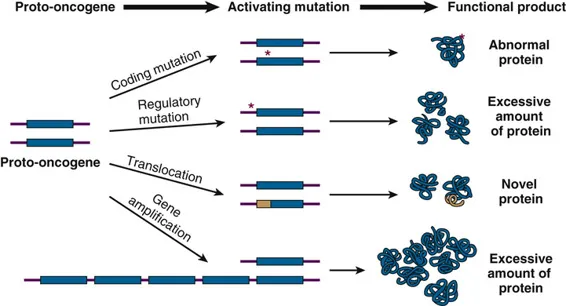
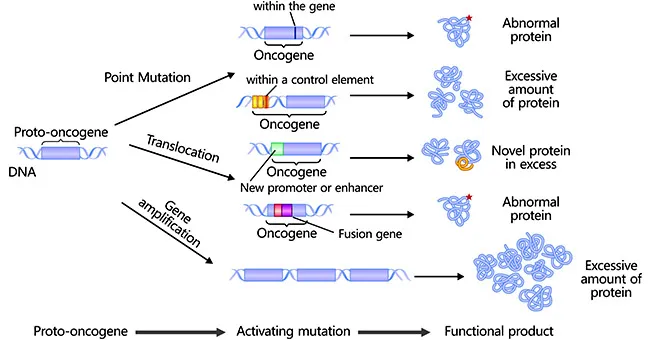
Oncogenes and proto-oncogenes US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Oncogenes and proto-oncogenes. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Oncogenes and proto-oncogenes US Medical PG Question 1: A 33-year-old woman comes to the physician 1 week after noticing a lump in her right breast. Fifteen years ago, she was diagnosed with osteosarcoma of her left distal femur. Her father died of an adrenocortical carcinoma at the age of 41 years. Examination shows a 2-cm, firm, immobile mass in the lower outer quadrant of the right breast. A core needle biopsy of the mass shows adenocarcinoma. Genetic analysis in this patient is most likely to show a defect in which of the following genes?
- A. BRCA1
- B. KRAS
- C. TP53 (Correct Answer)
- D. Rb
- E. PTEN
Oncogenes and proto-oncogenes Explanation: ***TP53***
- This patient's presentation with **early-onset breast cancer**, a history of **osteosarcoma** at a young age, and a father's death from **adrenocortical carcinoma** at 41 years strongly suggests **Li-Fraumeni syndrome**.
- Li-Fraumeni syndrome is an autosomal dominant disorder caused by a germline mutation in the **tumor suppressor gene TP53**, increasing the risk for multiple primary cancers at a young age.
*BRCA1*
- While **BRCA1 mutations** are associated with an increased risk of breast and ovarian cancer, they are not typically linked to osteosarcoma or adrenocortical carcinoma.
- The constellation of cancers in this patient is more indicative of Li-Fraumeni syndrome than solely a BRCA1-related cancer syndrome.
*KRAS*
- **KRAS** is an oncogene commonly mutated in several cancers, including pancreatic, colorectal, and lung cancer, but is not primarily associated with either Li-Fraumeni syndrome or the specific tumors seen in this family history.
- Mutations in KRAS are typically somatic mutations acquired during a person's lifetime, not germline mutations causing inherited cancer syndromes like the one suggested here.
*Rb*
- Mutations in the **retinoblastoma (Rb) gene** are associated with retinoblastoma and an increased risk of osteosarcoma, but not typically with adrenocortical carcinoma or breast cancer as part of a classic inherited syndrome.
- The combination of breast cancer, osteosarcoma, and adrenocortical carcinoma points more specifically to TP53.
*PTEN*
- **PTEN mutations** are associated with Cowden syndrome, which increases the risk for breast cancer, thyroid cancer, and endometrial cancer, along with benign growths.
- However, Cowden syndrome does not typically include osteosarcoma or adrenocortical carcinoma as prominent features, making PTEN less likely than TP53 for this specific family history.
