Secondary immunodeficiencies US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Secondary immunodeficiencies. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Secondary immunodeficiencies US Medical PG Question 1: A 13-month-old boy is referred to an immunologist with recurrent otitis media, bacterial sinus infections, and pneumonia, which began several months earlier. He is healthy now, but the recurrent nature of these infections are troubling to his parents and they are hoping to find a definitive cause. The boy was born at 39 weeks gestation via spontaneous vaginal delivery. He is up to date on all vaccines and is meeting all developmental milestones. The patient has five older siblings, but none of them had similar recurrent illnesses. Clinical pathology results suggest very low levels of serum immunoglobulin. As you discuss options for diagnosis with the patient’s family, which of the following tests should be performed next?
- A. CSF gram staining
- B. Urine protein screening
- C. Stool cultures
- D. Flow cytometry (Correct Answer)
- E. Genetic analysis
Secondary immunodeficiencies Explanation: ***Flow cytometry***
- Flow cytometry is essential for evaluating **lymphocyte subsets** (B cells, T cells, NK cells) and their maturation, which is crucial for diagnosing **primary immunodeficiencies** like X-linked agammaglobulinemia (XLA).
- Given the history of recurrent bacterial infections and **very low serum immunoglobulin levels**, assessing B cell numbers and T cell populations would directly help identify defects in humoral immunity.
*CSF gram staining*
- **CSF gram staining** is used to diagnose **bacterial meningitis** at the time of an active infection.
- The patient is currently healthy, and the test would not identify the underlying cause of recurrent infections or low immunoglobulin levels.
*Urine protein screening*
- **Urine protein screening** is used to detect **kidney disease** or other conditions causing proteinuria.
- It is not relevant to investigating recurrent bacterial infections or low serum immunoglobulin levels, which point towards an immune system defect.
*Stool cultures*
- **Stool cultures** are performed to identify **gastrointestinal infections** (e.g., bacterial, parasitic).
- While infections can occur in immunodeficient patients, this test is not a primary diagnostic tool for the underlying **immunodeficiency** causing recurrent otitis media, sinus infections, and pneumonia.
*Genetic analysis*
- **Genetic analysis** can confirm certain **primary immunodeficiency diagnoses** once specific defects are suspected (e.g., mutations in *BTK* for XLA).
- However, flow cytometry is typically the next step to broadly characterize the immune cell populations and narrowed down differential diagnoses before proceeding with targeted genetic testing.
Secondary immunodeficiencies US Medical PG Question 2: A 3-month-old boy presents to his pediatrician with persistent diarrhea, oral candidiasis, and signs and symptoms of respiratory syncytial virus (RSV) pneumonia. He is very lean with weight in the 10th percentile. His blood pressure is 105/64 mm Hg and heart rate is 84/min. He is being evaluated for an immunodeficiency. Laboratory results for HIV are negative by polymerase chain reaction (PCR). Which of the following is the most likely cause of this child’s presentation?
- A. Grossly reduced levels of B cells
- B. An X-linked inheritance of HLA genes
- C. Selective IgA deficiency
- D. Defective T cell function (Correct Answer)
- E. Defective isotype switching
Secondary immunodeficiencies Explanation: ***Defective T cell function***
- The presentation with recurrent infections (oral candidiasis, RSV pneumonia), persistent diarrhea, and **failure to thrive (lean, 10th percentile weight)** in a young infant, despite negative HIV PCR, strongly suggests a **severe combined immunodeficiency (SCID)**.
- **T-cell dysfunction is the hallmark of SCID**, leading to a broad susceptibility to opportunistic pathogens and impaired immune responses.
*Grossly reduced levels of B cells*
- While some immunodeficiencies involve B-cell defects (e.g., **X-linked agammaglobulinemia**), the primary clinical picture here (severe viral and fungal infections, failure to thrive) points more strongly to a profound T-cell defect affecting both humoral and cellular immunity.
- Reduced B cells alone would primarily result in recurrent bacterial infections rather than the observed opportunistic infections.
*An X-linked inheritance of HLA genes*
- **HLA genes (Major Histocompatibility Complex)** are located on **chromosome 6**, not the X chromosome, and are crucial for antigen presentation, not typically associated with X-linked inheritance patterns.
- Defects in HLA (e.g., bare lymphocyte syndrome) can impair T-cell function but are not solely X-linked and would still fall under the umbrella of defective T-cell function.
