Neoplasia

On this page
🧬 The Neoplastic Revolution: Cellular Rebellion Unleashed
Neoplasia transforms normal cells into rogue populations that defy growth controls, invade tissues, and reshape entire organ systems. You'll trace the molecular battle between oncogenes and tumor suppressors, master the patterns that distinguish benign from malignant behavior, and build the diagnostic frameworks that guide treatment decisions. This lesson integrates genetics, pathology, and clinical reasoning to reveal cancer not as a single disease but as an ecosystem of cellular rebellion. By the end, you'll command the language, logic, and evidence base that define modern oncology practice.
📌 Remember: NEOPLASM - New growth, Encapsulated (benign), Organized (benign), Persistent, Lack of function, Autonomous, Spontaneous regression rare, Monoclonal origin
The neoplastic spectrum spans from benign growths that respect tissue boundaries to aggressive malignancies that invade distant organs. Benign tumors grow slowly (<2 cm/year), remain localized, and rarely recur after complete excision (<5% recurrence). Malignant tumors demonstrate rapid growth (>5 cm/year), tissue invasion, metastatic potential, and 10-year survival rates varying from 90% (early-stage) to <10% (metastatic disease).
Cellular transformation involves 6-10 genetic alterations accumulating over 10-30 years. The process requires inactivation of tumor suppressor genes (p53, Rb, APC) and activation of oncogenes (RAS, MYC, HER2). p53 mutations occur in >50% of human cancers, while RAS mutations affect 30% of malignancies.
⭐ Clinical Pearl: Tumor doubling time predicts behavior-benign lesions double every 100-300 days, while aggressive malignancies double every 25-50 days
| Characteristic | Benign | Malignant | Clinical Significance | |---|---|---|---|---| | Growth Rate | Slow (<2 cm/year) | Rapid (>5 cm/year) | Predicts urgency | | Differentiation | Well-differentiated | Poorly differentiated | Affects prognosis | | Invasion | No | Yes | Determines staging | | Metastasis | Never | Common (>60%) | Defines treatment | | Recurrence | Rare (<5%) | Common (>30%) | Guides follow-up |Neoplastic cells acquire hallmark capabilities: sustained proliferation, growth suppressor evasion, apoptosis resistance, replicative immortality, angiogenesis induction, and invasion/metastasis activation. Telomerase reactivation occurs in 85% of cancers, enabling unlimited replicative potential.
💡 Master This: Neoplasia classification depends on tissue of origin (epithelial vs mesenchymal), behavior (benign vs malignant), and differentiation grade (well, moderate, poor)-these three parameters determine prognosis and treatment approach.
