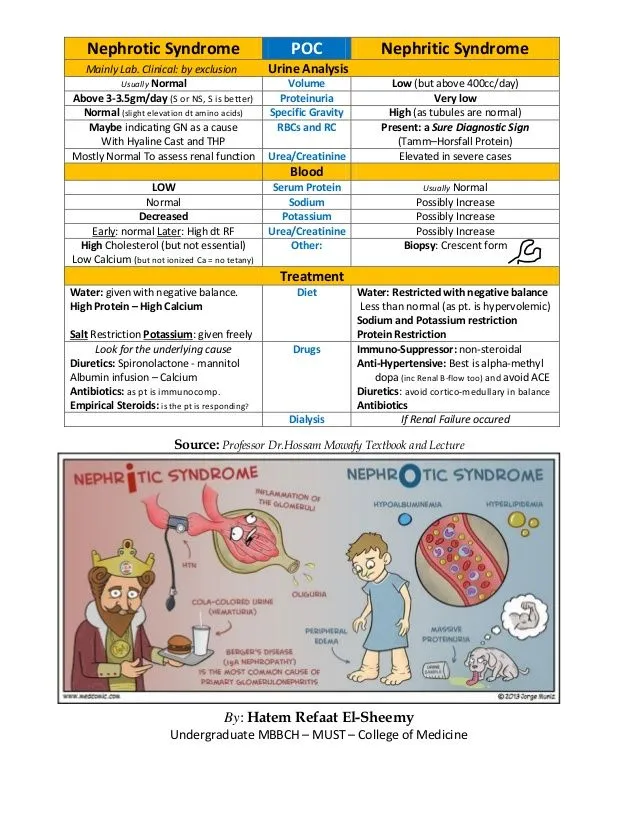
- Nephrotic syndrome: >3.5g/day proteinuria with hypoalbuminemia
- Nephritic syndrome: Hematuria with RBC casts and hypertension
- Acute presentations: <3 months duration with rapid decline
- Chronic presentations: >3 months with progressive scarring
- Tubular Disorders (25-30% of acute kidney injury)
- Acute tubular necrosis: 50-60% of hospital-acquired AKI
- Tubulointerstitial nephritis: 10-15% of chronic kidney disease
- Drug-induced: 70% from NSAIDs, antibiotics, PPIs
- Infectious: 20% bacterial, viral, or fungal etiology
| Pathology Type | Primary Location | Key Laboratory Finding | Timeline | Reversibility | ESRD Risk |
|---|---|---|---|---|---|
| Acute GN | Glomerulus | RBC casts, proteinuria | Days-weeks | 80-90% | <5% |
| RPGN | Glomerulus | Crescents >50% | Weeks | 30-40% | 60-70% |
| ATN | Tubules | Muddy brown casts | Hours-days | 90-95% | <2% |
| AIN | Interstitium | Eosinophiluria, WBC casts | Days-weeks | 70-80% | 10-15% |
| Chronic GN | Glomerulus | Progressive proteinuria | Months-years | <10% | 80-90% |
The pathophysiology of renal disease follows predictable patterns based on anatomical involvement. Glomerular diseases primarily affect filtration barrier integrity, leading to proteinuria and hematuria. Tubular disorders disrupt reabsorption and secretion, causing electrolyte imbalances and concentrating defects. Interstitial inflammation triggers fibrosis and progressive nephron loss.
💡 Master This: Every renal biopsy interpretation requires systematic evaluation - glomerular cellularity (<3 cells/capillary loop normal), tubular atrophy (<10% normal), interstitial fibrosis (<5% normal), and vascular sclerosis grading. These quantitative thresholds predict 5-year survival with 85% accuracy.
Understanding the temporal evolution of renal pathology enables early intervention. Acute processes show rapid onset within days to weeks, often with reversible injury if treated promptly. Chronic diseases develop over months to years, with irreversible structural changes including glomerulosclerosis, tubular atrophy, and interstitial fibrosis.
Connect this foundational understanding through detailed pathophysiological mechanisms to understand how specific disease processes target different nephron compartments.
