Pulmonary

On this page
🫁 The Respiratory Command Center: Pulmonary System Mastery
You'll master the pulmonary system from airway anatomy through ventilation mechanics to pattern recognition that distinguishes obstructive from restrictive disease at the bedside. This lesson builds your diagnostic framework for interpreting pulmonary function tests, imaging, and blood gases, then equips you with evidence-based treatment algorithms for asthma, COPD, pneumonia, and respiratory failure. By integrating multi-system connections and rapid-fire clinical tools, you'll develop the systematic thinking that transforms scattered facts into confident, precise clinical decisions when your patient can't breathe.
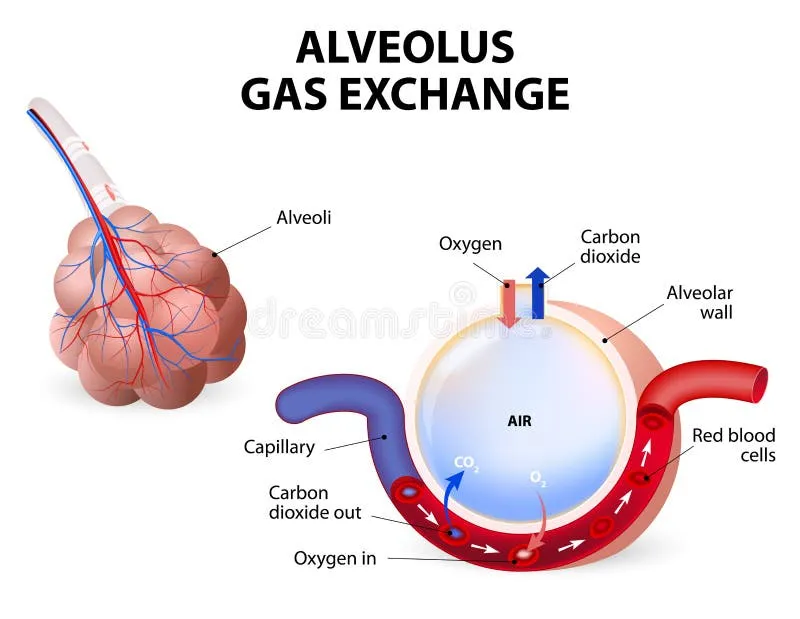
The respiratory system operates as your body's most sophisticated gas exchange facility, moving 8,000-10,000 liters of air daily through 300 million alveoli with a combined surface area of 70 square meters. Understanding this architectural marvel reveals why certain diseases target specific anatomical zones and how compensatory mechanisms maintain life even with 50% functional lung loss.
📌 Remember: ABCDE for respiratory zones - Alveolar (300M units), Bronchiolar (30,000 terminal), Conducting (23 generations), Dead space (150ml), Exchange surface (70m²)
Pulmonary Architecture: The Multi-Zone Framework
-
Conducting Zone (Generations 0-16)
- Trachea to terminal bronchioles: 16 generations
- Dead space volume: 150ml in average adult
- Primary function: Air conditioning and filtration
- Humidification to 100% relative humidity
- Temperature regulation to 37°C
- Particle filtration down to 2-5 microns
-
Respiratory Zone (Generations 17-23)
- Respiratory bronchioles to alveolar sacs: 7 generations
- Gas exchange surface: 70 square meters
- Alveolar count: 300 million functional units
- Type I pneumocytes: 95% surface area coverage
- Type II pneumocytes: 5% surface, 100% surfactant production
- Alveolar macrophages: 15 million active scavengers
| Zone | Generations | Primary Function | Key Measurements | Clinical Significance |
|---|---|---|---|---|
| Conducting | 0-16 | Air transport | 150ml dead space | V/Q mismatch source |
| Transitional | 17-19 | Mixed function | 50ml volume | Early disease target |
| Respiratory | 20-23 | Gas exchange | 3000ml capacity | ARDS primary site |
| Vascular | All zones | Perfusion | 5L/min flow | PE impact zone |
| Pleural | External | Mechanics | -5cmH2O pressure | Pneumothorax risk |
The blood-gas barrier measures only 0.5 microns thick, consisting of alveolar epithelium, basement membrane, and capillary endothelium. This ultra-thin interface enables 250ml O₂ and 200ml CO₂ exchange per minute at rest, scaling to >3000ml O₂ during maximal exercise.
