Apoptosis and cancer US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Apoptosis and cancer. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Apoptosis and cancer US Medical PG Question 1: A 58-year-old woman presents to a physician with a painless swelling behind her right ear, which she noticed 1 month ago. She has no other complaint nor does she have any specific medical condition. On physical examination, her vital signs are stable. An examination of the right post-auricular area shows enlarged lymph nodes, which are non-tender and rubbery in consistency, with normal overlying skin. A detailed general examination reveals the presence of one enlarged axillary lymph node on the left side with similar features. Complete blood counts are within normal limits but atypical lymphocytes are present on the peripheral blood smear. The patient’s serum lactate dehydrogenase level is slightly elevated. Excisional biopsy of the lymph node is performed and histopathological examination of the tissue yields a diagnosis of follicular lymphoma. Further cytogenetic studies reveal that the condition is associated with overexpression of the BCL-2 gene. Which of the following cytogenetic abnormalities is most likely to be present?
- A. t(3;14)(q27;q32)
- B. t(11;18)(q21;q21)
- C. t(8;14)(q24;q32)
- D. t(11;14)(q13;q32)
- E. t(14;18)(q32;q21) (Correct Answer)
Apoptosis and cancer Explanation: ***t(14;18)(q32;q21)***
- This translocation is the **hallmark of follicular lymphoma**, present in approximately **85-90% of cases**.
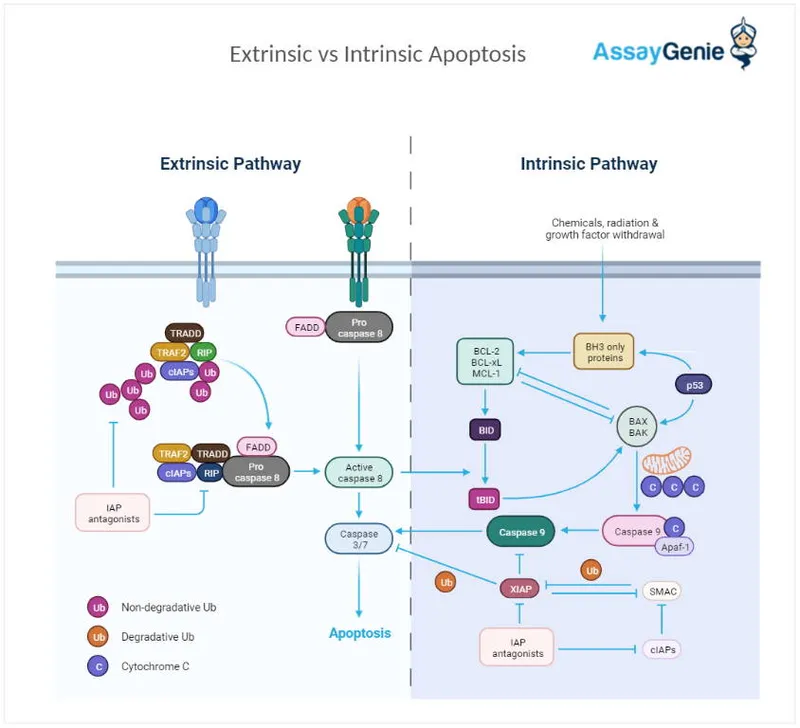
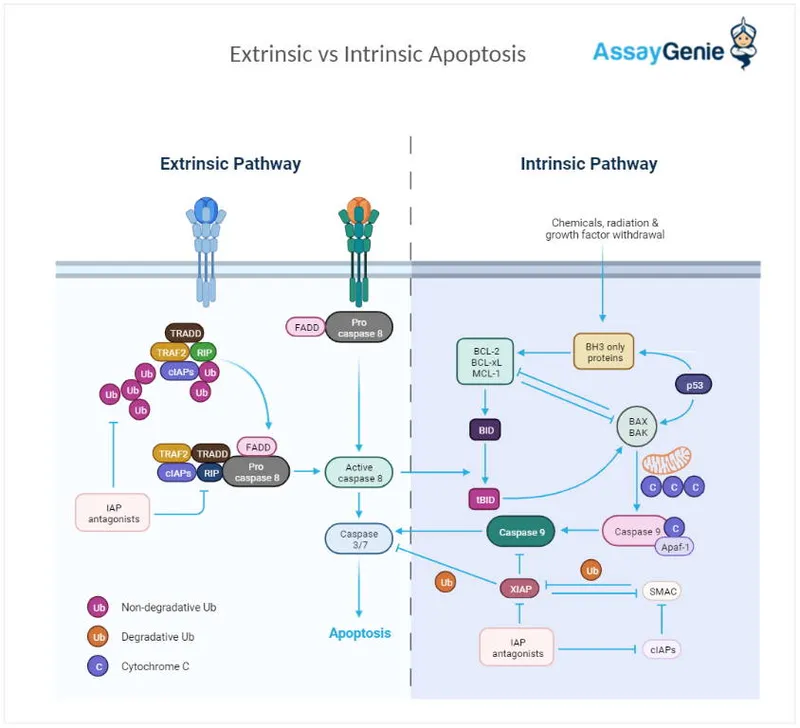
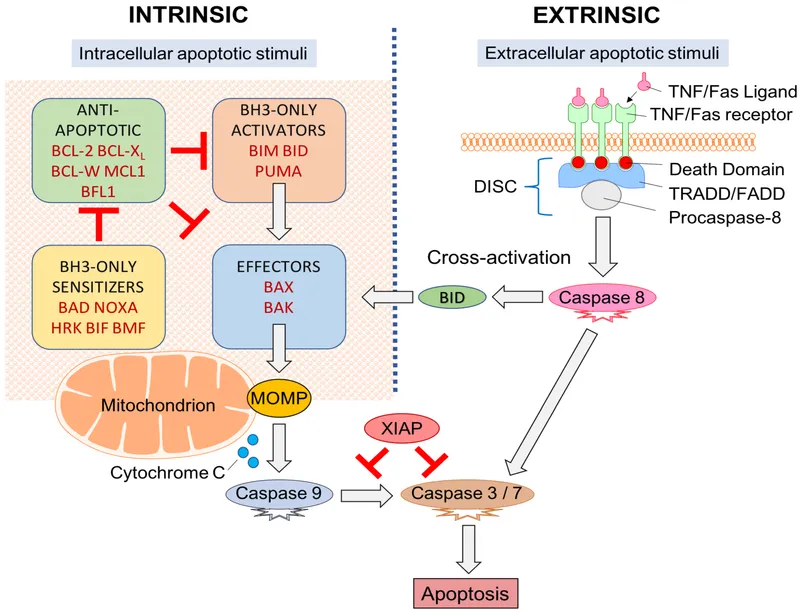
- The **BCL-2 gene** on chromosome **18q21** is translocated to the **immunoglobulin heavy chain (IGH) locus** on chromosome **14q32**, leading to **BCL-2 overexpression**.
- BCL-2 is an **anti-apoptotic protein** that promotes **B-cell survival** by inhibiting programmed cell death, resulting in accumulation of malignant lymphocytes.
*t(3;14)(q27;q32)*
- This translocation involves the **BCL6 gene** on chromosome **3q27** and the immunoglobulin heavy chain locus on chromosome **14q32**.
- It is more commonly associated with **diffuse large B-cell lymphoma (DLBCL)** and occasionally seen in follicular lymphomas with transformation.
- BCL6 rearrangements promote lymphomagenesis through dysregulation of germinal center B-cell differentiation, but this is **not the mechanism** in primary follicular lymphoma with BCL-2 overexpression.
*t(11;18)(q21;q21)*
- This translocation is characteristic of **extranodal marginal zone lymphoma** of mucosa-associated lymphoid tissue (**MALT lymphoma**).