Understanding neoplastic nomenclature provides the foundation for recognizing tumor behavior patterns and predicting clinical outcomes across all organ systems.
🧬 The Neoplastic Revolution: Cellular Rebellion Unleashed
⚔️ Cellular Warfare: The Oncogene-Tumor Suppressor Battle
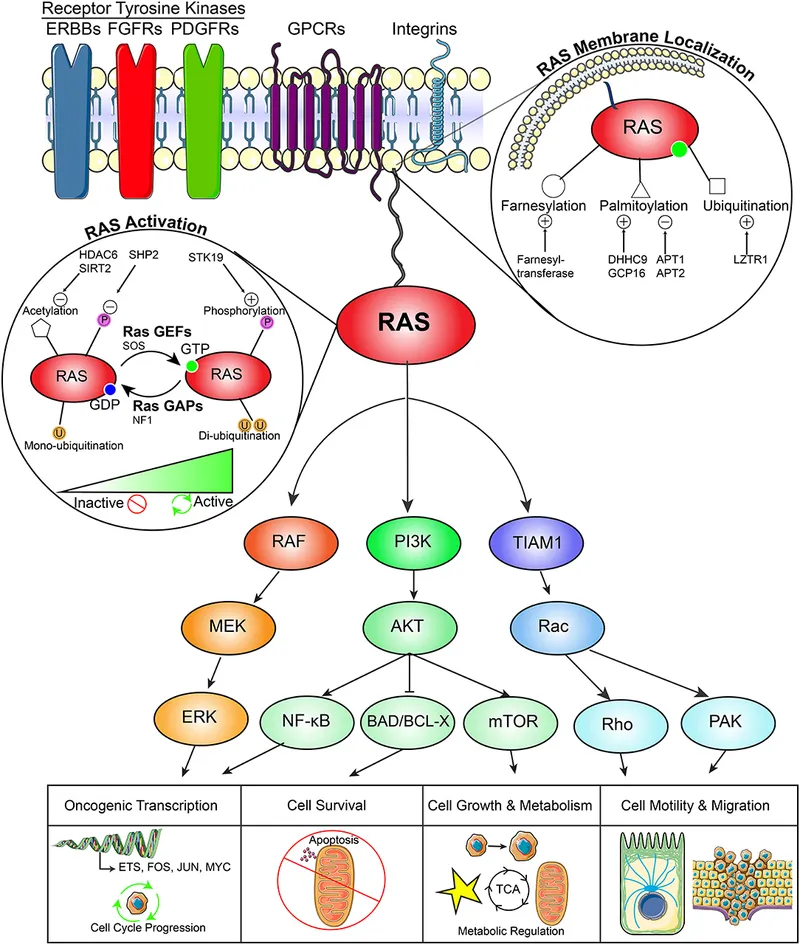
Proto-oncogenes function as cellular accelerators, promoting growth when appropriately activated. RAS proteins regulate 30% of cellular proliferation signals, while MYC transcription factors control 15% of all genes. Normal RAS activation lasts <5 minutes, but mutant RAS remains constitutively active.
📌 Remember: ONCOGENES - Overexpressed, Normal function accelerated, Constitutively active, One hit sufficient (dominant), Growth promotion, Early cancer events, Needs activation, Excess function, Stimulatory
Tumor suppressor genes function as cellular brakes, preventing inappropriate proliferation. p53 (chromosome 17p13) acts as "guardian of the genome," detecting DNA damage and initiating cell cycle arrest or apoptosis. Rb protein (chromosome 13q14) controls G1/S checkpoint, preventing DNA replication in damaged cells.
| Gene | Location | Function | Cancer Association | Mutation Frequency |
|---|---|---|---|---|
| p53 | 17p13 | DNA damage response | >50% all cancers | Most common |
| Rb | 13q14 | Cell cycle control | Retinoblastoma, sarcomas | 30% cancers |
| APC | 5q21 | Wnt pathway regulation | Colorectal cancer | 80% CRC |
| BRCA1/2 | 17q21/13q12 | DNA repair | Breast/ovarian cancer | 5-10% hereditary |
| VHL | 3p25 | Hypoxia response | Renal cell carcinoma | 90% clear cell RCC |
⭐ Clinical Pearl: Li-Fraumeni syndrome (p53 germline mutations) causes 50% cancer risk by age 30 and 90% lifetime risk, demonstrating tumor suppressor gene importance
Knudson's model predicts that hereditary cancers occur 10-20 years earlier than sporadic forms and often present as bilateral or multifocal disease. Retinoblastoma exemplifies this pattern: hereditary cases average 13 months onset with 90% bilateral involvement, while sporadic cases average 24 months with 10% bilateral disease.
💡 Master This: Oncogene activation requires gain-of-function mutations (dominant effect), while tumor suppressor inactivation requires loss-of-function mutations (recessive effect)-this fundamental difference explains inheritance patterns and therapeutic targeting strategies.
The oncogene-tumor suppressor paradigm establishes the molecular framework for understanding cancer genetics and targeted therapy development across all malignancy types.