🔬 The Renal Pathology Battlefield: Where Kidneys Fight for Survival
⚡ Glomerular Warfare: The Filtration Barrier Under Siege
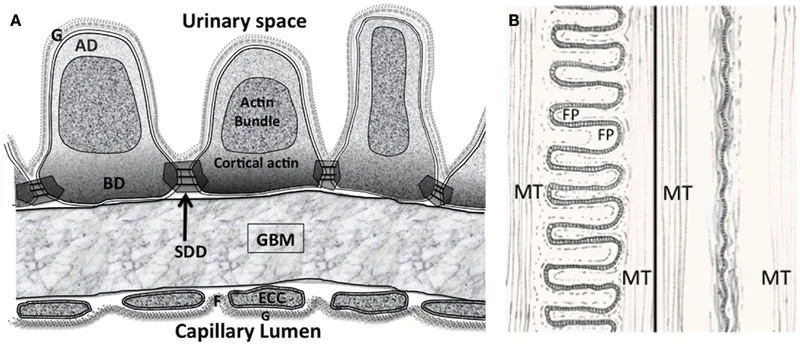
The glomerular filtration barrier consists of three critical components: fenestrated endothelium (70-100nm pores), glomerular basement membrane (300-350nm thick), and podocyte foot processes with 25-60nm slit diaphragms. Each layer contributes specific filtration properties - size selectivity, charge selectivity, and dynamic regulation.
📌 Remember: CHARGE - Capillary endothelium (size barrier), Heparan sulfate (charge barrier), Albumin exclusion, Renal basement membrane (structural), Glycocalyx protection, Epithelial slits (final filter). This sequence explains why albumin (3.6nm radius) normally shows <0.2% filtration despite size compatibility.
- Nephrotic Syndrome Mechanisms (>3.5g/day proteinuria)
- Podocyte injury: Foot process effacement in >80% of cases
- Basement membrane defects: Spike-and-dome deposits in membranous nephropathy
- Minimal change disease: Normal light microscopy, 100% foot process effacement
- FSGS: Segmental sclerosis affecting <50% of glomerular tuft initially
- Charge barrier loss: Heparan sulfate depletion reduces negative charge repulsion
- Nephritic Syndrome Mechanisms (Hematuria with RBC casts)
- Endocapillary proliferation: >3 cells per capillary loop
- Basement membrane breaks: Subendothelial immune deposits
- Post-infectious GN: Subepithelial humps in 60-70% of cases
- Anti-GBM disease: Linear IgG deposition with crescents in >50%
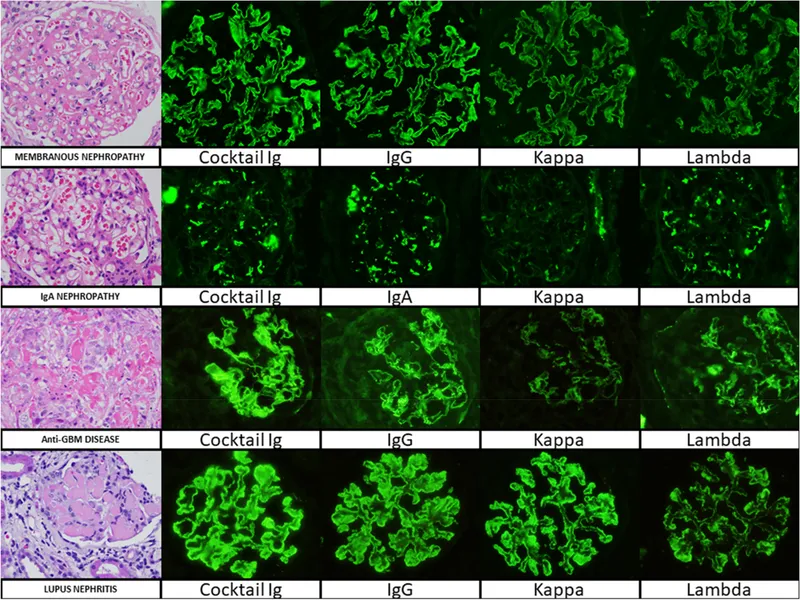
The immune-mediated mechanisms dominate glomerular pathology, with three distinct patterns: immune complex deposition (70% of cases), anti-GBM antibodies (5%), and pauci-immune ANCA-associated disease (25%). Each pattern produces characteristic histological and clinical signatures.