Oncogenes and proto-oncogenes US Medical PG Question 2: A scientist is researching the long term effects of the hepatitis viruses on hepatic tissue. She finds that certain strains are oncogenic and increase the risk of hepatocellular carcinoma. However, they appear to do so via different mechanisms. Which of the following answer choices correctly pairs the hepatitis virus with the correct oncogenic process?
- A. Hepatitis A virus - chronic inflammation
- B. Hepatitis C virus - chronic inflammation
- C. Hepatitis E virus - integration of viral DNA into host hepatocyte genome
- D. Hepatitis B virus - integration of viral DNA into host hepatocyte genome (Correct Answer)
- E. Hepatitis A virus - integration of viral DNA into host hepatocyte genome
Oncogenes and proto-oncogenes Explanation: ***Hepatitis B virus - integration of viral DNA into host hepatocyte genome***
- **Hepatitis B virus (HBV)** is a **DNA virus** that can integrate its genetic material into the host hepatocyte genome, leading to genomic instability and promoting oncogenesis.
- This integration, along with chronic inflammation and the production of viral regulatory proteins, contributes significantly to the development of **hepatocellular carcinoma (HCC)**.
*Hepatitis A virus - chronic inflammation*
- **Hepatitis A virus (HAV)** is an **RNA virus** that causes **acute hepatitis** but does not lead to chronic infection or chronic inflammation.
- Due to its acute and self-limiting nature, HAV is **not associated with hepatocellular carcinoma**.
*Hepatitis C virus - integration of viral DNA into host hepatocyte genome*
- **Hepatitis C virus (HCV)** is an **RNA virus** and therefore does not integrate its DNA into the host genome (as it has no DNA phase).
- HCV causes HCC primarily through **chronic inflammation**, **fibrosis**, and **cirrhosis**, not DNA integration.
*Hepatitis E virus - integration of viral DNA into host hepatocyte genome*
- **Hepatitis E virus (HEV)** is an **RNA virus** that typically causes acute, self-limiting hepatitis and does not integrate its genetic material into the host genome.
- While HEV can cause chronic infection in immunocompromised individuals, it is **not generally recognized as an oncogenic virus** leading to HCC.
*Hepatitis A virus - integration of viral DNA into host hepatocyte genome*
- **Hepatitis A virus (HAV)** is an **RNA virus**, meaning it does not have a DNA stage and therefore cannot integrate DNA into the host genome.
- HAV causes **acute, self-limiting infections** and is definitively **not associated with hepatocellular carcinoma**.
Oncogenes and proto-oncogenes US Medical PG Question 3: A 58-year-old male undergoes a surveillance colonoscopy in which a 2 cm adenoma is identified and removed. Had this adenoma not been excised, the patient would have been at risk of progression to carcinoma. Which of the following is the final mutational step in the progression from adenoma to carcinoma?
- A. p53 inactivation (Correct Answer)
- B. APC mutation
- C. COX-2 overexpression
- D. SMAD 2/4 loss
- E. K-ras mutation
Oncogenes and proto-oncogenes Explanation: ***p53 inactivation***
- **p53 loss of function** is typically the final genetic event in the **adenoma-to-carcinoma sequence**, facilitating unrestricted cell growth and preventing apoptosis in dysplastic cells.
- The **p53 tumor suppressor gene** normally checkpoints cell division and induces programmed cell death, making its inactivation critical for malignant transformation.
*APC mutation*
- **APC (adenomatous polyposis coli) mutation** is often the **initiating event** in colorectal adenoma formation, leading to aberrant crypt foci and polyp formation.
- While critical for early tumor genesis, it does not represent the final step in progression to invasive carcinoma.
*COX-2 overexpression*
- **Cyclooxygenase-2 (COX-2) overexpression** leads to increased prostaglandin production, which can promote cell proliferation, angiogenesis, and inhibit apoptosis.
