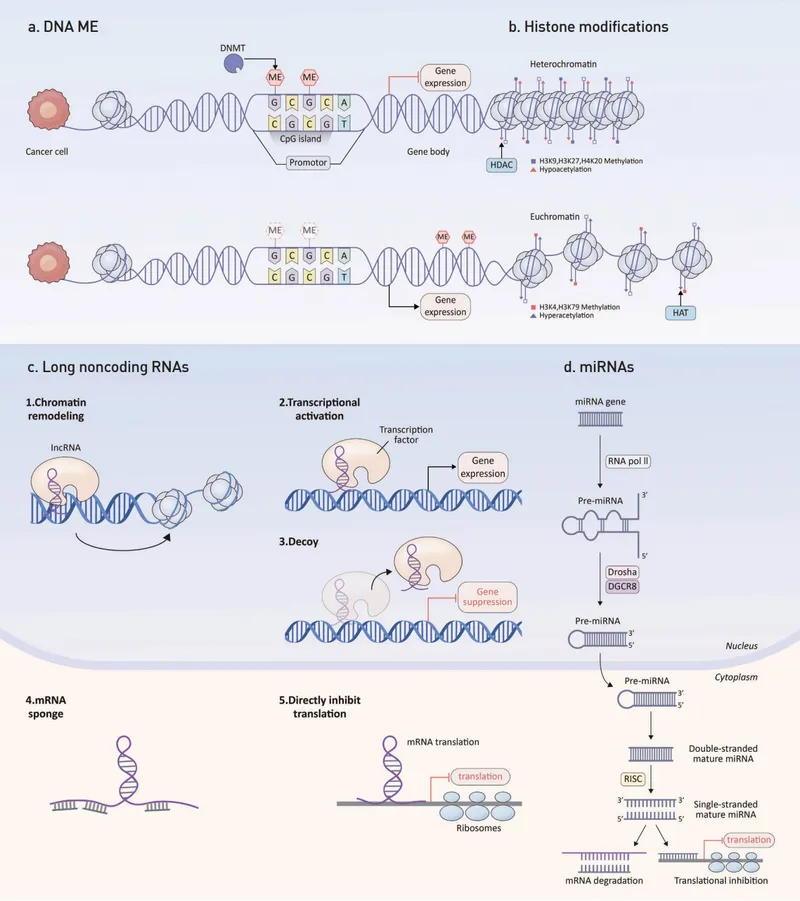
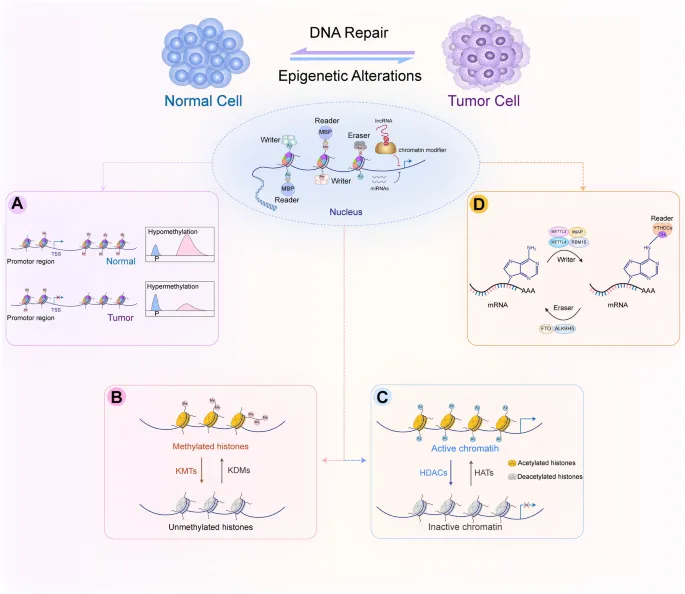
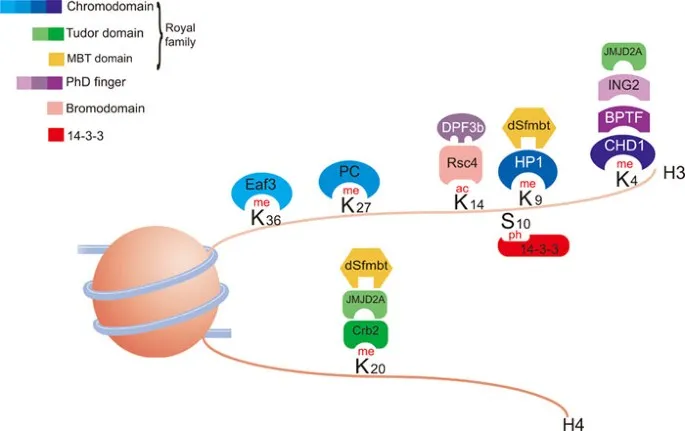
Epigenetic mechanisms in cancer US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Epigenetic mechanisms in cancer. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Epigenetic mechanisms in cancer US Medical PG Question 1: A 65-year-old man comes to the physician because of a 1-month history of progressive back pain. He has also had a 5-kg (11-lb) weight loss over the past 3 months. His only medications are a daily multivitamin and ibuprofen, which he takes daily for the back pain. Physical examination shows tenderness to palpation over the lower spine and the left iliac crest. His hemoglobin concentration is 9.3 g/dL, his serum calcium concentration is 12 mg/dL, and his serum creatinine concentration is 2.1 mg/dL. A bone marrow biopsy shows 21% plasma cells. A diagnosis of multiple myeloma is established. In preparation for an autologous hematopoietic stem cell transplantation, the patient receives a myeloablative treatment regimen that includes busulfan. Which of the following drugs acts via a similar mechanism of action to busulfan?
- A. Etoposide
- B. Vemurafenib
- C. Vincristine
- D. Cytarabine
- E. Lomustine (Correct Answer)
Epigenetic mechanisms in cancer Explanation: ***Lomustine***
- Both **busulfan** and **lomustine** are **alkylating agents**. They act by transferring **alkyl groups** to DNA, leading to cross-linking of DNA strands and inhibition of DNA synthesis and function.
- This **DNA damage** results in cell cycle arrest and apoptosis, particularly in rapidly dividing cells like cancer cells.
*Etoposide*
- **Etoposide** is a **topoisomerase II inhibitor** that prevents DNA relegation after strand breaks, leading to DNA damage and cell death.
- While it also targets DNA, its mechanism is distinct from the alkylation process of busulfan.
*Vemurafenib*