💡 Master This: Alveolar pressure equation PAO₂ = FiO₂(PB-PH₂O) - PaCO₂/RQ where PB=760mmHg, PH₂O=47mmHg, RQ=0.8 - this calculation predicts A-a gradient abnormalities in >90% of pulmonary diseases
Understanding respiratory zone architecture connects directly to how restrictive diseases target alveolar walls while obstructive diseases primarily affect conducting airways, setting the foundation for systematic pulmonary pathology recognition.
🫁 The Respiratory Command Center: Pulmonary System Mastery
⚙️ The Ventilation Engine: Mechanical Mastery Systems

📌 Remember: DIME for inspiration muscles - Diaphragm (75% work), Intercostals (20% work), Minor accessories (5% work), Expiration passive at rest
Ventilation Mechanics: The Pressure-Volume Relationship
-
Inspiratory Mechanics
- Diaphragmatic descent: 1-2cm quiet, 10cm deep breathing
- Pleural pressure change: -5cmH2O to -8cmH2O
- Alveolar pressure drop: 0 to -1cmH2O
- Air flow rate: 500ml in 2 seconds
- Peak flow: 300ml/second during quiet breathing
- Resistance: 1-2cmH2O/L/sec in healthy lungs
-
Expiratory Mechanics
- Passive elastic recoil: >75% of expiratory force
- Pleural pressure return: -8cmH2O to -5cmH2O
- Active muscle recruitment: >25% vital capacity efforts
- Abdominal muscles: +20cmH2O pressure generation
- Internal intercostals: rib depression and chest compression
- Expiratory reserve: 1200ml additional volume
| Parameter | Quiet Breathing | Deep Breathing | Forced Expiration | Clinical Threshold |
|---|---|---|---|---|
| Tidal Volume | 500ml | 3000ml | Variable | <300ml concerning |
| Pleural Pressure | -5 to -8cmH2O | -15 to -30cmH2O | +20 to +40cmH2O | >-2cmH2O abnormal |
| Flow Rate | 300ml/sec | 1500ml/sec | 8000ml/sec | <200ml/sec impaired |
| Resistance | 1-2cmH2O/L/sec | 2-3cmH2O/L/sec | 3-5cmH2O/L/sec | >5cmH2O/L/sec obstructed |
| Work of Breathing | 0.5J/L | 2-3J/L | 10-15J/L | >5J/L at rest pathologic |
The compliance-resistance relationship determines ventilatory efficiency through the equation Time Constant = Compliance × Resistance. Normal lungs have time constants of 0.1-0.2 seconds, allowing 95% equilibration within 3 time constants or 0.6 seconds.
💡 Master This: Laplace's Law governs alveolar stability: P = 2γ/r where γ=surfactant tension and r=radius - without surfactant reducing surface tension from 70 to 25 dynes/cm, small alveoli would collapse into larger ones, causing atelectasis
Understanding ventilation mechanics reveals why restrictive diseases increase work of breathing through reduced compliance, while obstructive diseases increase work through elevated resistance, connecting mechanical principles to clinical presentations in the next framework.