| Disease Pattern | IF Staining | EM Deposits | Clinical Syndrome | Progression Rate | Treatment Response |
|---|---|---|---|---|---|
| Minimal Change | Negative | None | Pure nephrotic | >95% remission | 90% steroid responsive |
| Membranous | Granular IgG | Subepithelial | Nephrotic + thrombosis | 30% spontaneous remission | 60% immunosuppression |
| Post-infectious | Granular C3 | Subepithelial humps | Nephritic | 95% recovery | Supportive care |
| Anti-GBM | Linear IgG | GBM thickening | RPGN + pulmonary | <10% recovery | Plasmapheresis urgent |
| ANCA | Negative/minimal | None | Necrotizing GN | 70% remission | Cyclophosphamide |
The complement cascade plays crucial roles in glomerular injury, with alternative pathway activation in C3 glomerulopathy and classical pathway involvement in lupus nephritis. Understanding complement patterns guides both diagnosis and targeted therapy selection.
💡 Master This: Glomerular crescents represent irreversible injury when >50% of glomeruli are affected, with <20% chance of renal recovery. Early recognition within 6-8 weeks of onset allows aggressive immunosuppression to prevent ESRD in 60-70% of cases.
The temporal progression of glomerular disease follows predictable stages: acute injury (days-weeks), subacute inflammation (weeks-months), and chronic sclerosis (months-years). Intervention during acute phases offers maximal therapeutic benefit with reversibility potential.
Connect this glomerular foundation through tubular and interstitial mechanisms to understand how different nephron compartments interact during disease progression.
⚡ Glomerular Warfare: The Filtration Barrier Under Siege
🔧 Tubular Sabotage: When the Kidney's Plumbing Fails
The nephron's four distinct tubular segments each perform specialized functions: proximal tubule (65% sodium reabsorption), loop of Henle (25% sodium, concentrating mechanism), distal convoluted tubule (10% sodium, calcium regulation), and collecting duct (5% sodium, acid-base balance).
📌 Remember: PROXIMAL - Protein reabsorption (99%), Renal gluconeogenesis, Organic anion secretion, Xenobiotic elimination, Isotonic reabsorption, Mitochondria-rich, Acidification (80% bicarbonate), Large volume (65% filtrate).
- Acute Tubular Necrosis Mechanisms (50-60% of hospital AKI)
- Ischemic ATN: >30 minutes hypoperfusion causes ATP depletion
- Nephrotoxic ATN: Direct cellular toxicity from drugs, contrast, pigments
- Aminoglycosides: Proximal tubule accumulation, 5-10 days onset
- Contrast agents: Osmotic injury plus vasoconstriction, 24-48 hours onset
- Rhabdomyolysis: Myoglobin precipitation at pH <5.6, heme toxicity
- Tubulointerstitial Nephritis (10-15% of CKD)
- Acute interstitial nephritis: Allergic reaction in 70%, eosinophiluria in 80%
- Chronic interstitial nephritis: Progressive fibrosis with tubular atrophy
- Drug-induced: NSAIDs (40%), antibiotics (25%), PPIs (15%)
- Infectious: Bacterial (60%), viral (30%), fungal (10%)
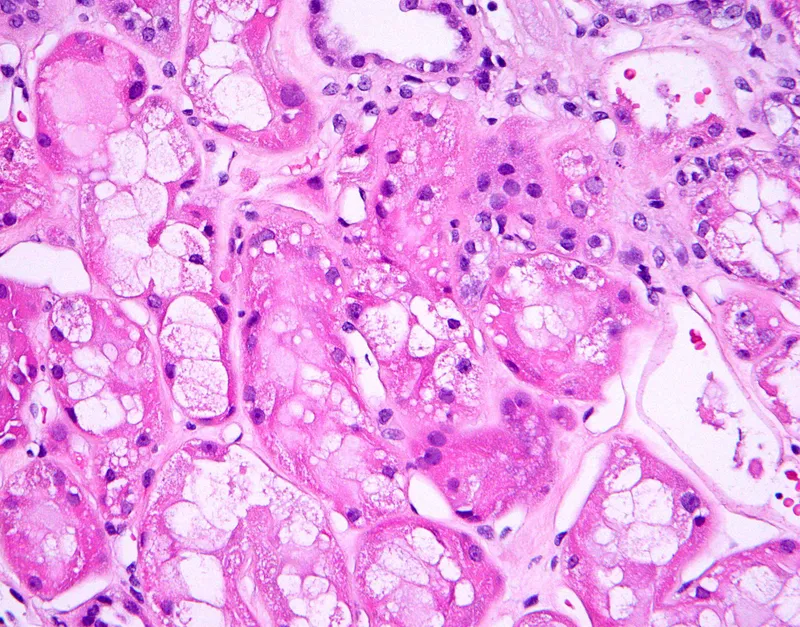
The pathophysiological cascade in ATN follows predictable stages: initiation (hours), extension (days), maintenance (days-weeks), and recovery (weeks-months). Each phase shows distinct cellular and molecular changes that guide therapeutic timing.