- **Vemurafenib** is a **BRAF kinase inhibitor** used in melanoma treatment. It specifically targets the **BRAF V600E mutation**.
- Its mechanism involves blocking signal transduction pathways critical for cell proliferation, rather than directly damaging DNA.
*Vincristine*
- **Vincristine** is a **vinca alkaloid** that acts as a **microtubule inhibitor**, preventing the formation of the **mitotic spindle** during cell division.
- This leads to metaphase arrest and apoptosis, a mechanism fundamentally different from DNA alkylation.
*Cytarabine*
- **Cytarabine** is an **antimetabolite**, specifically a **pyrimidine analog**, that inhibits **DNA polymerase**.
- It gets incorporated into DNA, leading to chain termination and inhibition of DNA synthesis and repair, making its action different from direct DNA alkylation.
Epigenetic mechanisms in cancer US Medical PG Question 2: An investigator studying epigenetic mechanisms isolates histone proteins, the structural motifs involved in DNA binding and regulation of transcription. The peptide bonds of histone proteins are hydrolyzed and one type of amino acid is isolated. At normal body pH, this amino acid has a net charge of +1 . The investigator performs titration of this amino acid and obtains the graph shown. The isolated amino acid is most likely which of the following?
- A. Proline
- B. Lysine (Correct Answer)
- C. Aspartate
- D. Serine
- E. Histidine
Epigenetic mechanisms in cancer Explanation: ***Lysine***
- Histones are **positively charged** proteins rich in **basic amino acids** like lysine and arginine, which allows them to bind tightly to the negatively charged DNA.
- The titration curve shown with three distinct pKa values and a net charge of +1 at normal body pH (around 7.4) is characteristic of **lysine**, which has both an alpha-amino group (pKa ~9-10) and a basic side chain (pKa ~10.5).
*Proline*
- **Proline is a nonpolar** amino acid that does not contribute significantly to the positive charge of histones required for DNA binding.