⚙️ The Ventilation Engine: Mechanical Mastery Systems
🎯 The Pattern Recognition Matrix: Clinical Correlation Frameworks
📌 Remember: VINDICATE for pulmonary differentials - Vascular (PE, edema), Infectious (pneumonia, TB), Neoplastic (lung cancer), Degenerative (COPD), Iatrogenic (drug-induced), Congenital (CF), Autoimmune (sarcoid), Trauma (pneumothorax), Endocrine (rare causes)
Clinical Assessment Framework: The SOAP-V2 Method
-
Symptom Pattern Analysis
- Dyspnea onset: Acute (<24hrs) vs Chronic (>3 months)
- Cough characteristics: Productive vs dry, timing patterns
- Chest pain: Pleuritic vs dull, location specificity
- Pleuritic pain: Sharp, inspiratory, suggests pleural irritation
- Dull pain: Constant, pressure-like, suggests parenchymal disease
- Hemoptysis volume: <30ml minor, >100ml major bleeding
-
Objective Findings Integration
- Vital signs: RR >24, O2 sat <92%, HR >100 suggest severity
- Physical examination: Inspection, palpation, percussion, auscultation
- Laboratory correlation: ABG, CBC, inflammatory markers
- A-a gradient: Normal <15mmHg, >20mmHg suggests V/Q mismatch
- D-dimer: >500ng/ml raises PE suspicion but low specificity
- Procalcitonin: >0.5ng/ml suggests bacterial infection

| Clinical Pattern | Key Features | Diagnostic Tests | Time to Diagnosis | Mortality Risk |
|---|---|---|---|---|
| Acute Dyspnea | Onset <24hrs | CXR, ABG, BNP | <2 hours | Variable |
| Chronic Dyspnea | Onset >3 months | PFTs, HRCT, Echo | Days to weeks | Generally low |
| Hemoptysis | Blood in sputum | Bronchoscopy, CT-PA | <24 hours | 5-15% if massive |
| Chest Pain | Pleuritic pattern | CXR, CT-PA, ECG | <4 hours | <5% if non-cardiac |
| Hypoxemia | O2 sat <92% | ABG, CXR, Echo | <1 hour | 10-30% severe cases |
Physiologic Pattern Recognition: The V/Q Framework
- V/Q Mismatch Patterns
- Dead space increase: PE, emphysema, pulmonary hypertension
- Shunt increase: Pneumonia, ARDS, atelectasis
- Mixed patterns: COPD exacerbation, heart failure
- Normal V/Q ratio: 0.8-1.0 in healthy zones
- Dead space: V/Q >1.0, ventilation without perfusion
- Shunt: V/Q <0.1, perfusion without ventilation
💡 Master This: Shunt equation Qs/Qt = (CcO₂ - CaO₂)/(CcO₂ - CvO₂) where normal shunt <5%, >15% shunt causes refractory hypoxemia, and >30% shunt requires mechanical ventilation
Understanding clinical patterns through systematic frameworks enables rapid differentiation between obstructive, restrictive, vascular, and infectious pulmonary diseases, setting the foundation for targeted diagnostic approaches in the next analytical framework.
🎯 The Pattern Recognition Matrix: Clinical Correlation Frameworks
🔬 The Diagnostic Discrimination Engine: Systematic Analysis Protocols
📌 Remember: COMPARE for systematic discrimination - Clinical timeline, Oxygen response, Morphology on imaging, Physiologic patterns, Age demographics, Risk factors, Evidence-based markers
Obstructive vs Restrictive: The Fundamental Divide
-
Obstructive Disease Signatures
- FEV₁/FVC ratio: <70% (normal >80%)
- Total lung capacity: Normal or increased
- Residual volume: Increased >120% predicted
- Air trapping: RV/TLC >35% (normal <25%)
- Hyperinflation: TLC >120% predicted
- Flow limitation: FEV₁ <80% predicted with poor bronchodilator response
-
Restrictive Disease Signatures
- FEV₁/FVC ratio: Normal >80% or increased
- Total lung capacity: Decreased <80% predicted
- Lung compliance: Reduced <100ml/cmH2O
- Chest wall restriction: Normal DLCO, reduced lung volumes
- Parenchymal restriction: Reduced DLCO <80%, bilateral infiltrates
- Neuromuscular restriction: Normal imaging, weak inspiratory pressures
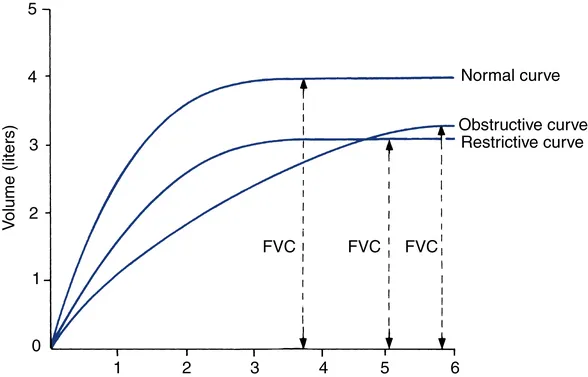
| Parameter | Normal Values | Obstructive Pattern | Restrictive Pattern | Mixed Pattern |
|---|---|---|---|---|
| FEV₁/FVC | >80% | <70% | >80% or increased | <70% |
| TLC | 6000ml ±20% | Normal or ↑ | <80% predicted | Variable |
| RV/TLC | <25% | >35% | Normal or ↓ | >35% |
| DLCO | >80% predicted | Variable | <80% predicted | <80% predicted |
| Compliance | 200ml/cmH2O | Normal or ↑ | <100ml/cmH2O | <100ml/cmH2O |
Acute vs Chronic Presentations: Timeline Discrimination
-
Acute Presentations (<24 hours)