- It is an important factor in tumor growth and progression but occurs earlier in the sequence and is not the terminal mutational step for carcinoma.
*SMAD 2/4 loss*
- **SMAD 2/4 loss of function** disrupts the **TGF-β signaling pathway**, which normally inhibits cell growth and promotes differentiation.
- This event typically occurs in the late adenoma stage, contributing to dysplasia, but **p53 inactivation** is considered the final critical step for full malignant transformation.
*K-ras mutation*
- **K-ras mutation** is a well-known event in the **adenoma-to-carcinoma sequence**, occurring earlier than p53 inactivation, usually in intermediate-sized adenomas.
- It leads to constitutive activation of the RAS/MAPK pathway, promoting cell growth and survival, but generally before full malignant transformation.
Oncogenes and proto-oncogenes US Medical PG Question 4: A 41-year-old man with HIV comes to the physician because of rectal bleeding and itching for 2 weeks. During this period, he has also had pain with defecation. Four months ago, he was diagnosed with anogenital warts that were treated with cryotherapy. Over the past year, he has been sexually active with 3 male partners. He uses condoms inconsistently. Current medications are zidovudine, emtricitabine, and efavirenz. Digital rectal examination and anoscopy show an exophytic mass on the anal margin that is protruding into the anal canal. The mass is tender to palpation and bleeds easily on contact. Laboratory studies show a leukocyte count of 7,600/mm3 and a CD4+ T-lymphocyte count of 410/mm3 (N ≥ 500). A biopsy specimen of the lesion shows a well-differentiated squamous cell carcinoma. Which of the following cellular processes was most likely involved in the pathogenesis of this patient's malignancy?
- A. Activation of c-myc gene
- B. Inactivation of TP53 gene (Correct Answer)
- C. Activation of TAX gene
- D. Inactivation of VHL gene
- E. Inactivation of WT1 gene
Oncogenes and proto-oncogenes Explanation: ***Inactivation of TP53 gene***
- This patient's **squamous cell carcinoma** (SCC) of the anus is strongly associated with **human papillomavirus (HPV) infection**, which is common in HIV-positive sexually active men. HPV oncoproteins, particularly E6, promote the degradation of the **TP53 tumor suppressor protein**.
- Inactivating mutations or degradation of **TP53** remove a critical checkpoint in the cell cycle, allowing cells with DNA damage to proliferate uncontrollably and contributing to carcinogenesis.
*Activation of c-myc gene*
- The **c-myc proto-oncogene** is involved in cell proliferation, differentiation, and apoptosis, and its activation is commonly seen in lymphomas (e.g., Burkitt lymphoma) and other cancers.
- While *c-myc* activation can contribute to various malignancies, it is not the **primary molecular mechanism** linked to HPV-associated anal squamous cell carcinoma.
*Activation of TAX gene*
- The **TAX gene** is a transforming gene of **human T-cell lymphotropic virus type 1 (HTLV-1)**, responsible for T-cell leukemia/lymphoma.
- This patient's presentation with anal squamous cell carcinoma, rather than a hematologic malignancy, makes HTLV-1 and TAX gene activation an unlikely cause.
*Inactivation of VHL gene*
- The **VHL (Von Hippel-Lindau) gene** is a tumor suppressor gene whose inactivation is strongly associated with **renal cell carcinoma** (clear cell type) and other tumors like pheochromocytoma and hemangioblastoma.
- Inactivation of **VHL** is not a primary mechanism in the development of anal squamous cell carcinoma.
*Inactivation of WT1 gene*
- The **WT1 (Wilms tumor 1) gene** is a tumor suppressor gene primarily associated with **Wilms tumor**, a kidney cancer that typically affects children.
- Inactivation of **WT1** is not a known pathogenic mechanism for anal squamous cell carcinoma in adults.