*Selective IgA deficiency*
- Patients with selective IgA deficiency are often **asymptomatic** or experience **recurrent sinopulmonary and gastrointestinal infections** but typically do not present with severe opportunistic infections like RSV pneumonia and persistent candidiasis with failure to thrive.
- **T-cell function is preserved** in selective IgA deficiency, making the severe presentation less likely.
*Defective isotype switching*
- This primarily affects the ability of B cells to produce different classes of antibodies **(IgG, IgA, IgE)**, often due to defects in T follicular helper cells or B cell intrinsic defects.
- While it can lead to recurrent infections, particularly bacterial, it does not typically cause the severe, opportunistic infections and failure to thrive seen with primary T-cell defects like SCID.
Secondary immunodeficiencies US Medical PG Question 3: A young infant is brought to an immunologist because of recurrent infections, which have not resolved despite appropriate medical treatment. On reviewing her medical history, the immunologist notes that the child has had frequent disseminated mycobacterial infections. He suspects a possible immunodeficiency. What is the most likely cause of this patient's immunodeficiency?
- A. ATM gene defect
- B. LFA-1 integrin defect
- C. B-cell maturation defect
- D. Interferon-gamma signaling defect (Correct Answer)
- E. BTK gene defect
Secondary immunodeficiencies Explanation: ***Interferon-gamma signaling defect***
- **Interferon-gamma (IFN-γ)** is crucial for activating macrophages to kill intracellular pathogens like mycobacteria. A defect in its signaling pathway leads to impaired macrophage function and severe, recurrent mycobacterial infections.
- This condition is often referred to as **Mendelian susceptibility to mycobacterial diseases (MSMD)**.
*ATM gene defect*
- An **ATM gene defect** is associated with **ataxia-telangiectasia**, which primarily presents with cerebellar ataxia, telangiectasias, and immunodeficiency characterized by **recurrent sinopulmonary infections** and an increased risk of lymphomas, not typically disseminated mycobacterial infections.
- The immunodeficiency in ataxia-telangiectasia often involves **T-cell and IgA deficiency**.
*LFA-1 integrin defect*
- A defect in **LFA-1 integrin** causes **leukocyte adhesion deficiency type 1 (LAD-1)**, characterized by recurrent bacterial infections, impaired wound healing, delayed umbilical cord separation, and **leukocytosis**.
- The primary defect is in leukocyte extravasation and adhesion, leading to impaired pus formation, not specifically disseminated mycobacterial disease.
*B-cell maturation defect*
- A **B-cell maturation defect** typically leads to **antibody deficiencies**, resulting in recurrent infections, particularly with **encapsulated bacteria** (e.g., Streptococcus pneumoniae, Haemophilus influenzae).
- It would not primarily manifest as recurrent disseminated mycobacterial infections, as macrophage-mediated immunity is more critical for mycobacteria.
*BTK gene defect*
- A **BTK gene defect** causes **X-linked agammaglobulinemia (XLA)**, characterized by a virtual absence of B cells and severe deficiency of all immunoglobulin classes.
- Patients present with recurrent bacterial infections starting around 6 months of age, once maternal antibodies wane, but **disseminated mycobacterial infections** are not the hallmark.
Secondary immunodeficiencies US Medical PG Question 4: A 37-year old man is being evaluated due to a recent history of fatigue that started 3 weeks ago. The patient presents with a history of HIV, which was first diagnosed 7 years ago. He has been on an antiretroviral regimen and takes it regularly. His CD4+ count is 350 cells/mm3. According to the patient, his partner passed away from a "blood cancer", and he is worried that his fatigue might be connected to a similar pathology. The physician clarifies that there is an increased risk for HIV patients to develop certain kinds of lymphomas. Which one of the conditions below is the patient more likely to develop based on his medical history?
- A. Diffuse large B cell lymphoma (Correct Answer)
- B. Follicular lymphoma
- C. Burkitt’s lymphoma
- D. Extranodal marginal zone lymphoma
- E. Small lymphocytic lymphoma
Secondary immunodeficiencies Explanation: ***Diffuse large B cell lymphoma***
- **Diffuse large B-cell lymphoma (DLBCL)** is the most common type of lymphoma diagnosed in HIV-positive patients, accounting for about 50% of cases.