- Life-threatening: PE, pneumothorax, ARDS, massive hemoptysis
- Urgent evaluation: Pneumonia, asthma exacerbation, heart failure
- Diagnostic timeline: <2 hours for severe, <6 hours for moderate
- CT-PA sensitivity: >95% for PE when high clinical suspicion
- CXR sensitivity: >90% for pneumothorax >20%
- ABG turnaround: <30 minutes for acid-base status
-
Chronic Presentations (>3 months)
- Progressive diseases: IPF, COPD, lung cancer, pulmonary hypertension
- Stable conditions: Asthma, bronchiectasis, sarcoidosis
- Diagnostic approach: Systematic workup over days to weeks
- HRCT sensitivity: >95% for interstitial lung disease
- PFT reproducibility: Within 5% on repeat testing
- Biomarker stability: Consistent patterns over multiple measurements
💡 Master This: Acute-on-chronic presentations require dual assessment - identify acute trigger (infection, PE, pneumothorax) while managing underlying chronic disease (COPD, ILD), as >60% of respiratory ICU admissions involve chronic disease exacerbations
Understanding systematic discrimination enables rapid differentiation between similar presentations using quantitative thresholds and evidence-based criteria, connecting diagnostic precision to treatment algorithm selection in the next therapeutic framework.
🔬 The Diagnostic Discrimination Engine: Systematic Analysis Protocols
💊 The Treatment Algorithm Matrix: Evidence-Based Intervention Protocols

📌 Remember: BREATHE for treatment priorities - Bronchodilators first, Respiratory support, Etiologic treatment, Anti-inflammatory agents, Thromboprophylaxis, Heart failure management, Education and prevention
Acute Respiratory Failure: The LMNOP Protocol
-
Oxygen Therapy Protocols
- Target saturation: 92-96% (COPD: 88-92%)
- Nasal cannula: 1-6L/min provides 24-44% FiO₂
- High-flow nasal cannula: Up to 60L/min with up to 100% FiO₂
- HFNC benefits: PEEP effect 3-5cmH2O, dead space washout
- Success rate: >70% in acute hypoxemic respiratory failure
- Escalation criteria: No improvement in 2-4 hours
-
Non-Invasive Ventilation (NIV)
- CPAP indications: Cardiogenic pulmonary edema, OSA, post-extubation
- BiPAP indications: COPD exacerbation, neuromuscular disease
- Success predictors: pH >7.25, GCS >13, cooperative patient
- CPAP pressure: 5-15cmH2O titrated to comfort and oxygenation
- BiPAP settings: IPAP 12-20cmH2O, EPAP 5-10cmH2O
- Failure rate: <20% when appropriate selection criteria applied
| Intervention | Indication | Target Parameters | Success Rate | Escalation Criteria |
|---|---|---|---|---|
| Nasal Cannula | Mild hypoxemia | SpO₂ 92-96% | >90% | No improvement 1hr |
| High-Flow O₂ | Moderate hypoxemia | SpO₂ >90%, RR <25 | 70-80% | No improvement 2-4hrs |
| CPAP | Pulmonary edema | PCWP <18mmHg | >85% | Worsening in 2hrs |
| BiPAP | COPD, acidosis | pH >7.35, PCO₂ <60 | 75-85% | pH <7.25 despite NIV |
| Intubation | NIV failure | Controlled ventilation | >95% | Last resort |
Pharmacologic Intervention Algorithms: The SMART Protocol
-
Bronchodilator Therapy
- SABA (albuterol): 2.5-5mg nebulized q20min × 3 for acute exacerbations
- LABA combinations: Formoterol/budesonide for asthma maintenance
- Anticholinergics: Ipratropium 500mcg nebulized q6h for COPD
- Onset: SABA 5-15min, anticholinergics 15-30min
- Duration: SABA 4-6hrs, LABA 12hrs, tiotropium 24hrs
- Combination benefit: 15-25% additional FEV₁ improvement
-
Anti-Inflammatory Protocols
- Systemic steroids: Prednisone 40-60mg daily for 5-7 days
- Inhaled steroids: High-dose during acute exacerbations
- Steroid-sparing agents: Azathioprine, methotrexate for chronic disease
- Response timeline: Systemic 6-12hrs, inhaled 2-4 weeks
- Tapering protocol: Reduce by 50% every 3-5 days if stable
- Side effect monitoring: Glucose, blood pressure, bone density
💡 Master This: Combination therapy with LABA + ICS reduces exacerbation rates by >40% compared to monotherapy, while triple therapy (LABA + LAMA + ICS) provides additional 15-20% benefit in severe COPD with eosinophilia >300 cells/μL
Understanding evidence-based treatment algorithms enables systematic intervention with quantitative targets and response monitoring, connecting therapeutic precision to multi-system integration in the next advanced framework.