Oncogenes and proto-oncogenes US Medical PG Question 5: A 2-year-old boy from a rural community is brought to the pediatrician after his parents noticed a white reflection in both of his eyes in recent pictures. Physical examination reveals bilateral leukocoria, nystagmus, and inflammation. When asked about family history of malignancy, the father of the child reports losing a brother to an eye tumor when they were children. With this in mind, which of the following processes are affected in this patient?
- A. Base excision repair
- B. Regulation of the G1-S transition (Correct Answer)
- C. DNA mismatch repair
- D. Stem cell self-renewal
- E. Nucleotide excision repair
Oncogenes and proto-oncogenes Explanation: ***Regulation of the G1-S transition***
- This patient's symptoms (bilateral **leukocoria**, **nystagmus**, family history of eye tumor) are characteristic of **retinoblastoma**, which is often caused by a mutation in the **RB1 gene**.
- The **RB1 gene** product (retinoblastoma protein) is a key **tumor suppressor** that regulates the G1-S cell cycle transition, and its dysfunction leads to uncontrolled cell proliferation.
*Base excision repair*
- This process is primarily involved in repairing damaged bases in DNA, often due to oxidation or alkylation.
- Defects in base excision repair are typically associated with conditions such as **MUTYH-associated polyposis**, not retinoblastoma.
*DNA mismatch repair*
- This system corrects errors that occur during DNA replication, such as incorrect base pairings or small insertions/deletions.
- Impairment of mismatch repair is a hallmark of **Lynch syndrome** (hereditary nonpolyposis colorectal cancer), which does not present with retinoblastoma.
*Stem cell self-renewal*
- While uncontrolled self-renewal can contribute to cancer, retinoblastoma is specifically linked to defects in the **RB1 gene**, which is a cell cycle regulator, not directly a primary regulator of stem cell self-renewal itself.
- Loss of G1-S checkpoint control is a more direct and proximal cause of the tumor formation in retinoblastoma.
*Nucleotide excision repair*
- This pathway is responsible for repairing bulkier DNA lesions, such as those caused by UV radiation.
- Deficiencies in nucleotide excision repair lead to diseases like **xeroderma pigmentosum**, characterized by extreme sensitivity to sunlight and increased skin cancer risk, which is unrelated to the presented case.
Oncogenes and proto-oncogenes US Medical PG Question 6: A 55-year-old man with a history of sun exposure presents with a slowly growing, pearly nodule with telangiectasias on his nose. The lesion occasionally bleeds when traumatized. Biopsy shows basaloid cells arranged in palisading patterns. Which of the following mutations is most likely involved in the pathogenesis?
- A. P53 mutation
- B. PTCH1 gene mutation (Correct Answer)
- C. EGFR mutation
- D. KIT mutation
Oncogenes and proto-oncogenes Explanation: **PTCH1 gene mutation**
- The clinical presentation of a **pearly nodule with telangiectasias** on the **nose**, history of **sun exposure**, and **basaloid cells arranged in palisading patterns** on biopsy are classic for **basal cell carcinoma (BCC)** [1].
- Mutations in the **PTCH1 gene**, a tumor suppressor gene involved in the **Hedgehog signaling pathway**, are found in the majority of sporadic BCCs and are central to its pathogenesis [2,3].
*P53 mutation*
- While **P53 mutations** are common in many cancers, including **squamous cell carcinoma** [3], they are not the primary driver mutation for basal cell carcinoma in the way PTCH1 mutations are.
- Loss of P53 function typically leads to uncontrolled cell growth and reduced apoptosis, but it's a general cancer mechanism rather than a specific one for BCC.
*EGFR mutation*
- **EGFR mutations** are primarily associated with certain types of **lung adenocarcinoma** and **glioblastoma**, not basal cell carcinoma.
- These mutations lead to constitutive activation of the **epidermal growth factor receptor** signaling pathway, promoting cell proliferation and survival in those specific cancers.