- Furthermore, its unique cyclic structure incorporates its amino group into the ring, impacting its pKa relative to other primary amino acids but not making it a primary basic residue in the histone context.
*Aspartate*
- **Aspartate is an acidic amino acid** with a negatively charged side chain at physiological pH, which would repel DNA rather than bind to it.
- Its titration curve would show a net negative charge at normal body pH, not a positive one.
*Serine*
- **Serine is a polar, uncharged** amino acid and would not contribute the necessary positive charge for histone-DNA interaction.
- Its side chain lacks an ionizable group within the physiological pH range, so its titration curve would only show two pKa values (for the carboxyl and amino groups) and a net charge of 0 at neutral pH.
*Histidine*
- While **histidine is a basic amino acid**, its side chain pKa is around 6.0, meaning it is only partially protonated and positively charged at physiological pH.
- A protein rich in **histidine** would not consistently carry a strong positive charge across the typical physiological pH range as effectively as one rich in lysine or arginine.
Epigenetic mechanisms in cancer US Medical PG Question 3: A 5-year-old African-American boy is brought to the physician because of fatigue and night sweats for the past month. During this time, he has also lost 3 kg (6.6 lbs). Before the onset of symptoms, he had been healthy except for a febrile seizure as an infant. His brother had chickenpox 2 months ago. He is at the 75th percentile for height and 50th percentile for weight. He appears markedly fatigued. His temperature is 38°C (100.4°F), pulse is 95/min, respirations are 19/min, and blood pressure is 100/60 mm Hg. Lung and cardiac examination is normal. There are enlarged, nontender lymph nodes bilaterally in the neck. The abdomen is soft and nontender. A complete blood count shows:
Leukocyte count 8,000/mm³
Hemoglobin 9.1 g/dL
Hematocrit 26.9%
Platelet count 34,000/mm³
Serum
Na+ 135 mEq/L
K+ 4.5 mEq/L
Cl- 101 mEq/L
HCO3- 27 mEq/L
Urea nitrogen 9 mg/dL
Creatinine 0.7 mg/dL
Ca2+ 8.8 mg/dL
PCR testing demonstrates a 9:22 chromosomal translocation. The patient is diagnosed with Philadelphia chromosome-positive acute lymphoblastic leukemia. Which of the following is the most appropriate targeted therapy component?
- A. Cladribine
- B. Imatinib (Correct Answer)
- C. Hydroxyurea
- D. All-trans retinoic acid
- E. Transfuse platelets
Epigenetic mechanisms in cancer Explanation: ***Imatinib***
- The patient has **Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL)**, indicated by the **9:22 chromosomal translocation** (BCR-ABL fusion gene).
- **Imatinib** is a tyrosine kinase inhibitor (TKI) that specifically targets the **BCR-ABL fusion protein**, making it the most appropriate targeted therapy for Ph+ ALL.
*Cladribine*
- **Cladribine** is a purine analog primarily used in the treatment of **hairy cell leukemia** and some forms of lymphoma.
- It is not a targeted therapy for the **BCR-ABL fusion gene** in Ph+ ALL.
*Hydroxyurea*
- **Hydroxyurea** is a myelosuppressive agent used to rapidly lower high blood counts in conditions like **chronic myeloid leukemia (CML)** or **myeloproliferative neoplasms**.
- It does not target the specific genetic abnormality of Ph+ ALL.
*All-trans retinoic acid*
- **All-trans retinoic acid (ATRA)** is a form of vitamin A used in the treatment of **acute promyelocytic leukemia (APL)**.
- ATRA induces differentiation of promyelocytes and is not effective for Ph+ ALL.
*Transfuse platelets*
- While the patient has **thrombocytopenia** (platelet count 34,000/mm³), **platelet transfusion** is a supportive measure, not a targeted therapy for leukemia.
- It addresses a complication of the disease rather than the underlying oncogenic driver.
Epigenetic mechanisms in cancer US Medical PG Question 4: An investigator is studying the effects of zinc deprivation on cancer cell proliferation. It is hypothesized that because zinc is known to be a component of transcription factor motifs, zinc deprivation will result in slower tumor growth. To test this hypothesis, tumor cells are cultured on media containing low and high concentrations of zinc. During the experiment, a labeled oligonucleotide probe is used to identify the presence of a known transcription factor. The investigator most likely used which of the following laboratory techniques?