💊 The Treatment Algorithm Matrix: Evidence-Based Intervention Protocols
🌐 The Pulmonary Integration Network: Multi-System Mastery Connections
📌 Remember: CARDIAC for pulmonary-cardiac integration - Cor pulmonale development, Arrhythmia triggers, Right heart strain, Diastolic dysfunction, Ischemia from hypoxemia, Afterload changes, Cardiac output effects
Cardiopulmonary Integration: The Heart-Lung Axis
-
Right Heart-Lung Interactions
- Pulmonary vascular resistance: Normal 100-200 dynes·sec·cm⁻⁵
- Cor pulmonale development: PVR >400 dynes·sec·cm⁻⁵
- Right heart strain markers: BNP >400pg/ml, troponin elevation
- Acute cor pulmonale: Massive PE, severe ARDS
- Chronic cor pulmonale: COPD, pulmonary fibrosis, pulmonary hypertension
- Compensatory mechanisms: RV hypertrophy, tricuspid regurgitation
-
Left Heart-Lung Interactions
- Cardiogenic pulmonary edema: PCWP >18mmHg
- Heart failure with preserved EF: >50% of heart failure patients
- Diastolic dysfunction: E/e' ratio >15 predicts elevated filling pressures
- Hydrostatic pressure: >25mmHg causes alveolar flooding
- Oncotic pressure: <15mmHg worsens fluid extravasation
- Lymphatic clearance: Overwhelmed at >3× normal flow
| Integration | Normal Values | Mild Dysfunction | Moderate Dysfunction | Severe Dysfunction |
|---|---|---|---|---|
| PVR | 100-200 dynes·sec·cm⁻⁵ | 200-300 | 300-400 | >400 |
| PCWP | 8-12mmHg | 12-18mmHg | 18-25mmHg | >25mmHg |
| BNP | <100pg/ml | 100-400pg/ml | 400-1000pg/ml | >1000pg/ml |
| RV/LV ratio | <0.6 | 0.6-0.9 | 0.9-1.2 | >1.2 |
| Cardiac Index | >2.5L/min/m² | 2.0-2.5 | 1.5-2.0 | <1.5 |
Renal-Pulmonary Integration: The Acid-Base Network
-
Acid-Base Compensation Patterns
- Respiratory acidosis: Acute HCO₃⁻ +1 per 10mmHg PCO₂ rise
- Respiratory alkalosis: Acute HCO₃⁻ -2 per 10mmHg PCO₂ fall
- Metabolic compensation: Expected PCO₂ = 1.5 × [HCO₃⁻] + 8 ± 2
- Renal compensation: Takes 3-5 days for full effect
- Respiratory compensation: Occurs within hours
- Mixed disorders: >30% of ICU patients have complex patterns
-
Fluid-Electrolyte Integration
- Diuretic effects: Loop diuretics cause metabolic alkalosis
- Hyperventilation: Respiratory alkalosis causes hypokalemia
- Renal failure: Metabolic acidosis drives compensatory hyperventilation
- Potassium shifts: pH 0.1 unit change = K⁺ 0.6mEq/L opposite shift
- Calcium effects: Alkalosis increases protein binding, decreases ionized Ca²⁺
- Phosphate buffer: Most important intracellular acid-base buffer
💡 Master This: Winter's formula predicts expected PCO₂ in metabolic acidosis: PCO₂ = 1.5 × [HCO₃⁻] + 8 ± 2 - deviations >2mmHg suggest mixed acid-base disorders requiring separate evaluation of respiratory and metabolic components
Understanding multi-system integration reveals how pulmonary diseases affect cardiovascular performance, renal function, and acid-base balance, connecting isolated organ dysfunction to systemic disease patterns that require comprehensive management approaches in the final mastery framework.