⚔️ Cellular Warfare: The Oncogene-Tumor Suppressor Battle
🎯 Pattern Recognition Mastery: Decoding Neoplastic Behavior
Morphologic Assessment Framework:
- Cellular Level: Nuclear size variation (>3:1 ratio), chromatin pattern (coarse, irregular), nucleolar prominence (>2 per nucleus)
- Tissue Level: Loss of polarity, architectural distortion, stromal invasion
- Organ Level: Mass effect, functional impairment, distant spread
📌 Remember: MALIGNANT - Metastasis potential, Anaplastic features, Large nuclei, Invasive growth, Growth factor independence, Necrosis common, Asymmetric division, Nuclear pleomorphism, Telomerase active
Grading Systems quantify differentiation degree:
- Grade 1 (Well-differentiated): <10% mitoses, minimal atypia, 90% 5-year survival
- Grade 2 (Moderately differentiated): 10-20% mitoses, moderate atypia, 70% 5-year survival
- Grade 3 (Poorly differentiated): >20% mitoses, marked atypia, 40% 5-year survival
- Grade 4 (Undifferentiated): >30% mitoses, severe atypia, <20% 5-year survival
Staging Systems assess anatomic extent:
- T (Primary tumor): T1 (<2 cm), T2 (2-5 cm), T3 (>5 cm), T4 (invasion)
- N (Regional nodes): N0 (none), N1 (1-3 nodes), N2 (4-9 nodes), N3 (>10 nodes)
- M (Metastasis): M0 (none), M1 (distant spread)
Clinical Behavior Patterns:
- Benign: Slow growth (months-years), well-circumscribed, <5% recurrence after excision
- Locally Aggressive: Intermediate growth, infiltrative borders, 20-30% recurrence
- Malignant: Rapid growth (weeks-months), invasive, >60% metastatic potential
⭐ Clinical Pearl: Mitotic index >10 per 10 HPF strongly suggests malignancy, while atypical mitoses (tripolar, ring forms) are pathognomonic for cancer
Immunohistochemical Markers enhance diagnostic precision:
- Ki-67: Proliferation index (<5% benign, >20% malignant)
- p53: Overexpression in >50% malignancies
- Cytokeratins: Epithelial differentiation markers
- Vimentin: Mesenchymal origin indicator
💡 Master This: Combine morphology (cellular and architectural features), behavior (growth rate and invasion), and markers (immunohistochemistry and molecular) for definitive neoplastic classification-no single parameter suffices for accurate diagnosis.
Pattern recognition mastery enables rapid assessment of neoplastic potential and guides appropriate management decisions across all clinical scenarios.
🎯 Pattern Recognition Mastery: Decoding Neoplastic Behavior
⚖️ Diagnostic Discrimination: The Differential Matrix
Primary Discrimination Framework:
- Benign vs Malignant: Growth rate, invasion, metastasis potential
- Primary vs Metastatic: Organ-specific markers, clinical history, multiplicity
- Epithelial vs Mesenchymal: Cytokeratin vs vimentin expression
- Well vs Poorly Differentiated: Architectural preservation, cellular atypia
| Parameter | Benign | Low-Grade Malignant | High-Grade Malignant | Metastatic |
|---|---|---|---|---|
| Growth Rate | <1 cm/year | 1-3 cm/year | >5 cm/year | Variable |
| Mitotic Index | <2/10 HPF | 2-10/10 HPF | >20/10 HPF | >15/10 HPF |
| Nuclear Grade | 1 (uniform) | 2 (mild atypia) | 3-4 (severe atypia) | Variable |
| Necrosis | Absent | Focal (<10%) | Extensive (>30%) | Common |
| Invasion | None | Microscopic | Gross invasion | Present |
Organ-Specific Considerations:
- Breast: ER/PR/HER2 status determines treatment (70% ER+, 15% HER2+, 15% triple-negative)
- Lung: EGFR mutations (15% adenocarcinomas), ALK rearrangements (5%)
- Colon: Microsatellite instability (15% cases), KRAS mutations (40%)
- Prostate: Gleason score (3+3=6 low-risk, 4+5=9 high-risk)
Molecular Discrimination Tools:
- Next-Generation Sequencing: Identifies 300+ cancer genes simultaneously
- Fluorescence In-Situ Hybridization: Detects specific translocations (>95% sensitivity)
- Immunohistochemistry Panels: 10-15 markers for lineage determination
- Flow Cytometry: DNA ploidy analysis (diploid vs aneuploid)
📌 Remember: METASTASIS - Multiple lesions, Epithelial markers retained, Tissue architecture disrupted, Atypical location, Size variation, Tumor emboli, Angioinvasion, Similar histology, Immunohistochemistry helpful, Search for primary
Differential Diagnosis Algorithms:
- Round Cell Tumors: CD99 (Ewing's), Desmin (rhabdomyosarcoma), LCA (lymphoma)
- Spindle Cell Lesions: SMA (smooth muscle), S-100 (nerve), CD34 (solitary fibrous tumor)
- Epithelioid Tumors: Cytokeratin (carcinoma), Melanoma markers (S-100, Melan-A)
⭐ Clinical Pearl: Immunohistochemistry panels achieve >90% diagnostic accuracy when combining 3-4 specific markers, but single markers rarely provide definitive diagnosis
Clinical Context Integration:
- Age: Pediatric tumors (<18 years) favor sarcomas, adult tumors (>40 years) favor carcinomas
- Location: Organ-specific tumor predilections guide differential diagnosis
- Presentation: Rapid growth suggests high-grade malignancy, slow growth suggests benign process
💡 Master This: Effective differential diagnosis requires systematic integration of morphology, immunohistochemistry, molecular features, and clinical context-each parameter narrows possibilities until definitive diagnosis emerges through convergent evidence.