- A. ELISA
- B. PCR
- C. Western blot
- D. Northern blot
- E. Southwestern blot (Correct Answer)
Epigenetic mechanisms in cancer Explanation: ***Southwestern blot***
- A **Southwestern blot** specifically identifies **DNA-binding proteins** (such as transcription factors) by detecting their ability to bind to specific **labeled DNA oligonucleotide probes**
- The technique involves: protein separation by gel electrophoresis → transfer to membrane → probing with **labeled double-stranded DNA oligonucleotide**
- This directly answers the question: using a labeled oligonucleotide probe to identify a transcription factor
*ELISA*
- **ELISA** detects and quantifies proteins using **antibody-antigen interactions**, not DNA-binding activity
- While it could detect the presence of a transcription factor protein, it cannot assess the protein's ability to bind to specific DNA sequences
- Does not utilize oligonucleotide probes for detection
*PCR*
- **PCR** amplifies specific **DNA sequences** but does not detect or characterize proteins
- This technique would amplify DNA, not identify DNA-binding proteins
- Not applicable for detecting transcription factor presence or function
*Western blot*
- **Western blot** detects specific proteins using **antibodies**, not oligonucleotide probes
- While it could confirm transcription factor protein presence, it cannot assess DNA-binding capability
- Uses antibody-based detection, not nucleotide probe-based detection
*Northern blot*
- **Northern blot** detects specific **RNA molecules**, not DNA-binding proteins
- Uses labeled DNA or RNA probes to detect RNA, not to detect proteins that bind DNA
- Wrong target molecule (RNA vs. proteins)
Epigenetic mechanisms in cancer US Medical PG Question 5: A 62-year-old man with small cell lung cancer undergoes radiation therapy. His oncologist explains that radiation causes DNA damage and double strand breaks and this damage stops the cancer cells from growing because they can no longer replicate their DNA. One key mediator of this process is a cell cycle regulator called P53, which is upregulated after DNA damage and helps to trigger cell cycle arrest and apoptosis. One mechanism by which P53 activity is increased is a certain chromatin modification that loosens DNA coiling allowing for greater transcription of the proteins within that region of DNA. Which of the following enzymes most likely causes the chromatin modification described in this case?
- A. Histone deacetylase
- B. Histone acetyltransferase (Correct Answer)
- C. Histone methyltransferase
- D. DNA methyltransferase
- E. Xist
Epigenetic mechanisms in cancer Explanation: ***Histone acetyltransferase***
- This enzyme **acetylates histone proteins**, neutralizing their positive charge and thereby weakening their interaction with negatively charged DNA.
- This modification leads to a more **relaxed chromatin structure (euchromatin)**, making DNA more accessible for **transcription**, which is consistent with the upregulation of P53.
*Histone deacetylase*
- This enzyme **removes acetyl groups from histones**, making them more positively charged and increasing their affinity for DNA.
- This results in **condensed chromatin (heterochromatin)**, which generally **represses gene transcription**.
*Histone methyltransferase*
- This enzyme **adds methyl groups to histones**, which can either activate or repress gene transcription depending on the specific **lysine or arginine residue** methylated and the number of methyl groups added.
- While methylation is a chromatin modification, the question specifically describes a process of **loosening DNA coiling for greater transcription**, which is more characteristic of acetylation.
*DNA methyltransferase*
- This enzyme **adds methyl groups directly to DNA**, typically at **CpG sites**, leading to **gene silencing** by hindering transcription factor binding or recruiting repressor complexes.
- This modification primarily affects DNA directly, not histone proteins, and generally **inhibits gene expression**.
*Xist*
- **Xist (X-inactive specific transcript)** is a **long non-coding RNA** that plays a crucial role in **X-chromosome inactivation** in females.
- It functions by coating one of the X chromosomes, leading to its transcriptional silencing, rather than directly modifying chromatin for general gene upregulation.
Epigenetic mechanisms in cancer US Medical PG Question 6: An investigator is studying DNA repair processes in an experimental animal. The investigator inactivates a gene encoding a protein that physiologically excises nucleotides from damaged, bulky, helix-distorting DNA strands. A patient with a similar defect in this gene is most likely to present with which of the following findings?