- The increased risk of DLBCL in HIV patients is related to chronic immune stimulation and dysregulation, often exacerbated by co-infection with viruses like **Epstein-Barr virus (EBV)**.
*Follicular lymphoma*
- **Follicular lymphoma** is generally *less common* in HIV-positive patients compared to the general population.
- Its incidence does not significantly increase in the context of HIV infection.
*Burkitt’s lymphoma*
- **Burkitt's lymphoma** is also more common in HIV patients, but typically presents in those with *more severe immunosuppression* (lower CD4 counts) and is specifically associated with **Epstein-Barr virus (EBV)** co-infection.
- While a possibility, DLBCL is the *overall most likely* lymphoma.
*Extranodal marginal zone lymphoma*
- **Extranodal marginal zone lymphoma** is *not typically associated* with an increased incidence in HIV-positive individuals.
- It often correlates with chronic inflammation or specific infections (e.g., *H. pylori* in gastric MALT lymphoma).
*Small lymphocytic lymphoma*
- **Small lymphocytic lymphoma (SLL)**, which is essentially the nodal form of chronic lymphocytic leukemia (CLL), is *not increased* in incidence in HIV-positive patients.
- CLL/SLL is generally considered to be *less common* or have no increased risk in HIV-infected individuals.
Secondary immunodeficiencies US Medical PG Question 5: A 71-year-old African American man is brought to the emergency department with a worsening productive cough and dyspnea for 2 days. He has had generalized bone pain for 2 months. He was admitted for pyelonephritis last month. He also received outpatient treatment for pneumonia almost 2 months ago. Over the past 2 months, he has been taking over-the-counter ibuprofen for pain as needed. He appears anxious. The vital signs include: temperature 38.8°C (101.8°F), pulse 95/min, respiratory rate 20/min, and blood pressure 155/90 mm Hg. The conjunctivae are pale. Crackles are heard in the right lower lobe. The cardiac examination shows no abnormalities. The laboratory studies show the following:
Hemoglobin 9 g/dL
Mean corpuscular volume 95 μm3
Leukocyte count 13,500/mm3
Segmented neutrophils 75%
Lymphocytes 25%
Platelet count 240,000/mm3
ESR 85 mm/hr
Serum
Na+ 135 mEq/L
K+ 4.2 mEq/L
Cl− 113 mEq/L
HCO3− 20 mEq/L
Ca+ 12.4 mg/dL
Albumin 4 g/dL
Urea nitrogen 38 mg/dL
Creatinine 2.2 mg/dL
A chest X-ray shows a right lower lobe opacity and blurring of the ipsilateral diaphragmatic dome. Skull and pelvic X-rays are performed (see image). Which of the following is the most likely underlying cause of this patient’s recent infections?
- A. T cell dysfunction
- B. Unresolved pneumonia
- C. Advanced age
- D. Hypogammaglobulinemia (Correct Answer)
- E. NSAID-induced chronic kidney disease
Secondary immunodeficiencies Explanation: ***Hypogammaglobulinemia***
- The patient's recurrent bacterial infections (pneumonia, pyelonephritis), **bone pain**, hypercalcemia (**Ca+ 12.4 mg/dL**), anemia (**Hb 9 g/dL**), and **acute kidney injury** (**creatinine 2.2 mg/dL**) are classic findings of **multiple myeloma**.
- In multiple myeloma, abnormal **plasma cells** produce monoclonal immunoglobulins, leading to **hypogammaglobulinemia** of the other immunoglobulin classes, which impairs the immune response to encapsulated bacteria and increases the risk of recurrent bacterial infections.
*T cell dysfunction*
- While T-cell dysfunction can lead to recurrent infections, it is not the primary immune defect seen in multiple myeloma; rather, **B-cell dysregulation** and **hypogammaglobulinemia** are more characteristic.
- T-cell dysfunction is more commonly associated with opportunistic infections, viral, or fungal pathogens, rather than the recurrent bacterial infections described.
*Unresolved pneumonia*
- While the patient has a current pneumonia and a history of recent pneumonia, the underlying cause of repeated infections and the constellation of other symptoms (bone pain, hypercalcemia, anemia, kidney injury) point to a systemic issue like multiple myeloma rather than just an isolated, unresolved infection.
- The patient’s history of pyelonephritis further supports a generalized compromise in immunity, suggesting a broader problem than just a persistent lung infection.