Diagnostic discrimination mastery enables confident classification of challenging cases and optimal treatment selection based on precise tumor characterization.
⚖️ Diagnostic Discrimination: The Differential Matrix
🎯 Treatment Paradigms: Evidence-Based Cancer Management
Surgical Principles:
- R0 Resection: Negative margins (>1 cm for most tumors), 90% local control
- R1 Resection: Microscopic positive margins, 60-70% local control
- R2 Resection: Gross residual disease, <30% local control
- Lymph Node Sampling: >12 nodes for adequate staging in most cancers
Chemotherapy Protocols:
- Neoadjuvant: Pre-operative treatment, 20-30% pathologic complete response rates
- Adjuvant: Post-operative treatment, 15-25% survival improvement
- Palliative: Symptom control, 6-12 month median survival extension
- Dose Intensity: >85% planned dose delivery maintains efficacy
📌 Remember: TREATMENT - Tumor stage guides approach, Response assessment mandatory, Evidence-based protocols, Adjuvant therapy improves survival, Targeted agents for biomarkers, Multimodal combinations, Early detection crucial, Neoadjuvant downstages, Toxicity monitoring essential
Radiation Therapy Parameters:
- Conventional Fractionation: 1.8-2.0 Gy daily, 5 days/week, 6-7 weeks total
- Hypofractionation: >2.5 Gy per fraction, shorter treatment course
- Stereotactic Radiosurgery: 8-24 Gy single fraction, >90% local control
- Brachytherapy: High-dose rate (>12 Gy/hour) or low-dose rate (<2 Gy/hour)
| Treatment Modality | Early Stage Efficacy | Advanced Stage Efficacy | 5-Year Survival Impact |
|---|---|---|---|
| Surgery Alone | 80-95% | 20-40% | +60-80% |
| Radiation Alone | 70-90% | 15-30% | +40-60% |
| Chemotherapy Alone | 30-60% | 10-25% | +10-30% |
| Combined Modality | 85-98% | 30-50% | +70-85% |
| Targeted Therapy | 60-80% | 20-40% | +20-50% |
- HER2-Positive Breast Cancer: Trastuzumab improves survival by 30-40%
- EGFR-Mutant Lung Cancer: Tyrosine kinase inhibitors achieve 70% response rates
- BRAF-Mutant Melanoma: BRAF/MEK inhibitors provide 60% response rates
- Microsatellite Instability: Immune checkpoint inhibitors achieve 40-60% responses
⭐ Clinical Pearl: Performance status (ECOG 0-1 vs 2-4) predicts treatment tolerance and survival more strongly than age-ECOG 0-1 patients tolerate full-dose therapy with <10% severe toxicity
Response Assessment Criteria:
- Complete Response: 100% tumor disappearance, 5-year survival >80%
- Partial Response: >30% tumor shrinkage, median survival 12-18 months
- Stable Disease: <30% change, median survival 6-12 months
- Progressive Disease: >20% growth, median survival 3-6 months
Immunotherapy Advances:
- PD-1/PD-L1 Inhibitors: 20-40% response rates across multiple tumor types
- CAR-T Cell Therapy: 80-90% complete remission in refractory hematologic malignancies
- Tumor-Infiltrating Lymphocytes: 50-70% response rates in melanoma
- Cancer Vaccines: 10-30% response rates, primarily preventive benefit
💡 Master This: Treatment success requires precise staging, biomarker-driven selection, evidence-based protocols, and systematic monitoring-personalized medicine integrates tumor biology with patient factors to optimize outcomes while minimizing toxicity.