- A. Ataxic gait and facial telangiectasias
- B. Malignant breast and ovarian growths
- C. Leukocoria and a painful bone mass
- D. Colorectal and endometrial cancers
- E. Dry skin and increased photosensitivity (Correct Answer)
Epigenetic mechanisms in cancer Explanation: ***Dry skin and increased photosensitivity***
- The description of excising **nucleotides from damaged, bulky, helix-distorting DNA strands** points to a defect in **Nucleotide Excision Repair (NER)**.
- Patients with defects in NER, such as those with **xeroderma pigmentosum**, are highly susceptible to UV-induced DNA damage, leading to **dry skin, increased photosensitivity**, and a high risk of skin cancers.
*Ataxic gait and facial telangiectasias*
- This constellation of symptoms is characteristic of **ataxia-telangiectasia**, a disorder caused by mutations in the **ATM gene**, which is involved in **DNA double-strand break repair**.
- While a DNA repair defect, it's not primarily linked to the excision of bulky, helix-distorting DNA strands.
*Malignant breast and ovarian growths*
- These cancers are commonly associated with mutations in the **BRCA1 and BRCA2 genes**, which play crucial roles in **homologous recombination repair of DNA double-strand breaks**.
- This type of repair is distinct from the excision of bulky, helix-distorting DNA strands described in the question.
*Leukocoria and a painful bone mass*
- **Leukocoria** can indicate **retinoblastoma**, linked to mutations in the **RB1 tumor suppressor gene**, which regulates the cell cycle but isn't primarily a DNA repair gene.
- A painful bone mass could suggest **osteosarcoma**, which is sometimes seen in retinoblastoma patients but not directly related to the specific DNA repair defect described.
*Colorectal and endometrial cancers*
- These cancers are hallmarks of **Lynch syndrome (hereditary nonpolyposis colorectal cancer - HNPCC)**, which is caused by defects in **Mismatch Repair (MMR)** genes (e.g., MLH1, MSH2, MSH6, PMS2).
- Mismatch repair corrects errors that arise during DNA replication, which is different from excising bulky, helix-distorting DNA damage.
Epigenetic mechanisms in cancer US Medical PG Question 7: A 32-year-old woman visits her primary care provider with the results of a recent colonoscopy, which was ordered after 3 episodes of rectal bleeding in the last month. Her grandmother, mother, and sister all have been diagnosed with nonpolyposis colorectal cancer, at ages 65, 50, and 40 years, respectively. Colonoscopy for this patient revealed a large, flat, right-sided adenoma. Histopathological examination of the lesion showed villous histology and high-grade dysplasia. Which of the following helps explain the condition of this patient?
- A. Chromosomal instability
- B. Chemical carcinogenicity
- C. DNA hypermethylation
- D. Environmental carcinogenicity
- E. Microsatellite instability (Correct Answer)
Epigenetic mechanisms in cancer Explanation: ***Microsatellite instability***
- The patient's **family history** of early-onset, **nonpolyposis colorectal cancer** in three first-degree relatives across three generations strongly suggests a **hereditary syndrome**, specifically **Lynch syndrome (HNPCC)**, which is characterized by **microsatellite instability**.
- **Lynch syndrome** results from germline mutations in **DNA mismatch repair genes** (e.g., MLH1, MSH2, MSH6, PMS2), leading to accumulation of mutations in microsatellite regions and increased cancer risk. The patient's large, flat, right-sided adenoma with villous histology and high-grade dysplasia is also typical of Lynch syndrome-associated lesions.
*Chromosomal instability*
- **Chromosomal instability** (CIN) is characteristic of the **adenoma-carcinoma sequence** seen in sporadic colorectal cancer, involving large-scale chromosomal alterations (aneuploidy, translocations, deletions).
- While CIN is common in sporadic colorectal cancers, the strong family history and early onset in this patient point away from the typical CIN pathway as the primary cause.
*Chemical carcinogenicity*
- **Chemical carcinogenicity** refers to cancer development due to exposure to specific chemicals, which is generally associated with sporadic cancers and lacks the clear hereditary pattern seen here.
- While environmental factors can influence cancer risk, the striking familial presentation in this case makes a solely chemical cause unlikely.