*Advanced age*
- While advanced age is a risk factor for many conditions, including multiple myeloma and increased susceptibility to infections, it is not an underlying specific cause of the recurrent infections in this context.
- The patient's specific clinical and lab findings (e.g., hypercalcemia, anemia, kidney injury, bone pain) are highly suggestive of a distinct pathology beyond simply older age.
*NSAID-induced chronic kidney disease*
- The patient's use of **ibuprofen** could potentially contribute to his kidney injury; however, it does not explain the **hypercalcemia**, **anemia**, **bone pain**, or, most importantly, the **recurrent bacterial infections**.
- NSAID-induced nephropathy typically presents with different laboratory findings and does not cause the profound immune dysfunction observed in this patient.
Secondary immunodeficiencies US Medical PG Question 6: A 33-year-old man is brought into the emergency department with fever, lethargy, and confusion. He is a cachectic man in acute distress, unable to respond to questions or follow commands. His friend confides that the patient has been sexually active with multiple male partners and was diagnosed with HIV several months ago, but was lost to follow up. Based on prior records, his most recent CD4 count was 65 cells/uL. Which of the following is the most appropriate next step in management?
- A. CT head without contrast (Correct Answer)
- B. Lumbar puncture
- C. Recheck CD4 and HIV viral load serologies
- D. MRI brain with contrast
- E. Neurological exam with fundoscopy
Secondary immunodeficiencies Explanation: ***CT head without contrast***
- With signs of **increased intracranial pressure** (lethargy, confusion, inability to follow commands), performing a non-contrast CT head is crucial to rule out a **mass lesion** or **herniation risk** before any invasive procedures like a lumbar puncture.
- This patient's severely low **CD4 count** (65 cells/uL) puts him at very high risk for opportunistic central nervous system infections such as **toxoplasmosis** or **PML**, or even CNS lymphoma, which can cause mass lesions.
*Lumbar puncture*
- A **lumbar puncture** is contraindicated in the presence of signs suggestive of increased intracranial pressure until a **mass lesion** has been excluded by imaging.
- Performing a lumbar puncture in such a situation could precipitate **brain herniation**, which can be fatal.
*Recheck CD4 and HIV viral load serologies*
- While important for long-term management, rechecking these labs is not the most **immediate next step** for an acutely ill patient with severe neurological symptoms.
- The patient requires urgent diagnosis and treatment for his acute condition, which could be life-threatening, before focusing on **baseline serologies**.
*MRI brain with contrast*
- An **MRI brain with contrast** provides more detailed imaging than a CT, but a non-contrast CT is faster and sufficient for initial screening for mass lesions or herniation risk.
- In an emergency setting with an unstable patient, the **rapid accessibility** of CT makes it the preferred initial imaging modality.
*Neurological exam with fundoscopy*
- A neurological exam and fundoscopy are important components of the work-up but are **diagnostic steps**, not a management step.
- These exams will help localize the lesion and assess for **papilledema**, but imaging is required to confirm the presence of a mass or rule out herniation risk.
Secondary immunodeficiencies US Medical PG Question 7: You are seeing a 4-year-old boy in clinic who is presenting with concern for a primary immune deficiency. He has an unremarkable birth history, but since the age of 6 months he has had recurrent otitis media, bacterial pneumonia, as well as two episodes of sinusitis, and four episodes of conjunctivitis. He has a maternal uncle who died from sepsis secondary to H. influenza pneumonia. If you drew blood work for diagnostic testing, which of the following would you expect to find?
- A. Abnormally low number of T cells
- B. Abnormally high number of B cells
- C. Elevated immunoglobulin levels
- D. Abnormally low number of B cells (Correct Answer)
- E. Abnormally high number of T cells
Secondary immunodeficiencies Explanation: ***Abnormally low number of B cells***
- The recurrent bacterial infections (otitis media, pneumonia, sinusitis, conjunctivitis) and the family history of death from *H. influenza* pneumonia suggest a **primary B-cell immunodeficiency**, such as **X-linked agammaglobulinemia (XLA)**.
- In XLA, there is a block in B-cell development, leading to a profound absence of mature B cells and immunoglobulins.
*Abnormally low number of T cells*
- This would point towards a **T-cell immunodeficiency** or a **combined immunodeficiency**, typically presenting with opportunistic infections, viral, or fungal infections, rather than predominantly bacterial infections.