Evidence-based treatment paradigms enable optimal therapeutic selection and improved survival outcomes across all cancer types through systematic, data-driven approaches.
🎯 Treatment Paradigms: Evidence-Based Cancer Management
🔗 Systems Integration: The Cancer Ecosystem
Hallmarks Integration Matrix:
- Proliferative Signaling: Growth factor independence through autocrine loops (>80% cancers)
- Growth Suppressor Evasion: p53/Rb pathway disruption (>90% cancers)
- Apoptosis Resistance: BCL-2 family dysregulation (>70% cancers)
- Replicative Immortality: Telomerase activation (85% cancers)
- Angiogenesis: VEGF pathway activation (>95% solid tumors)
- Invasion/Metastasis: EMT program activation (>60% carcinomas)
Tumor Microenvironment Ecosystem:
- Cancer Cells: 10-40% of tumor mass, driver mutations in 3-8 genes
- Cancer-Associated Fibroblasts: 20-50% of tumor, growth factor production
- Immune Infiltrate: 5-30% of tumor, prognostic significance
- Vasculature: Abnormal architecture, increased permeability
- Extracellular Matrix: Altered composition, mechanical properties
Metabolic Reprogramming Networks:
- Warburg Effect: >90% cancers show aerobic glycolysis
- Glutamine Addiction: >60% cancers require glutamine supplementation
- Lipid Synthesis: De novo fatty acid synthesis in >80% cancers
- Nucleotide Metabolism: Enhanced purine/pyrimidine synthesis
📌 Remember: ECOSYSTEM - Epigenetic regulation, Cellular interactions, Oncogene networks, Stromal support, Yield growth factors, Signaling cascades, Tumor heterogeneity, Environmental pressures, Metabolic reprogramming
| System Component | Normal Function | Cancer Alteration | Therapeutic Target |
|---|---|---|---|
| Cell Cycle | Controlled progression | Checkpoint loss | CDK4/6 inhibitors |
| DNA Repair | Error correction | Deficiency/overactivity | PARP inhibitors |
| Metabolism | Balanced energy | Warburg effect | Metabolic inhibitors |
| Immunity | Surveillance | Evasion/suppression | Checkpoint inhibitors |
| Angiogenesis | Regulated vessel growth | Pathologic vessels | Anti-VEGF therapy |
- Tumor Initiation: Single cell with 2-3 driver mutations
- Clonal Expansion: 10^6-10^9 cells with shared mutations
- Branched Evolution: Multiple subclones with distinct mutations
- Metastatic Seeding: Rare cells (<0.01%) with metastatic capability
Therapeutic Resistance Networks:
- Primary Resistance: Pre-existing mutations in 10-30% patients
- Acquired Resistance: New mutations emerge in 6-18 months
- Adaptive Resistance: Pathway rewiring within days-weeks
- Microenvironmental Resistance: Stromal protection mechanisms
⭐ Clinical Pearl: Tumor heterogeneity explains why single-agent therapies achieve <20% cure rates, while combination approaches targeting multiple pathways achieve >60% response rates
Precision Medicine Integration:
- Genomic Profiling: 300+ gene panels identify actionable mutations in >60% patients
- Transcriptomic Analysis: Gene expression signatures predict treatment response
- Proteomic Assessment: Protein activity patterns guide targeted therapy
- Metabolomic Profiling: Metabolite signatures reveal pathway dependencies
Systems Pharmacology Approaches:
- Network-Based Drug Design: Target pathway hubs rather than single proteins
- Combination Therapy: Synergistic interactions achieve >10-fold efficacy improvement
- Adaptive Dosing: Real-time monitoring optimizes therapeutic windows
- Resistance Prevention: Multi-target approaches delay resistance emergence
💡 Master This: Cancer represents a complex adaptive system where emergent properties arise from multi-scale interactions-successful treatment requires systems-level understanding and network-based therapeutic strategies that account for tumor evolution and resistance mechanisms.