- It results in the **API2-MALT1 fusion gene**, which activates NF-κB signaling.
- This is distinct from the BCL-2 overexpression mechanism seen in follicular lymphoma.
*t(8;14)(q24;q32)*
- This translocation is the **hallmark of Burkitt lymphoma**, leading to **MYC gene overexpression**.
- The **MYC oncogene** on chromosome **8q24** is juxtaposed to the immunoglobulin heavy chain locus on chromosome **14q32**.
- MYC overexpression drives aggressive cell proliferation through dysregulation of cell cycle control, which is different from the anti-apoptotic mechanism of BCL-2 in follicular lymphoma.
*t(11;14)(q13;q32)*
- This translocation is the **hallmark of mantle cell lymphoma**, leading to **cyclin D1 (CCND1) overexpression**.
- The **CCND1 gene** on chromosome **11q13** is translocated to the immunoglobulin heavy chain locus on chromosome **14q32**.
- Cyclin D1 overexpression promotes cell cycle progression from G1 to S phase, which is a different pathogenic mechanism from BCL-2-mediated inhibition of apoptosis in follicular lymphoma.
Apoptosis and cancer US Medical PG Question 2: Researchers are investigating the mechanism of cell apoptosis and host defense in mice. They have observed that mice with certain gene deletions are not able to fight the induced viral infection. They identify a cell that is able to destroy target cells infected with viruses by exocytosis of granule contents, which induces the activation of caspases. Which type of cell is responsible for this process?
- A. CD8+ lymphocytes (Correct Answer)
- B. CD4+ lymphocytes
- C. Macrophages
- D. Neutrophils
- E. Eosinophils
Apoptosis and cancer Explanation: ***CD8+ lymphocytes***
- **CD8+ lymphocytes**, or **cytotoxic T lymphocytes (CTLs)**, are specialized to recognize and kill **virus-infected cells** and cancer cells.
- They achieve this by releasing cytotoxic granules containing **perforin** and **granzymes**, which enter the target cell and activate **caspases**, leading to **apoptosis**.
- Note: **Natural killer (NK) cells** also use a similar granule-mediated mechanism, but CD8+ T cells provide **antigen-specific** recognition via MHC class I.
*CD4+ lymphocytes*
- **CD4+ lymphocytes**, or **helper T cells**, primarily coordinate immune responses by secreting **cytokines** and activating other immune cells, rather than directly killing infected cells.
- They are crucial for both humoral and cell-mediated immunity but do not typically induce apoptosis via granule exocytosis.
*Macrophages*
- **Macrophages** are phagocytic cells that engulf and digest pathogens, cellular debris, and foreign substances.
- While they can present antigens and participate in immune responses, their primary role in antiviral defense is **phagocytosing infected cells** and presenting antigens, not inducing apoptosis via granule exocytosis.
*Neutrophils*
- **Neutrophils** are key components of the innate immune system, primarily involved in fighting bacterial infections through **phagocytosis**, degranulation, and formation of **neutrophil extracellular traps (NETs)**.
- They are not specialized for detecting and inducing apoptosis in virus-infected cells.
*Eosinophils*
- **Eosinophils** are primarily involved in the immune response against **parasitic infections** and allergic reactions.
- They release granules containing toxic proteins against parasites and contribute to inflammation, but they do not directly kill virus-infected cells via caspase activation.
Apoptosis and cancer US Medical PG Question 3: A 34-year-old woman comes to the physician for evaluation of a breast lump she noticed 2 days ago while showering. She has no history of major illness. Her mother died of ovarian cancer at age 38, and her sister was diagnosed with breast cancer at age 33. Examination shows a 1.5-cm, nontender, mobile mass in the upper outer quadrant of the left breast. Mammography shows pleomorphic calcifications. Biopsy of the mass shows invasive ductal carcinoma. The underlying cause of this patient's condition is most likely a mutation of a gene involved in which of the following cellular events?