*KIT mutation*
- **KIT mutations** are most commonly found in **gastrointestinal stromal tumors (GIST)** and certain types of **melanoma**.
- The KIT receptor tyrosine kinase plays a role in cell growth and differentiation in specific cell lineages, distinct from the epidermal cells involved in BCC.
**References:**
[1] Kumar V, Abbas AK, et al.. Robbins and Cotran Pathologic Basis of Disease. 9th ed. The Skin, pp. 1158-1162.
[2] Kumar V, Abbas AK, et al.. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Neoplasia, pp. 306-307.
[3] Cross SS. Underwood's Pathology: A Clinical Approach. 6th ed. Disorders Involving Inflammatory And Haemopoietic Cells, pp. 643-644.
Oncogenes and proto-oncogenes US Medical PG Question 7: A 16-year-old boy is brought to the physician because of a lesion that has been growing on his jaw over the past several months. He recently immigrated to the USA from Kenya with his family. Physical examination shows a 3-cm solid mass located above the left mandible. There is cervical lymphadenopathy. Biopsy of the mass shows sheets of lymphocytes and interspersed reactive histiocytes with abundant, clear cytoplasm and phagocytosed debris. Which of the following mechanisms is most likely directly responsible for the malignant transformation of this patient's cells?
- A. Defect in DNA repair
- B. Impairment of receptor function
- C. Inhibition of cell cycle arrest
- D. Integration of viral DNA (Correct Answer)
- E. Activation of transcription
Oncogenes and proto-oncogenes Explanation: **Integration of viral DNA**
- The clinical presentation (rapidly growing jaw mass in a young boy from Kenya) and histological findings (sheets of lymphocytes, "starry sky" appearance due to macrophages) are classic for **endemic Burkitt lymphoma**.
- Endemic Burkitt lymphoma is strongly associated with **Epstein-Barr virus (EBV)** infection. EBV DNA integrates into the host cell genome, promoting the characteristic **t(8;14) translocation** of *MYC* oncogene, leading to its overexpression and uncontrolled cell proliferation.
*Defect in DNA repair*
- While defects in DNA repair can lead to malignancy (e.g., in Lynch syndrome, xeroderma pigmentosum), it is not the primary mechanism of oncogenesis in Burkitt lymphoma.
- The hallmark of Burkitt lymphoma is a specific chromosomal translocation, not a generalized DNA repair defect.
*Impairment of receptor function*
- Impaired receptor function is associated with certain diseases (e.g., some autoimmune conditions, diabetes insipidus) but is not a direct mechanism for malignant transformation in Burkitt lymphoma.
- Malignancy typically arises from uncontrolled cell growth and division, not directly from receptor dysfunction.
*Inhibition of cell cycle arrest*
- While Burkitt lymphoma cells do evade cell cycle arrest, this is a **consequence** of the *MYC* oncogene overexpression, not the primary mechanism of malignant transformation itself.
- The **integration of viral DNA** leading to the *MYC* translocation is the upstream event that *causes* the inhibition of cell cycle arrest.
*Activation of transcription*
- **Activation of transcription** (specifically of the *MYC* oncogene) is a crucial step in the pathogenesis of Burkitt lymphoma, leading to uncontrolled cell growth.
- However, the direct cause of this dysregulated transcriptional activation is the **chromosomal translocation** resulting from viral DNA integration.
Oncogenes and proto-oncogenes US Medical PG Question 8: A 62-year-old man comes to the physician because of a growth on his penis that has been gradually increasing in size over the last year. He was diagnosed with HIV 10 years ago. He has been divorced for 25 years and has had “at least 30 sexual partners” since. Physical examination shows a nontender 2.5-cm ulcerated lesion with an erythematous base on the dorsum of the glans. There is firm left inguinal lymphadenopathy. A biopsy of the lesion shows small uniform basophilic cells with central necrosis that invade into the corpus cavernosum. This patient's condition is most likely associated with which of the following pathogens?