*DNA hypermethylation*
- **DNA hypermethylation** of promoter regions leading to gene silencing, particularly the **CpG island methylator phenotype (CIMP)**, is found in both sporadic colorectal cancers and some Lynch syndrome cases (especially those with MLH1 promoter methylation).
- However, **microsatellite instability** is the direct consequence of germline mutations in mismatch repair genes, which is the fundamental defect in Lynch syndrome suggested by the patient's family history.
*Environmental carcinogenicity*
- **Environmental carcinogenicity** broadly refers to cancer caused by external factors such as diet, smoking, or radiation.
- While environmental factors can play a role in all cancers, the strong autosomal dominant inheritance pattern and early onset of nonpolyposis colorectal cancer in this patient's family point towards a specific genetic predisposition rather than solely environmental causes.
Epigenetic mechanisms in cancer US Medical PG Question 8: A 16-year-old male presents to the emergency department complaining of episodes of pounding headache, chest fluttering, and excessive sweating. He has a past history of kidney stones that are composed of calcium oxalate. He does not smoke or drink alcohol. Family history reveals that his mother died of thyroid cancer. Vital signs reveal a temperature of 37.1°C (98.7°F), blood pressure of 200/110 mm Hg and pulse of 120/min. His 24-hour urine calcium, serum metanephrines, and serum normetanephrines levels are all elevated. Mutation of which of the following genes is responsible for this patient's condition?
- A. RET proto-oncogene (Correct Answer)
- B. BCL2
- C. BRAF
- D. BCR-ABL
- E. HER-2/neu (C-erbB2)
Epigenetic mechanisms in cancer Explanation: ***RET proto-oncogene***
- The patient's symptoms (pounding headache, chest fluttering, sweating, hypertension, tachycardia), elevated metanephrines, and a history of kidney stones (suggesting **hyperparathyroidism**) combined with a family history of **thyroid cancer** are classic for **Multiple Endocrine Neoplasia type 2A (MEN2A)**.
- **MEN2A** is caused by a germline mutation in the **RET proto-oncogene** and typically involves **medullary thyroid carcinoma**, **pheochromocytoma** (explaining the adrenal symptoms), and **primary hyperparathyroidism**.
*BCL2*
- The **BCL2 gene** is an **anti-apoptotic gene** primarily associated with lymphomas, particularly **follicular lymphoma**, where its overexpression promotes cell survival.
- Mutations or translocations involving BCL2 are not linked to endocrine disorders like MEN2A or the specific combination of symptoms seen in this patient.
*BRAF*
- The **BRAF gene** encodes a protein involved in cell growth signaling and is commonly mutated in various cancers, most notably **melanoma** and **papillary thyroid carcinoma**.
- While associated with thyroid cancer, a BRAF mutation does not explain the pheochromocytoma, hyperparathyroidism, or the specific family history indicative of MEN2A.
*BCR-ABL*
- The **BCR-ABL fusion gene** results from the **Philadelphia chromosome translocation (t(9;22))** and is the hallmark of **chronic myeloid leukemia (CML)** and some cases of acute lymphoblastic leukemia.
- This gene is a potent oncogene in hematopoietic malignancies and has no association with the endocrine tumors or symptoms described in the patient.
*HER-2/neu (C-erbB2)*
- **HER-2/neu (C-erbB2)** is an oncogene that encodes a receptor tyrosine kinase and is primarily associated with **breast cancer** and some **gastric cancers**, where its overexpression indicates a more aggressive tumor and guides targeted therapy.
- This gene is not implicated in the pathogenesis of MEN2A or the constellation of symptoms observed in this patient.
Epigenetic mechanisms in cancer US Medical PG Question 9: A 10-year-old boy is brought by his mother to his pediatrician for "skin growths." His mother reports that she started noticing small lumps arising from the patient's lips and eyelids several months ago. She also notes that he seems to suffer from frequent constipation and appears "weaker" than many of his peers. The boy's past medical history is unremarkable. His father and paternal grandmother have a history of medullary thyroid carcinoma. His height and weight are in the 85th and 45th percentiles, respectively. His temperature is 99°F (37.1°C), blood pressure is 110/65 mmHg, pulse is 90/min, and respirations are 18/min. On examination, he has an elongated face with protruding lips. There are numerous sessile painless nodules on the patient's lips, tongue, and eyelids. This patient's condition is most strongly associated with a mutation in which of the following genes?