- A. Repair of double-stranded DNA breaks (Correct Answer)
- B. Inhibition of programmed cell death
- C. Regulation of intercellular adhesion
- D. Activity of cytoplasmic tyrosine kinase
- E. Arrest of cell cycle in G1 phase
Apoptosis and cancer Explanation: ***Repair of double-stranded DNA breaks***
- The patient's **family history** (mother with ovarian cancer at 38, sister with breast cancer at 33) and early onset of **invasive ductal carcinoma** strongly suggest an inherited cancer syndrome.
- **BRCA1 and BRCA2 genes** are tumor suppressor genes responsible for repairing **double-stranded DNA breaks**, and mutations in these genes significantly increase the risk of breast and ovarian cancers.
*Inhibition of programmed cell death*
- Mutations leading to the **inhibition of programmed cell death (apoptosis)**, such as those affecting the **Bcl-2 gene**, can contribute to cancer by allowing damaged cells to survive and proliferate.
- While relevant to cancer pathogenesis, it is not the primary mechanism associated with the specific familial breast/ovarian cancer pattern seen here, which points more directly to DNA repair defects.
*Regulation of intercellular adhesion*
- Defects in **intercellular adhesion**, often involving **E-cadherin** (CDH1 gene) mutations, are associated with cancers like **lobular breast carcinoma** and **hereditary diffuse gastric cancer**.
- This patient has **invasive ductal carcinoma**, and the specific familial pattern is less characteristic of intercellular adhesion defects.
*Activity of cytoplasmic tyrosine kinase*
- Abnormal **cytoplasmic tyrosine kinase activity** is implicated in various cancers (e.g., **HER2/neu** amplification in breast cancer, **BCR-ABL** fusion in CML).
- While HER2/neu overexpression is common in breast cancer, it is typically a somatic mutation or amplification, and not the underlying germline defect explaining the strong family history of early-onset breast and ovarian cancer.
*Arrest of cell cycle in G1 phase*
- The **arrest of the cell cycle at the G1 phase** is mainly regulated by **p53** and **Rb tumor suppressor genes**, which prevent uncontrolled cell division.
- While mutations in these genes are crucial in many cancers, the specific familial pattern (breast and ovarian cancer) points more strongly to defects in homologous recombination via BRCA1/2, a different DNA repair pathway.
Apoptosis and cancer US Medical PG Question 4: A 67-year-old man comes to the physician for a follow-up examination after he was diagnosed with mantle cell lymphoma. The physician recommends a chemotherapeutic regimen containing bortezomib. Which of the following best describes the effect of this drug?
- A. Crosslinking of purine bases
- B. Preventing the relaxation of DNA supercoils
- C. Inhibition of tyrosine kinase receptors
- D. Accumulation of ubiquitinated proteins (Correct Answer)
- E. Stabilization of tubulin polymers
Apoptosis and cancer Explanation: ***Accumulation of ubiquitinated proteins***
- **Bortezomib** is a **proteasome inhibitor**, specifically targeting the 26S proteasome, which is responsible for degrading ubiquitinated proteins.
- Its inhibition leads to the accumulation of various **ubiquitinated proteins**, including pro-apoptotic factors, ultimately inducing **apoptosis** in cancer cells.
*Crosslinking of purine bases*
- This mechanism is characteristic of **alkylating agents** such as cyclophosphamide or cisplatin, which form covalent bonds with DNA, preventing replication and transcription.
- **Bortezomib** does not directly crosslink DNA bases; its primary action is on protein degradation pathways.
*Preventing the relaxation of DNA supercoils*
- This describes the mechanism of **topoisomerase inhibitors**, such as etoposide (topoisomerase II) or irinotecan (topoisomerase I), which block DNA replication and repair.
- Bortezomib has a distinct mechanism involving proteasome inhibition, not direct interaction with DNA or topoisomerases.