⭐ Clinical Pearl: Fractional excretion of sodium (FENa) distinguishes prerenal azotemia (<1%) from ATN (>2%) with 95% accuracy, but fractional excretion of urea (FEUrea <35%) provides better discrimination in patients receiving diuretics.
| Tubular Disorder | Primary Location | Key Urinalysis Finding | Electrolyte Pattern | Recovery Time | Mortality Risk |
|---|---|---|---|---|---|
| Ischemic ATN | Proximal + thick limb | Muddy brown casts | Hyperkalemia | 2-6 weeks | 20-30% |
| Nephrotoxic ATN | Proximal tubule | Granular casts | Normal K initially | 1-3 weeks | 10-15% |
| Acute AIN | Interstitium | WBC casts, eosinophils | Hyperkalemia, acidosis | 2-8 weeks | <5% |
| Chronic AIN | Interstitium + tubules | Bland sediment | Salt wasting | Irreversible | Variable |
| Contrast nephropathy | Proximal tubule | Granular casts | Transient oliguria | 3-7 days | 5-10% |
💡 Master This: Urinary biomarkers predict ATN severity and recovery - NGAL rises within 2 hours of injury (normal <150 ng/mL), KIM-1 indicates proximal tubule damage, and IL-18 correlates with dialysis requirement when >100 pg/mL.
The molecular mechanisms of tubular injury involve oxidative stress, inflammatory cascades, and cell cycle arrest. Understanding these pathways enables targeted interventions during the extension phase when injury amplification occurs.
- Biomarker Timeline for ATN diagnosis
- NGAL: Rises 2-6 hours, peaks 24 hours, predicts dialysis need
- Cystatin C: Increases 12-24 hours, less affected by muscle mass
- KIM-1: Specific for proximal tubule, elevated 24-48 hours
- Creatinine: Delayed rise 24-72 hours, insensitive to early injury
Connect this tubular understanding through pattern recognition frameworks to understand how different injury patterns create distinct clinical presentations.
🔧 Tubular Sabotage: When the Kidney's Plumbing Fails
🔍 Pattern Recognition Mastery: Decoding the Renal Detective Story
The urinalysis pattern provides the foundation for all renal diagnosis, with specific cast types indicating precise anatomical injury locations. RBC casts confirm glomerular bleeding, WBC casts suggest interstitial inflammation, and granular casts indicate tubular injury.
📌 Remember: CASTS - Cellular (glomerular/interstitial), Albumin (nephrotic), Sediment timing (<2 hours), Tubular (ATN/AIN), Specific gravity (>1.018 concentrating ability). Fresh specimens within 2 hours show 95% diagnostic accuracy versus <60% when delayed.
- Nephrotic Pattern Recognition (>3.5g/day proteinuria)
- Classic triad: Proteinuria + hypoalbuminemia + edema
- Extended pentad: Add hyperlipidemia + hypercoagulability
- Minimal change: Children (80%), steroid responsive (90%)
- FSGS: Adults (40%), steroid resistant (70%)
- Membranous: Adults (60%), thrombosis risk (25%)
- Nephritic Pattern Recognition (Hematuria + RBC casts)
- Acute nephritic: Sudden onset, hypertension, oliguria
- RPGN pattern: Creatinine doubling in <3 months
- Post-infectious: Children, recent strep (2-6 weeks)
- IgA nephropathy: Young adults, concurrent URI
- Anti-GBM: Pulmonary symptoms (60%), linear IF
The temporal pattern of presentation provides crucial diagnostic clues - acute onset (<days) suggests post-infectious or drug-induced causes, while insidious progression (months-years) indicates primary glomerular disease or systemic conditions.