- Examples include **Severe Combined Immunodeficiency (SCID)**, which often presents earlier and more severely.
*Abnormally high number of B cells*
- This is not characteristic of a primary immunodeficiency with recurrent bacterial infections; rather, it might be seen in certain autoimmune conditions or lymphoproliferative disorders.
- **High B cell counts** generally imply a functioning humoral immune system, which contradicts the infectious history.
*Elevated immunoglobulin levels*
- This finding would generally indicate a **functioning humoral immune response**, possibly due to chronic infection or an inflammatory process, but not a primary B-cell immunodeficiency causing recurrent bacterial infections.
- In conditions like **Common Variable Immunodeficiency (CVID)**, some immunoglobulin levels might be normal, but often key classes (like IgG, IgA, or IgM) are low.
*Abnormally high number of T cells*
- This finding is generally not associated with the pattern of recurrent bacterial infections described, which strongly points to a **humoral (antibody) deficiency**.
- **Elevated T cells** could be seen in some autoimmune conditions or certain viral infections, but not typically in a primary immunodeficiency characterized by recurrent bacterial infections.
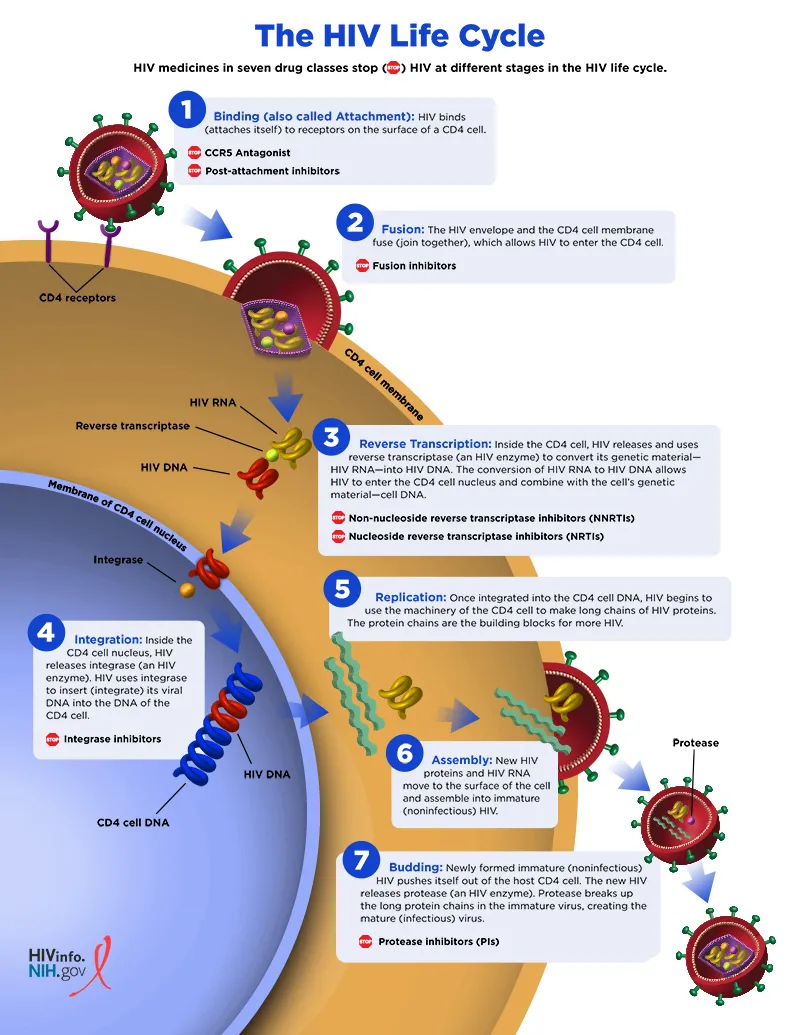
Secondary immunodeficiencies US Medical PG Question 8: An investigator is studying the mechanism of HIV infection in cells obtained from a human donor. The effect of a drug that impairs viral fusion and entry is being evaluated. This drug acts on a protein that is cleaved off of a larger glycosylated protein in the endoplasmic reticulum of the host cell. The protein that is affected by the drug is most likely encoded by which of the following genes?