*Inhibition of tyrosine kinase receptors*
- This is the action of **tyrosine kinase inhibitors**, a class of drugs like imatinib or gefitinib, that target specific signaling pathways involved in cell growth and proliferation.
- Bortezomib's anti-cancer effects are mediated through protein degradation pathways, not by inhibiting receptor tyrosine kinases.
*Stabilization of tubulin polymers*
- This mechanism is characteristic of **taxanes** (e.g., paclitaxel), which hyperstabilize microtubules, interfering with cell division.
- **Bortezomib** does not affect microtubule dynamics; its action is focused on the proteasomal degradation system.
Apoptosis and cancer US Medical PG Question 5: A 56-year-old woman presents to a physician for evaluation of a lump in her left breast. She noticed the lump last week while taking a shower. She says that the lump seemed to be getting larger, which worried her. The lump is not painful. The medical history is unremarkable. She has smoked cigarettes for the last 30 years. On examination, bilateral small nodules are present that are non-tender and immobile. A mammography confirms the masses and fine needle aspiration cytology of the lesions reveals malignant cells arranged in a row of cells. What is the most likely diagnosis?
- A. Fibroadenoma
- B. Mucinous carcinoma
- C. Inflammatory carcinoma
- D. Invasive lobular carcinoma (Correct Answer)
- E. Invasive ductal carcinoma
Apoptosis and cancer Explanation: ***Invasive lobular carcinoma***
- The classic presentation of **invasive lobular carcinoma** includes **bilateral, non-tender, immobile masses** and malignant cells arranged in a **single-file pattern** on FNA, often described as "**Indian files**".
- This type of cancer frequently presents with subtle thickening or diffuse induration rather than well-defined masses due to its infiltrative growth pattern.
*Fibroadenoma*
- **Fibroadenomas** are typically **benign, mobile, well-defined** masses, often described as "rubbery" or "slippery," unlike the immobile nodules described.
- While they can be firm, they do not show malignant cells on FNA or the classic "Indian file" arrangement.
*Mucinous carcinoma*
- **Mucinous carcinoma** is characterized by the presence of tumor cells floating in abundant **extracellular mucin**, which would be evident on FNA.
- This typically presents as a **soft, gelatinous mass**, which doesn't align with the description of firm, immobile nodules.
*Inflammatory carcinoma*
- **Inflammatory carcinoma** presents with characteristic inflammatory signs like **redness, warmth, swelling, and peau d'orange** (orange peel skin appearance) due to dermal lymphatic invasion.
- These prominent skin changes are not mentioned in the patient's presentation.
*Invasive ductal carcinoma*
- **Invasive ductal carcinoma** usually presents as a **solitary, firm, irregular mass** and on FNA typically shows malignant cells in **duct-like structures** or disorganized clusters, not characteristically in single-file lines.
- While it is the most common type of breast cancer, the specific "Indian file" arrangement points more strongly to lobular carcinoma.
Apoptosis and cancer US Medical PG Question 6: A 61-year-old Caucasian male presents to your office complaining of morning headaches of 6 weeks duration. A head MRI reveals a likely metastasis of unknown origin in the supratentorial region of the brain. On biopsy, the neoplastic mass is shown to have a mutation in BRAF, a protein kinase, in which a glutamic acid is substituted for valine at position 600 of the protein. Where did this metastasis most likely originate?
- A. Stomach
- B. Skin (Correct Answer)
- C. Breast
- D. Brain
- E. Bone
Apoptosis and cancer Explanation: ***Skin***
- A brain metastasis with a **V600E BRAF mutation** is highly suggestive of **melanoma**, a type of skin cancer.
- Melanoma frequently metastasizes to the **brain**, and the BRAF V600E mutation is a common and actionable target in advanced melanoma.
*Stomach*
- Stomach cancers (gastric adenocarcinomas) less commonly metastasize to the brain compared to melanoma.