⭐ Clinical Pearl: Protein selectivity index distinguishes minimal change disease (<0.1) from other causes (>0.2) with 90% accuracy, calculated as (IgG clearance/albumin clearance) × 100. This test predicts steroid responsiveness in 85% of cases.
| Clinical Pattern | Proteinuria | Hematuria | Hypertension | Renal Function | Most Likely Diagnosis |
|---|---|---|---|---|---|
| Pure nephrotic | >3.5g/day | Absent/minimal | <50% | Normal/mild ↓ | Minimal change |
| Nephrotic + hematuria | >3.5g/day | Microscopic | 60-70% | Mild-moderate ↓ | FSGS/Membranous |
| Acute nephritic | 1-3g/day | Gross | >90% | Acute ↓ | Post-infectious GN |
| RPGN | Variable | RBC casts | >80% | Rapid ↓ | Crescentic GN |
| Chronic nephritic | 1-2g/day | Persistent | 70-80% | Progressive ↓ | IgA nephropathy |
💡 Master This: Complement levels predict disease activity and guide monitoring - low C3 (<90 mg/dL) with normal C4 suggests alternative pathway activation in post-infectious GN, while low C3 and C4 indicates classical pathway involvement in lupus nephritis.
The histological pattern correlation requires understanding light microscopy, immunofluorescence, and electron microscopy findings. Each technique provides complementary information essential for definitive diagnosis and treatment selection.
- Immunofluorescence Patterns
- Linear IgG: Anti-GBM disease (>95% specific)
- Granular IgG/C3: Immune complex deposition (membranous, lupus)
- Mesangial IgA: IgA nephropathy (>90% sensitive)
- Negative/minimal: Pauci-immune (ANCA vasculitis, minimal change)
Connect this pattern recognition mastery through systematic discrimination frameworks to understand how subtle differences distinguish between similar-appearing conditions.
🔍 Pattern Recognition Mastery: Decoding the Renal Detective Story
⚖️ Diagnostic Discrimination: The Art of Renal Differential Diagnosis
The nephrotic syndrome differential exemplifies diagnostic complexity, where minimal change disease, FSGS, and early membranous nephropathy can present identically but require completely different treatment approaches and have vastly different prognoses.
📌 Remember: STEROID - Selectivity index (<0.1 minimal change), Time to remission (<8 weeks minimal change), Electron microscopy (foot process effacement), Response rate (>90% minimal change), Onset age (children minimal change), Immunofluorescence (negative minimal change), Duration of treatment (6 months initial).
- Nephrotic Syndrome Discrimination (All with >3.5g/day proteinuria)
- Minimal Change Disease: Negative IF, normal LM, 100% foot process effacement
- FSGS: Negative IF, segmental sclerosis, variable foot process changes
- Primary FSGS: Idiopathic, steroid responsive in 30%
- Secondary FSGS: Adaptive (obesity, drugs), steroid resistant
- Membranous Nephropathy: Granular IgG, subepithelial deposits, spike-dome
- Primary: PLA2R antibodies in 70%, spontaneous remission 30%
- Secondary: Malignancy (10%), autoimmune (15%), infections (5%)
The crescentic glomerulonephritis differential requires rapid discrimination to prevent irreversible kidney damage, with three distinct immunological mechanisms requiring different therapeutic approaches.
⭐ Clinical Pearl: Crescent percentage predicts renal outcome - <25% crescents have >90% renal survival, 25-50% crescents show 60% survival, and >50% crescents result in <20% renal recovery at 2 years.
| Crescentic GN Type | IF Pattern | Serology | Extrarenal Features | Treatment Response | Renal Survival |
|---|---|---|---|---|---|
| Anti-GBM | Linear IgG | Anti-GBM >95% | Pulmonary hemorrhage 60% | Poor if Cr >6 | <30% at 1 year |
| Immune Complex | Granular | ANA, anti-dsDNA | Systemic lupus 40% | Good if early | 70-80% at 5 years |
| ANCA | Negative | c-ANCA 90%, p-ANCA 70% | Upper respiratory 80% | Good with treatment | 80-85% at 5 years |
| Idiopathic | Negative | All negative | Rare | Variable | 50-60% at 5 years |
💡 Master This: Biomarker combinations improve diagnostic accuracy - NGAL + KIM-1 distinguishes ATN from prerenal azotemia with 95% sensitivity, while NGAL + IL-18 predicts dialysis requirement with 90% specificity when both elevated >3-fold.
The chronic kidney disease differential focuses on identifying reversible causes and slowing progression, with specific interventions targeting underlying mechanisms rather than symptomatic management alone.