- A. Chlamydia trachomatis
- B. Haemophilus ducreyi
- C. Epstein-Barr virus
- D. Human papillomavirus (Correct Answer)
- E. Neisseria gonorrhoeae
Oncogenes and proto-oncogenes Explanation: ***Human papillomavirus***
- The description of a slowly growing, ulcerated penile lesion with inguinal lymphadenopathy in an HIV-positive man with multiple sexual partners is highly suggestive of **penile squamous cell carcinoma**, which is strongly associated with **human papillomavirus (HPV)** infection.
- The biopsy findings of "small uniform basophilic cells with central necrosis invading into the corpus cavernosum" are consistent with a poorly differentiated squamous cell carcinoma, often linked to high-risk HPV types.
*Chlamydia trachomatis*
- This pathogen causes **urethritis**, **cervicitis**, and **lymphogranuloma venereum**, which presents with painful lymphadenopathy and anogenital ulcers, but typically not a slowly growing, ulcerated mass like the one described.
- The histological description does not fit the typical presentation of complications from *Chlamydia trachomatis* infection.
*Haemophilus ducreyi*
- This bacterium is the cause of **chancroid**, which presents as painful, ragged ulcers with tender inguinal lymphadenopathy.
- While it causes ulcers and lymphadenopathy, the clinical presentation and biopsy findings of a chronic, slowly enlarging, infiltrative lesion are not typical of chancroid.
*Epstein-Barr virus*
- While Epstein-Barr virus (EBV) is associated with several cancers, including **nasopharyngeal carcinoma**, **Burkitt lymphoma**, and **post-transplant lymphoproliferative disorder**, it is not a known cause of penile squamous cell carcinoma.
- The clinical and histological features do not align with EBV-associated malignancies.
*Neisseria gonorrhoeae*
- This bacterium primarily causes **urethritis**, **cervicitis**, and **disseminated gonococcal infection**.
- It does not cause chronic, slowly enlarging ulcerated lesions on the penis that progress to squamous cell carcinoma.
Oncogenes and proto-oncogenes US Medical PG Question 9: A research lab is investigating the rate of replication of a variety of human cells in order to better understand cancer metastasis. A particular cell line of interest is marked with a high concern for malignant potential due to its chromatin structure characteristics. Which of the following is most closely associated with an increased potential for malignancy?
- A. Methylated DNA
- B. H1 protein
- C. Nucleosomes
- D. Euchromatin (Correct Answer)
- E. Heterochromatin
Oncogenes and proto-oncogenes Explanation: ***Euchromatin***
- An increased amount of **euchromatin** suggests that the DNA is less condensed and more accessible for transcription and replication, which is characteristic of rapidly dividing cells, including cancer cells.
- Cancer cells often exhibit **deregulated gene expression** due to alterations in chromatin structure, leading to an increase in euchromatin and higher rates of protein synthesis necessary for rapid proliferation.
*Methylated DNA*
- While DNA methylation is an important **epigenetic modification** involved in cancer, specific patterns of methylation (e.g., hypermethylation of tumor suppressor genes or hypomethylation of oncogenes) are associated with malignancy, not methylation of all DNA.
- Global hypomethylation is linked to genomic instability in cancer, whereas hypermethylation often leads to gene silencing.
*H1 protein*
- **H1 histone protein**, also known as the linker histone, is responsible for compacting nucleosomes and is essential for forming higher-order chromatin structures.
- An abundance of H1 generally indicates a more condensed chromatin state, which would be *less* associated with the active transcription and replication seen in highly malignant cells.
*Nucleosomes*
- **Nucleosomes** are the fundamental building blocks of chromatin, consisting of DNA wrapped around histone proteins. They are present in all eukaryotic cells, irrespective of their malignant potential.
- While alterations in nucleosome positioning and modification can occur in cancer, the presence of nucleosomes themselves is not indicative of malignancy.