- While BRAF mutations can occur in gastric cancer, the **V600E mutation** is not typically a defining feature of gastric cancer metastases to the brain.
*Breast*
- Breast cancer can metastasize to the brain, but the presence of a **BRAF V600E mutation** is not a characteristic genetic alteration for breast cancer.
- Common mutations in breast cancer include those in **ER, PR, and HER2** receptors or **PIK3CA**, not BRAF V600E.
*Brain*
- The question states the mass is a **metastasis of unknown origin**, implying it did not originate in the brain itself.
- Primary brain tumors like **gliomas** would not be described as metastases and have a different mutational spectrum.
*Bone*
- Bone cancers (sarcomas) or metastases to the bone usually do not present with a **BRAF V600E mutation** as their primary driver for brain metastasis.
- While various cancers can metastasize to bone, the specific mutation points away from a bone origin.
Apoptosis and cancer US Medical PG Question 7: A cell biologist is studying the activity of a novel chemotherapeutic agent against a cancer cell line. After incubation with the agent and cell detachment from the tissue culture plate, the DNA is harvested from the cells and run on a gel. Of note, there are large bands at every multiple of 180 base pairs on the gel. Which of the following explains the pathophysiology of this finding?
- A. Protein denaturation
- B. Release of lysosomal enzymes
- C. Cellular swelling
- D. ATP depletion
- E. Caspase activation (Correct Answer)
Apoptosis and cancer Explanation: ***Caspase activation***
- The presence of large bands at multiples of **180 base pairs** on a gel indicates a characteristic ladder-like fragmentation pattern of DNA. This fragmentation is a hallmark of **apoptosis**, a form of programmed cell death.
- **Caspase activation**, particularly that of **endonucleases** like caspase-activated DNase (CAD), is responsible for cleaving DNA between nucleosomes, leading to these distinct 180-bp fragments.
*Protein denaturation*
- **Protein denaturation** involves the unfolding of proteins due to stressors but does not directly cause DNA fragmentation into specific band sizes.
- While it can be a part of apoptosis or necrosis, it's not the primary mechanism explaining the observed **DNA laddering**.
*Release of lysosomal enzymes*
- **Lysosomal enzymes** are typically released during **necrosis** or severe cellular injury, leading to widespread, indiscriminate degradation of cellular components, including DNA.
- This degradation would result in a **smear** rather than discrete bands on a gel, as the DNA would be randomly fragmented.
*Cellular swelling*
- **Cellular swelling** is an early, reversible sign of cell injury often associated with **necrosis** or hydropic change due to ion pump dysfunction.
- It does not directly lead to **DNA fragmentation** or the specific laddering pattern seen in apoptosis.
*ATP depletion*
- **ATP depletion** is a critical event in cell injury, often leading to activation of anaerobic glycolysis, failure of ion pumps, and ultimately cell death.
- While **ATP depletion** can contribute to necrosis, apoptosis often requires ATP for the energy-dependent cascade of caspase activation and DNA fragmentation.
Apoptosis and cancer US Medical PG Question 8: A 58-year-old man presents with lower back pain that started a couple of weeks ago and is gradually increasing in severity. At present, he rates the intensity of the pain as 6/10. There is no radiation or associated paresthesias. There is no history of trauma. Past medical history is significant for aggressive squamous cell carcinoma of the right lung status post surgical resection followed by adjunct chemotherapy and radiation therapy that was completed 6 months ago. A technetium bone scan reveals metastatic lesions in the lumbar vertebrae at levels L2–L4. The physician explains to the patient that these are likely metastatic lesions from his primary lung cancer. Which of the following best describes the mechanism that most likely led to the development of these metastatic lesions?