- CKD Progression Predictors
- Proteinuria: >1g/day doubles progression risk
- Hypertension: >130/80 accelerates decline by 30%
- Diabetes control: HbA1c >7% increases risk 2-fold
- ACE inhibitor use: Reduces progression by 40-50%
| CKD Cause | Proteinuria Pattern | Imaging Findings | Progression Rate | Reversible Factors | Specific Treatment |
|---|---|---|---|---|---|
| Diabetic nephropathy | Albumin >300mg/day | Normal size initially | 5-10 mL/min/year | Glycemic control | ACE-I/ARB |
| Hypertensive nephrosclerosis | <1g/day | Small kidneys | 2-5 mL/min/year | BP control | Multiple agents |
| ADPKD | Minimal | Multiple cysts | Variable | BP, UTI prevention | Tolvaptan |
| Chronic GN | 1-3g/day | Normal/small | 3-8 mL/min/year | Immunosuppression | Disease-specific |
⚖️ Diagnostic Discrimination: The Art of Renal Differential Diagnosis
🎯 Treatment Precision: Evidence-Based Renal Therapeutics
The immunosuppressive hierarchy in glomerular disease follows risk-benefit stratification, with corticosteroids as first-line for steroid-responsive conditions, cytotoxic agents for aggressive disease, and biologics for refractory cases.
📌 Remember: STEROIDS - Start high dose (1mg/kg/day), Taper slowly (over 6 months), Evaluate response (8 weeks), Resistance needs biopsy, Osteoporosis prophylaxis, Infection monitoring, Diabetes screening, Side effect management.
- Nephrotic Syndrome Treatment Protocols
- Minimal Change Disease: Prednisone 1mg/kg/day (max 80mg) for 4-6 weeks
- FSGS: Extended steroids 4-6 months, CNI if resistant
- Primary FSGS: Cyclosporine 3-5mg/kg/day target level 100-200
- Secondary FSGS: Treat underlying cause, ACE inhibition
- Membranous Nephropathy: Risk stratification based on anti-PLA2R levels
- Low risk: Conservative with ACE-I/ARB
- High risk: Rituximab 375mg/m² weekly × 4 doses
The RPGN treatment urgency requires immediate intervention within 24-48 hours to prevent irreversible kidney damage, with plasmapheresis for anti-GBM disease and high-dose steroids for ANCA vasculitis.
⭐ Clinical Pearl: Plasmapheresis timing in anti-GBM disease determines outcome - treatment within 14 days of onset achieves renal recovery in 60% of patients, while delay beyond 21 days reduces recovery to <10%.
| RPGN Type | First-Line Treatment | Dosing Protocol | Duration | Response Rate | Monitoring |
|---|---|---|---|---|---|
| Anti-GBM | Plasmapheresis + steroids | Daily × 14 days | 2-3 weeks | 30-40% | Anti-GBM levels |
| ANCA | Cyclophosphamide + steroids | 2mg/kg/day | 3-6 months | 70-80% | CBC, urinalysis |
| Lupus | Mycophenolate + steroids | 2-3g/day | 6 months | 60-70% | Anti-dsDNA, C3/C4 |
| Post-infectious | Supportive care | ACE-I, diuretics | Variable | 90-95% | Complement levels |
💡 Master This: ACE inhibitor/ARB therapy reduces proteinuria by 30-50% and slows CKD progression by 40% when systolic BP maintained <130 mmHg. Hyperkalemia risk increases when eGFR <30, requiring monitoring every 2-4 weeks during dose titration.
The acute kidney injury management requires immediate identification and correction of reversible factors, with renal replacement therapy decisions based on specific indications rather than arbitrary creatinine thresholds.