Systems integration mastery enables comprehensive cancer understanding and rational therapeutic design targeting network vulnerabilities rather than isolated pathways.
🔗 Systems Integration: The Cancer Ecosystem
🎯 Clinical Mastery Arsenal: Rapid-Fire Neoplasia Command
Essential Diagnostic Thresholds:
- Mitotic Index: >10/10 HPF = malignant, <2/10 HPF = benign
- Ki-67 Proliferation: >20% = high-grade, <5% = low-grade
- Nuclear-Cytoplasmic Ratio: >1:2 suggests malignancy
- Tumor Doubling Time: <50 days = aggressive, >300 days = indolent
📌 Remember: RAPID DX - Recognize patterns quickly, Assess grade/stage, Profile biomarkers, Identify treatment targets, Determine prognosis, Develop management plan, X-ray/imaging correlation
High-Yield Clinical Correlations:
- p53 Mutations: >50% all cancers, Li-Fraumeni syndrome = 90% lifetime risk
- BRCA1/2 Mutations: 80% breast cancer risk, 40% ovarian cancer risk
- Lynch Syndrome: 80% colorectal cancer risk, 60% endometrial cancer risk
- Von Hippel-Lindau: 90% renal cell carcinoma risk
Staging Survival Correlations:
- Stage I: >90% 5-year survival (most cancers)
- Stage II: 70-85% 5-year survival
- Stage III: 40-60% 5-year survival
- Stage IV: <20% 5-year survival
| Cancer Type | Early Detection Method | Screening Age | Survival Benefit |
|---|---|---|---|
| Breast | Mammography | 40-50 years | 30% mortality reduction |
| Cervical | Pap smear/HPV | 21 years | 80% mortality reduction |
| Colorectal | Colonoscopy | 45-50 years | 60% mortality reduction |
| Lung | Low-dose CT | 50-55 years | 20% mortality reduction |
| Prostate | PSA + DRE | 50-55 years | 10% mortality reduction |
Treatment Response Timeframes:
- Targeted Therapy: Response in 4-8 weeks, resistance in 6-18 months
- Immunotherapy: Response in 8-12 weeks, durable responses >2 years
- Chemotherapy: Response in 6-12 weeks, median duration 6-12 months
- Radiation: Response in 2-6 weeks, late effects months-years
Emergency Recognition Patterns:
- Tumor Lysis Syndrome: Uric acid >8 mg/dL, K+ >6 mEq/L, PO4 >4.5 mg/dL
- Hypercalcemia: Ca >12 mg/dL, altered mental status, kidney dysfunction
- Superior Vena Cava Syndrome: Facial swelling, dyspnea, collateral circulation
- Spinal Cord Compression: Back pain, neurologic deficits, bowel/bladder dysfunction
💡 Master This: Neoplasia expertise requires instant pattern recognition, quantitative threshold mastery, evidence-based decision algorithms, and systematic monitoring protocols-combine morphologic assessment, molecular profiling, and clinical correlation for optimal patient outcomes.
Prognosis Prediction Framework:
- Tumor Factors: Stage (40% prognostic weight), grade (25%), biomarkers (20%)
- Patient Factors: Performance status (10%), comorbidities (3%), age (2%)
- Treatment Factors: Complete resection (>90% local control), adequate dosing (>85% planned dose)
Clinical mastery arsenal enables rapid assessment, accurate diagnosis, and optimal treatment selection through systematic application of evidence-based principles and quantitative thresholds.
🎯 Clinical Mastery Arsenal: Rapid-Fire Neoplasia Command
Practice Questions: Neoplasia
Test your understanding with these related questions
A 33-year-old woman comes to the physician 1 week after noticing a lump in her right breast. Fifteen years ago, she was diagnosed with osteosarcoma of her left distal femur. Her father died of an adrenocortical carcinoma at the age of 41 years. Examination shows a 2-cm, firm, immobile mass in the lower outer quadrant of the right breast. A core needle biopsy of the mass shows adenocarcinoma. Genetic analysis in this patient is most likely to show a defect in which of the following genes?