🌐 The Pulmonary Integration Network: Multi-System Mastery Connections
🎯 The Clinical Mastery Arsenal: Rapid-Fire Reference Tools
📌 Remember: FAST-LUNG for emergency recognition - Failure to oxygenate, Acidosis severe, Shock present, Tachypnea >30, Low consciousness, Unstable vitals, No improvement, Gasping respirations
Essential Clinical Thresholds: The Numbers That Save Lives
-
Critical Oxygenation Thresholds
- SpO₂ <88%: Immediate intervention required
- PaO₂ <60mmHg: Respiratory failure definition
- A-a gradient >20mmHg: Significant V/Q mismatch
- FiO₂ 100% for 15min: PaO₂ should exceed 500mmHg
- Shunt >20%: Refractory hypoxemia likely
- DLCO <50%: Significant parenchymal disease
-
Ventilation Failure Markers
- PaCO₂ >50mmHg: Hypoventilation present
- pH <7.30: Severe respiratory acidosis
- Respiratory rate >30: Impending failure
- Minute ventilation >15L/min: Excessive work of breathing
- Dead space >60%: Severe V/Q mismatch
- Compliance <30ml/cmH2O: Severe restriction
| Emergency Threshold | Normal Range | Mild Abnormal | Moderate Abnormal | Severe/Critical |
|---|---|---|---|---|
| SpO₂ | 95-100% | 92-94% | 88-91% | <88% |
| PaO₂ | 80-100mmHg | 60-79mmHg | 40-59mmHg | <40mmHg |
| PaCO₂ | 35-45mmHg | 45-55mmHg | 55-70mmHg | >70mmHg |
| pH | 7.35-7.45 | 7.30-7.34 | 7.20-7.29 | <7.20 |
| Respiratory Rate | 12-20/min | 20-25/min | 25-30/min | >30/min |
Rapid Diagnostic Algorithms: The 5-Minute Assessment
-
Acute Dyspnea Protocol
- History: Onset, triggers, associated symptoms - <2 minutes
- Examination: Vitals, oxygen saturation, breath sounds - <2 minutes
- Initial tests: CXR, ABG, BNP if indicated - <30 minutes
- CXR interpretation: <5 minutes for pneumothorax, infiltrates, effusions
- ABG analysis: <2 minutes for acid-base status and oxygenation
- Point-of-care ultrasound: <5 minutes for pneumothorax, effusion, cardiac function
-
Treatment Decision Framework
- Oxygen therapy: Start immediately if SpO₂ <92%
- Bronchodilators: Within 15 minutes for wheeze or known COPD/asthma
- Antibiotics: Within 1 hour for suspected pneumonia with fever or infiltrate
- Steroid timing: Within 2 hours for COPD exacerbation or severe asthma
- Diuretic timing: Within 30 minutes for obvious pulmonary edema
- Anticoagulation: Within 4 hours for high-probability PE
💡 Master This: CURB-65 score predicts pneumonia mortality: Confusion, Urea >7mmol/L, Respiratory rate ≥30, Blood pressure <90/60, age ≥65 - score ≥2 requires hospitalization, ≥3 suggests ICU consideration
The clinical mastery arsenal provides instant access to critical thresholds, rapid assessment protocols, and evidence-based decision frameworks that enable expert-level pulmonary care through systematic pattern recognition and quantitative precision.
🎯 The Clinical Mastery Arsenal: Rapid-Fire Reference Tools
Practice Questions: Pulmonary
Test your understanding with these related questions
A 71-year-old man is admitted to the ICU with a history of severe pancreatitis and new onset difficulty breathing. His vital signs are a blood pressure of 100/60 mm Hg, heart rate of 100/min, respirations of 27/min, temperature of 36.7°C (98.1°F), and oxygen saturation of 85% on room air. Physical examination shows a cachectic male in severe respiratory distress. Rales are heard at the base of each lung. The patient is intubated and a Swan-Ganz catheter is inserted. Pulmonary capillary wedge pressure is 8 mm Hg. An arterial blood gas study reveals a PaO2: FiO2 ratio of 180. The patient is diagnosed with acute respiratory distress syndrome. In which of the following segments of the respiratory tract are the cells responsible for the symptoms observed in this patient found?