- A. Transcoelomic
- B. Lymphatic spread
- C. Collagenase produced by cancer cells dissolves the basement membrane and aids in cellular invasion
- D. Hematogenous spread (Correct Answer)
- E. PTH (parathormone)-related protein production by tumor cells
Apoptosis and cancer Explanation: ***Hematogenous spread***
- Lung cancer frequently metastasizes to bone via the **hematogenous (bloodstream) route**, especially to the spine, pelvis, and long bones.
- The rich vascular supply of the vertebrae makes them a common site for metastases from many primary cancers, including those of the lung.
*Transcoelomic*
- **Transcoelomic spread** occurs when tumor cells spread directly within body cavities, such as the peritoneal or pleural cavity.
- This mechanism is typical for cancers of organs within these cavities, like ovarian cancer spreading within the peritoneum, and is not the primary route for lung cancer to distant bone.
*Lymphatic spread*
- **Lymphatic spread** involves tumor cells traveling through the lymphatic system to regional lymph nodes.
- While lung cancer commonly spreads to mediastinal and hilar lymph nodes, it is usually not the direct mechanism for distant bone metastases, which typically involve the circulatory system.
*Collagenase produced by cancer cells dissolves the basement membrane and aids in cellular invasion*
- While **collagenase production** and **basement membrane degradation** are crucial steps in local tumor invasion and intravasation (entering blood or lymphatic vessels), they describe the *how* a cell invades, not the *route* of distant metastasis.
- This mechanism facilitates the initial escape of cancer cells from the primary tumor but does not define the subsequent spread to distant sites like bone.
*PTH (parathormone)-related protein production by tumor cells*
- **PTH-related protein (PTHrP) production** by tumor cells can lead to **hypercalcemia of malignancy** due to its osteolytic effects.
- While this is a common paraneoplastic syndrome associated with squamous cell carcinoma of the lung, it is a *consequence* or *effect* of the tumor and does not describe the *mechanism of metastasis* itself.
Apoptosis and cancer US Medical PG Question 9: A 58-year-old male undergoes a surveillance colonoscopy in which a 2 cm adenoma is identified and removed. Had this adenoma not been excised, the patient would have been at risk of progression to carcinoma. Which of the following is the final mutational step in the progression from adenoma to carcinoma?
- A. p53 inactivation (Correct Answer)
- B. APC mutation
- C. COX-2 overexpression
- D. SMAD 2/4 loss
- E. K-ras mutation
Apoptosis and cancer Explanation: ***p53 inactivation***
- **p53 loss of function** is typically the final genetic event in the **adenoma-to-carcinoma sequence**, facilitating unrestricted cell growth and preventing apoptosis in dysplastic cells.
- The **p53 tumor suppressor gene** normally checkpoints cell division and induces programmed cell death, making its inactivation critical for malignant transformation.
*APC mutation*
- **APC (adenomatous polyposis coli) mutation** is often the **initiating event** in colorectal adenoma formation, leading to aberrant crypt foci and polyp formation.
- While critical for early tumor genesis, it does not represent the final step in progression to invasive carcinoma.
*COX-2 overexpression*
- **Cyclooxygenase-2 (COX-2) overexpression** leads to increased prostaglandin production, which can promote cell proliferation, angiogenesis, and inhibit apoptosis.
- It is an important factor in tumor growth and progression but occurs earlier in the sequence and is not the terminal mutational step for carcinoma.
*SMAD 2/4 loss*
- **SMAD 2/4 loss of function** disrupts the **TGF-β signaling pathway**, which normally inhibits cell growth and promotes differentiation.
- This event typically occurs in the late adenoma stage, contributing to dysplasia, but **p53 inactivation** is considered the final critical step for full malignant transformation.
*K-ras mutation*
- **K-ras mutation** is a well-known event in the **adenoma-to-carcinoma sequence**, occurring earlier than p53 inactivation, usually in intermediate-sized adenomas.
- It leads to constitutive activation of the RAS/MAPK pathway, promoting cell growth and survival, but generally before full malignant transformation.
More Apoptosis and cancer US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.