- A. gag
- B. env (Correct Answer)
- C. tat
- D. pol
- E. rev
Secondary immunodeficiencies Explanation: ***env***
- The **env (envelope) gene** of HIV encodes for the precursor protein **gp160**, which is then cleaved by host cellular proteases into **gp120** and **gp41** within the endoplasmic reticulum.
- **gp120** and **gp41** together form the viral envelope glycoproteins responsible for viral binding to host cells and **fusion/entry**, making them the target of drugs that impair these processes.
*gag*
- The **gag (group-specific antigen) gene** encodes for structural proteins of the viral core, such as **p24 (capsid protein)**, p17 (matrix protein), and p7 (nucleocapsid protein).
- These proteins are primarily involved in the assembly of new virions and do not directly mediate viral fusion and entry.
*tat*
- The **tat (trans-activator of transcription) gene** encodes a regulatory protein that significantly enhances the transcription of viral genes.
- It plays a crucial role in the viral life cycle by increasing the efficiency of HIV gene expression, but it is not directly involved in viral fusion or entry.
*pol*
- The **pol (polymerase) gene** encodes for essential viral enzymes, including **reverse transcriptase**, integrase, and protease.
- These enzymes are critical for converting viral RNA into DNA, integrating viral DNA into the host genome, and cleaving viral polyproteins, respectively, but they are not involved in mediating viral entry.
*rev*
- The **rev (regulator of virion expression) gene** encodes a regulatory protein that facilitates the transport of unspliced and partially spliced viral RNAs from the nucleus to the cytoplasm.
- This transport is crucial for the synthesis of structural and enzymatic proteins and for packaging viral RNA into new virions, but it does not directly participate in viral fusion and entry.
Secondary immunodeficiencies US Medical PG Question 9: A 1-year-old infant is brought to the emergency department by his parents because of fever and rapid breathing for the past 2 days. He had a mild seizure on the way to the emergency department and developed altered sensorium. His mother states that the patient has had recurrent respiratory infections since birth. He was delivered vaginally at term and without complications. He is up to date on his vaccines and has met all developmental milestones. His temperature is 37.0°C (98.6°F), pulse rate is 200/min, and respirations are 50/min. He is lethargic, irritable, and crying excessively. Physical examination is notable for a small head, an elongated face, broad nose, low set ears, and cleft palate. Cardiopulmonary exam is remarkable for a parasternal thrill, grade IV pansystolic murmur, and crackles over both lung bases. Laboratory studies show hypocalcemia and lymphopenia. Blood cultures are drawn and broad-spectrum antibiotics are started, and the child is admitted to the pediatric intensive care unit. The intensivist suspects a genetic abnormality and a fluorescence in situ hybridization (FISH) analysis is ordered which shows 22q11.2 deletion. Despite maximal therapy, the infant succumbs to his illness. The parents of the child request an autopsy. Which of the following findings is the most likely to be present on autopsy?
- A. Aplastic thymus (Correct Answer)
- B. Hypercellular bone marrow
- C. Accessory spleen
- D. Hypertrophy of Hassall's corpuscles
- E. Absent follicles in the lymph nodes
Secondary immunodeficiencies Explanation: ***Aplastic thymus***
- This infant's presentation with 22q11.2 deletion, recurrent respiratory infections, hypocalcemia, and congenital heart disease (parasternal thrill, pansystolic murmur) is classic for **DiGeorge syndrome**.
- **DiGeorge syndrome** is characterized by thymic aplasia or hypoplasia, leading to **T-cell immunodeficiency**, and parathyroid hypoplasia, resulting in **hypocalcemia**.
*Hypercellular bone marrow*
- **Hypercellular bone marrow** indicates increased hematopoietic activity and is not a characteristic finding in DiGeorge syndrome.
- In immunodeficiency states like DiGeorge, the bone marrow itself is often normal or may show lymphoid depletion.
*Accessory spleen*
- **Accessory spleen** is a common congenital anomaly and is not specifically associated with DiGeorge syndrome or its immunodeficiency.
- While it can occur in individuals with DiGeorge syndrome, it is not a direct pathological consequence of the 22q11.2 deletion.
*Hypertrophy of Hassall's corpuscles*
- **Hassall's corpuscles** are found in the medulla of the thymus, and their hypertrophy would indicate an active or hyperplastic thymus, which is contrary to the **thymic aplasia/hypoplasia** seen in DiGeorge syndrome.
- In DiGeorge syndrome, the thymus is either absent or severely underdeveloped.