- RRT Indications (AEIOU)
- Acidosis: pH <7.1 or bicarbonate <10
- Electrolyte: K+ >6.5 with ECG changes
- Intoxication: Dialyzable toxins (methanol, lithium)
- Overload: Pulmonary edema refractory to diuretics
- Uremia: Pericarditis, encephalopathy, bleeding
| AKI Stage | Creatinine Criteria | Urine Output | Management | RRT Consideration | Recovery Rate |
|---|---|---|---|---|---|
| Stage 1 | 1.5-1.9× baseline | <0.5 mL/kg/h × 6h | Conservative | Rarely | >90% |
| Stage 2 | 2.0-2.9× baseline | <0.5 mL/kg/h × 12h | Optimize hemodynamics | Consider | 70-80% |
| Stage 3 | >3× baseline | <0.3 mL/kg/h × 24h | Aggressive support | Often required | 50-60% |
🎯 Treatment Precision: Evidence-Based Renal Therapeutics
🔗 Systemic Integration: The Kidney's Multi-Organ Network
The renin-angiotensin-aldosterone system represents the kidney's most powerful systemic influence, with renin release from juxtaglomerular cells triggering cardiovascular, fluid balance, and electrolyte responses that affect every organ system.
📌 Remember: RAAS CASCADE - Renin (kidney), Angiotensinogen (liver), ACE (lung), Systemic vasoconstriction, Cardiac remodeling, Aldosterone (adrenal), Sodium retention, Cardiovascular disease, Adaptive responses, Disease progression, End-organ damage.
- Cardiovascular-Renal Integration (Cardiorenal Syndrome)
- Type 1: Acute cardiac → acute renal (25-30% of heart failure)
- Type 2: Chronic cardiac → chronic renal (60% of CHF patients)
- Reduced ejection fraction: eGFR decline 3-5 mL/min/year
- Preserved ejection fraction: Diastolic dysfunction with volume overload
- Type 3: Acute renal → acute cardiac (fluid overload, electrolytes)
- Type 4: Chronic renal → chronic cardiac (LVH, CAD, arrhythmias)
- CKD Stage 3: CVD risk increased 2-fold
- CKD Stage 5: CVD mortality increased 10-20 fold
The endocrine integration involves multiple hormonal axes, with CKD causing secondary hyperparathyroidism, anemia from EPO deficiency, and metabolic acidosis affecting protein metabolism.
⭐ Clinical Pearl: Anemia in CKD appears when eGFR <30, with hemoglobin declining 0.2g/dL per 10 mL/min GFR decrease. ESA therapy targets hemoglobin 10-11g/dL to reduce cardiovascular events while avoiding thrombotic complications seen with higher targets.
| CKD Stage | eGFR Range | Cardiovascular Risk | Bone Disease Prevalence | Anemia Prevalence | Key Interventions |
|---|---|---|---|---|---|
| Stage 3a | 45-59 | 1.4× normal | 15-20% | 5-10% | BP control, ACE-I |
| Stage 3b | 30-44 | 1.8× normal | 30-40% | 20-30% | Phosphate binders |
| Stage 4 | 15-29 | 2.8× normal | 60-70% | 50-60% | ESA, vitamin D |
| Stage 5 | <15 | 5-10× normal | >90% | >80% | RRT preparation |
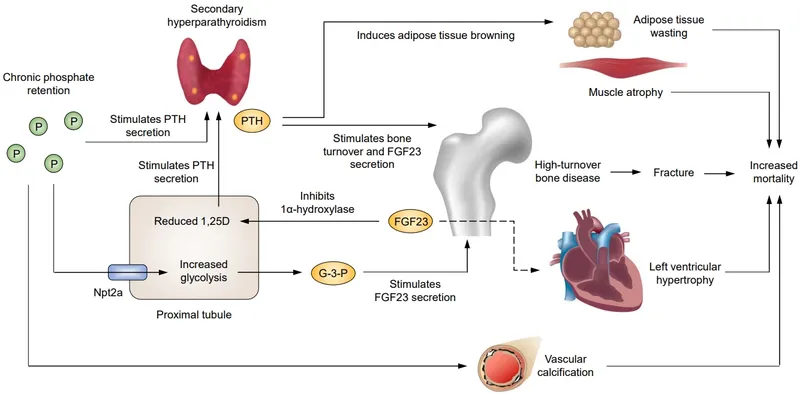
💡 Master This: FGF23 rises earliest in CKD progression, increasing 100-fold by Stage 4, and independently predicts cardiovascular mortality. Phosphate binders should target serum phosphate <4.6 mg/dL to prevent vascular calcification and reduce mortality by 22%.
The hematologic integration extends beyond anemia to include platelet dysfunction, bleeding tendency, and immune suppression, with uremic toxins affecting multiple cell lines and coagulation pathways.