*Heterochromatin*
- **Heterochromatin** is a highly condensed form of chromatin that is typically transcriptionally inactive or silenced.
- A high proportion of heterochromatin would suggest a cell with reduced gene expression and replication activity, which is generally *not* characteristic of rapidly dividing malignant cells.
Oncogenes and proto-oncogenes US Medical PG Question 10: A 62-year-old man comes to the physician because of easy bruising and recurrent nosebleeds over the past 4 months. During the same time period, the patient has felt weak and has had a 10-kg (22-lb) weight loss. Physical examination shows mucosal pallor and bruising on the upper and lower extremities in various stages of healing. The spleen is palpated 4 cm below the left costal margin. Laboratory studies show anemia and thrombocytopenia. A photomicrograph of a peripheral blood smear is shown. Histologic examination of a bone marrow biopsy in this patient is most likely to show which of the following findings?
- A. Neoplastic granulocytes with low leukocyte alkaline phosphatase score
- B. Neoplastic lymphocytes that stain positive for tartrate-resistant acid phosphatase (Correct Answer)
- C. Neoplastic myeloid cells that stain positive for myeloperoxidase
- D. Neoplastic lymphoid cells that stain positive for terminal deoxynucleotidyl transferase activity
- E. Dysplastic erythroid cells that stain positive for iron
Oncogenes and proto-oncogenes Explanation: ***Neoplastic lymphocytes that stain positive for tartrate-resistant acid phosphatase***
- The patient's symptoms of **easy bruising, recurrent nosebleeds, weakness, weight loss, mucosal pallor, and thrombocytopenia**, along with **splenomegaly**, are highly suggestive of a **hematologic malignancy**. The peripheral blood smear shows **lymphocytes with characteristic cytoplasmic projections ("hairy cells")**.
- **Hairy cell leukemia (HCL)** is a **B-cell lymphoma** characterized by the proliferation of these specific lymphocytes. These cells are known to stain positively for **tartrate-resistant acid phosphatase (TRAP)**, a diagnostic hallmark of this condition.
*Neoplastic granulocytes with low leukocyte alkaline phosphatase score*
- This description is characteristic of **chronic myeloid leukemia (CML)**, which typically presents with **markedly elevated white blood cell counts** (granulocytosis) and a low leukocyte alkaline phosphatase (LAP) score.
- The patient's presentation of **pancytopenia** (anemia, thrombocytopenia) and lack of **marked leukocytosis** makes CML less likely.
*Neoplastic myeloid cells that stain positive for myeloperoxidase*
- **Myeloperoxidase (MPO)** positivity is a key feature of **acute myeloid leukemia (AML)**, specifically distinguishing myeloid blasts from lymphoid blasts.
- While AML can cause **pancytopenia** and **splenomegaly**, the specific morphology of **hairy cells** in the peripheral smear and the absence of overt blasts point away from AML.
*Neoplastic lymphoid cells that stain positive for terminal deoxynucleotidyl transferase activity*
- **Terminal deoxynucleotidyl transferase (TdT)** is an enzyme found in immature lymphocytes and is a marker for **acute lymphoblastic leukemia (ALL)** and some lymphomas.
- The patient's age (62 years old) makes ALL less common than in children, and the clinical picture, particularly the **massive splenomegaly without significant lymphadenopathy** often seen in ALL, is more characteristic of hairy cell leukemia.
*Dysplastic erythroid cells that stain positive for iron*
- This finding is suggestive of **myelodysplastic syndromes (MDS)**, particularly refractory anemia with ring sideroblasts, where there is ineffective erythropoiesis and iron deposition in mitochondria of erythroid precursors (ring sideroblasts).
- While MDS can lead to **anemia** and **thrombocytopenia**, the distinctive morphology of **hairy cells** in the peripheral smear and the prominent **splenomegaly** are not typical features of MDS.
More Oncogenes and proto-oncogenes US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.