*Absent follicles in the lymph nodes*
- **Absent follicles in the lymph nodes** would indicate a B-cell deficiency, as follicles are primarily composed of B lymphocytes.
- DiGeorge syndrome primarily affects **T-cell development** due to thymic abnormalities, not B-cell development or lymph node follicular formation directly.
Secondary immunodeficiencies US Medical PG Question 10: A 27-year-old woman presents to the emergency department complaining of a left-sided headache and right-sided blurry vision. She states that 2 weeks ago she developed dark urine and abdominal pain. She thought it was a urinary tract infection so she took trimethoprim-sulfamethoxazole that she had left over. She planned on going to her primary care physician today but then she developed headache and blurry vision so she came to the emergency department. The patient states she is otherwise healthy. Her family history is significant for a brother with sickle cell trait. On physical examination, there is mild abdominal tenderness, and the liver edge is felt 4 cm below the right costal margin. Labs are drawn as below:
Hemoglobin: 7.0 g/dL
Platelets: 149,000/mm^3
Reticulocyte count: 5.4%
Lactate dehydrogenase: 3128 U/L
Total bilirubin: 2.1 mg/dL
Indirect bilirubin: 1.4 mg/dL
Aspartate aminotransferase: 78 U/L
Alanine aminotransferase: 64 U/L
A peripheral smear shows polychromasia. A Doppler ultrasound of the liver shows decreased flow in the right hepatic vein. Magnetic resonance imaging of the brain is pending. Which of the following tests, if performed, would most likely identify the patient’s diagnosis?
- A. Flow cytometry (Correct Answer)
- B. Glucose-6-phosphate-dehydrogenase levels
- C. Anti-histone antibodies
- D. Bone marrow biopsy
- E. Hemoglobin electrophoresis
Secondary immunodeficiencies Explanation: ***Flow cytometry***
- The patient's symptoms (headache, blurry vision, dark urine, abdominal pain, hepatomegaly) along with laboratory findings of **hemolytic anemia** (low hemoglobin, elevated reticulocyte count, high LDH, elevated indirect bilirubin) and signs of **thrombosis** (decreased hepatic vein flow, neurological symptoms) are highly suggestive of **paroxysmal nocturnal hemoglobinuria (PNH)**.
- **Flow cytometry** is the gold standard for diagnosing PNH by detecting the absence of **CD55** and **CD59** on red blood cells, granulocytes, and monocytes, indicating a deficiency in the **GPI anchor protein**.
*Glucose-6-phosphate-dehydrogenase levels*
- **G6PD deficiency** typically presents with hemolytic anemia triggered by **oxidant stressors** (like trimethoprim-sulfamethoxazole) but does not typically cause **thrombosis** or widespread organ involvement (e.g., hepatic vein thrombosis, neurological symptoms) as seen in this patient.
- Measuring G6PD levels would be appropriate if G6PD deficiency was suspected, but the clinical picture points more strongly to PNH due to the thrombotic events.
*Anti-histone antibodies*
- **Anti-histone antibodies** are primarily associated with drug-induced **lupus erythematosus**, which can manifest with various systemic symptoms, but not typically with severe hemolytic anemia and thrombotic microangiopathy in this specific pattern.
- While drug exposure is present (trimethoprim-sulfamethoxazole), the overall clinical and lab findings (especially the severe hemolytic picture and thrombosis) are not characteristic of drug-induced lupus in this context.
*Bone marrow biopsy*
- A **bone marrow biopsy** might show findings consistent with increased erythropoiesis due to hemolysis but is not a primary diagnostic test for PNH or its associated thrombotic complications.
- While it could be part of an evaluation for underlying bone marrow disorders, it would not directly confirm a diagnosis of PNH, which requires specific surface marker detection.
*Hemoglobin electrophoresis*
- **Hemoglobin electrophoresis** is used to diagnose **hemoglobinopathies** such as **sickle cell disease** or **thalassemia**. The patient's brother has sickle cell trait, but the patient's symptoms, particularly the prominent hemolytic anemia and thrombotic events, are not typical of a hemoglobinopathy in this acute presentation.
- While it could rule out a hemoglobinopathy, it wouldn't explain the full spectrum of symptoms, especially the thrombosis and the specific pattern of hemolysis (e.g., elevated LDH, indirect bilirubin).
More Secondary immunodeficiencies US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.