- Uremic Bleeding Mechanisms
- Platelet dysfunction: Adhesion and aggregation defects
- von Willebrand factor: Decreased activity and abnormal multimers
- Anemia: Reduced red cell mass affects platelet-vessel interaction
- Medications: Anticoagulants and antiplatelet agents
| Uremic Complication | Pathophysiology | Clinical Manifestation | Treatment | Monitoring |
|---|---|---|---|---|
| Bleeding | Platelet dysfunction | Mucosal bleeding, bruising | Desmopressin, dialysis | Bleeding time |
| Infection | Immune suppression | Increased infection rate | Vaccination, prophylaxis | WBC function |
| Malnutrition | Chronic inflammation | Protein-energy wasting | Nutritional support | Albumin, prealbumin |
| Neuropathy | Uremic toxins | Peripheral and autonomic | Adequate dialysis | Nerve conduction |
🔗 Systemic Integration: The Kidney's Multi-Organ Network
🎯 Clinical Mastery Arsenal: Your Renal Pathology Command Center
📌 Essential Numbers Arsenal: GFR <60 = CKD, Proteinuria >3.5g = nephrotic, Creatinine doubling <3 months = RPGN, FENa <1% = prerenal, Crescents >50% = poor prognosis, Anti-GBM = plasmapheresis within 14 days, NGAL >150 = ATN likely.
- Rapid Diagnostic Framework
- Step 1: Urinalysis pattern (<2 hours fresh)
- Step 2: Proteinuria quantification (24-hour or spot ratio)
- Step 3: Serological workup (ANA, ANCA, anti-GBM, complement)
- Step 4: Imaging assessment (ultrasound first-line)
- Step 5: Biopsy indication (unexplained AKI, nephrotic adults, RPGN)
⭐ High-Yield Clinical Pearls:
- Muddy brown casts = ATN (95% specific)
- RBC casts = glomerulonephritis (pathognomonic)
- Eosinophiluria >5% = AIN (80% sensitive)
- Linear IF = anti-GBM (>95% specific)
- Mesangial IgA = IgA nephropathy (diagnostic)
| Clinical Scenario | Key Diagnostic Test | Critical Threshold | Immediate Action | Time Window |
|---|---|---|---|---|
| Acute oliguria | FENa | <1% vs >2% | Volume expansion vs nephrology | <6 hours |
| Gross hematuria | Urinalysis + culture | RBC casts present | Nephrology consult | <24 hours |
| Nephrotic syndrome | 24-hour protein | >3.5g/day | Thrombosis prophylaxis | Immediate |
| RPGN | Crescent % | >50% | Aggressive immunosuppression | <48 hours |
| Contrast exposure | NGAL | >150 ng/mL | Hydration protocol | <2 hours |
- Minimal change: Steroids 1mg/kg × 4-6 weeks
- FSGS: Extended steroids 4-6 months or CNI
- Membranous: Risk stratify → rituximab if high risk
- Anti-GBM: Plasmapheresis daily + steroids
- ANCA: Cyclophosphamide 2mg/kg + steroids
The monitoring protocols ensure treatment safety and efficacy assessment, with specific parameters tracked at defined intervals based on disease type and therapy intensity.
⚠️ Critical Safety Monitoring:
- Cyclophosphamide: CBC weekly, hemorrhagic cystitis risk
- Calcineurin inhibitors: Levels + nephrotoxicity monitoring
- Rituximab: Hypogammaglobulinemia, PML risk
- Plasmapheresis: Coagulation, electrolytes, access complications
Master these frameworks, and you transform complex renal pathology into systematic clinical excellence, enabling rapid diagnosis, optimal treatment, and superior patient outcomes across the full spectrum of kidney disease.
🎯 Clinical Mastery Arsenal: Your Renal Pathology Command Center
Practice Questions: Renal pathology
Test your understanding with these related questions
A 22-year-old man comes to the physician because of a 2-week history of cough and decreased urination. The cough was initially nonproductive, but in the last few days he has coughed up small amounts of blood-tinged sputum with clots. He has not had any fevers, chills, or weight loss. He has smoked one pack of cigarettes daily for 5 years. Pulse is 115/min and blood pressure is 125/66 mm Hg. Physical examination shows dried blood around the lips. Serum studies show a creatinine of 2.9 mg/dL. Results of a serum antineutrophil cytoplasm antibody test are negative. A biopsy specimen of the kidney is most likely to show which of the following light microscopy findings?