- A. NF1
- B. MEN1
- C. RET (Correct Answer)
- D. NF2
- E. c-KIT
Epigenetic mechanisms in cancer Explanation: ***RET***
- The constellation of **skin growths** on the lips and eyelids (neuromas), **constipation** (ganglioneuromatosis), and a family history of **medullary thyroid carcinoma** (MTC) strongly suggests **Multiple Endocrine Neoplasia type 2B (MEN2B)**.
- MEN2B is caused by a germline mutation in the **RET proto-oncogene**, which is a receptor tyrosine kinase involved in cell growth and differentiation.
*NF1*
- Mutations in the **NF1 gene** cause **Neurofibromatosis type 1**, characterized by **café-au-lait spots**, neurofibromas (subcutaneous, not typically mucosal), iris Lisch nodules, and optic pathway gliomas.
- While it involves skin growths (neurofibromas), the specific mucosal neuromas, elongated facies, and family history of MTC are not typical features.
*MEN1*
- **MEN1 syndrome** is caused by mutations in the **MEN1 gene** and is associated with tumors of the **parathyroid**, **anterior pituitary**, and **pancreatic islet cells** (the 3 Ps).
- This patient's presentation of mucosal neuromas and medullary thyroid carcinoma is not characteristic of MEN1.
*NF2*
- Mutations in the **NF2 gene** cause **Neurofibromatosis type 2**, classically characterized by **bilateral vestibular schwannomas**, meningiomas, and ependymomas.
- Skin manifestations are less prominent and different from what is described, and MTC is not associated.
*c-KIT*
- Mutations in the **c-KIT gene** are primarily associated with **gastrointestinal stromal tumors (GIST)** and certain types of mastocytosis.
- It is not linked to the constellation of mucosal neuromas, elongated facies, or medullary thyroid carcinoma seen in this patient.
Epigenetic mechanisms in cancer US Medical PG Question 10: A 56-year-old woman presents to a physician for evaluation of a lump in her left breast. She noticed the lump last week while taking a shower. She says that the lump seemed to be getting larger, which worried her. The lump is not painful. The medical history is unremarkable. She has smoked cigarettes for the last 30 years. On examination, bilateral small nodules are present that are non-tender and immobile. A mammography confirms the masses and fine needle aspiration cytology of the lesions reveals malignant cells arranged in a row of cells. What is the most likely diagnosis?
- A. Fibroadenoma
- B. Mucinous carcinoma
- C. Inflammatory carcinoma
- D. Invasive lobular carcinoma (Correct Answer)
- E. Invasive ductal carcinoma
Epigenetic mechanisms in cancer Explanation: ***Invasive lobular carcinoma***
- The classic presentation of **invasive lobular carcinoma** includes **bilateral, non-tender, immobile masses** and malignant cells arranged in a **single-file pattern** on FNA, often described as "**Indian files**".
- This type of cancer frequently presents with subtle thickening or diffuse induration rather than well-defined masses due to its infiltrative growth pattern.
*Fibroadenoma*
- **Fibroadenomas** are typically **benign, mobile, well-defined** masses, often described as "rubbery" or "slippery," unlike the immobile nodules described.
- While they can be firm, they do not show malignant cells on FNA or the classic "Indian file" arrangement.
*Mucinous carcinoma*
- **Mucinous carcinoma** is characterized by the presence of tumor cells floating in abundant **extracellular mucin**, which would be evident on FNA.
- This typically presents as a **soft, gelatinous mass**, which doesn't align with the description of firm, immobile nodules.
*Inflammatory carcinoma*
- **Inflammatory carcinoma** presents with characteristic inflammatory signs like **redness, warmth, swelling, and peau d'orange** (orange peel skin appearance) due to dermal lymphatic invasion.
- These prominent skin changes are not mentioned in the patient's presentation.
*Invasive ductal carcinoma*
- **Invasive ductal carcinoma** usually presents as a **solitary, firm, irregular mass** and on FNA typically shows malignant cells in **duct-like structures** or disorganized clusters, not characteristically in single-file lines.
- While it is the most common type of breast cancer, the specific "Indian file" arrangement points more strongly to lobular carcinoma.
More Epigenetic mechanisms in cancer US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.