Myeloproliferative neoplasms US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Myeloproliferative neoplasms. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Myeloproliferative neoplasms US Medical PG Question 1: A 40-year-old man comes to the physician because of fatigue, increased sweating, and itching in his legs for the past 2 years. He has chronic bronchitis. He has smoked two packs of cigarettes daily for 24 years and drinks one to two beers every night. His only medication is a tiotropium bromide inhaler. His vital signs are within normal limits. He is 175 cm (5 ft 9 in) tall and weighs 116 kg (256 lb); BMI is 38 kg/m2. Physical examination shows facial flushing and bluish discoloration of the lips. Scattered expiratory wheezing and rhonchi are heard throughout both lung fields. Abdominal examination shows no abnormalities. Laboratory studies show:
Erythrocyte count 6.9 million/mm3
Hemoglobin 20 g/dL
Mean corpuscular volume 91 μm3
Leukocyte count 13,000/mm3
Platelet count 540,000/mm3
Serum
Ferritin 8 ng/mL
Iron 48 μg/dL
Iron binding capacity 402 μg/dL (N: 251 - 406 μg/dL)
Which of the following is the most appropriate next step in treatment?
- A. Weight loss
- B. Inhaled budesonide
- C. Hydroxyurea
- D. Allogeneic stem cell transplantation
- E. Phlebotomy (Correct Answer)
Myeloproliferative neoplasms Explanation: ***Phlebotomy***
- This patient has **secondary erythrocytosis** due to **chronic hypoxemia** from COPD (chronic bronchitis, 48 pack-year smoking history, cyanosis, wheezing).
- Severe erythrocytosis (Hb 20 g/dL, Hct likely >60%) causes **hyperviscosity syndrome** manifesting as fatigue, headaches, and pruritus.
- **Phlebotomy** is indicated when Hct >55% in secondary polycythemia to reduce thrombotic risk and improve symptoms related to hyperviscosity.
- The goal is to reduce hematocrit to <55% while addressing the underlying hypoxemia with oxygen therapy and COPD optimization.
- Iron deficiency (ferritin 8 ng/mL) may be present from prior phlebotomy or dietary causes; iron should NOT be supplemented as it would worsen erythrocytosis.
*Weight loss*
- While obesity (BMI 38) contributes to hypoventilation and worsens hypoxemia, weight loss is a long-term intervention that does not address the **acute hyperviscosity** and thrombotic risk.
- Weight loss would be an appropriate adjunctive measure but not the most immediate next step.
*Inhaled budesonide*
- Inhaled corticosteroids are used for COPD patients with frequent exacerbations or asthma-COPD overlap.
- This patient is already on appropriate bronchodilator therapy (tiotropium); adding corticosteroids does not address the primary issue of **severe erythrocytosis and hyperviscosity**.
- Optimizing COPD therapy and oxygen supplementation would be more appropriate than adding inhaled steroids at this point.
*Hydroxyurea*
- Hydroxyurea is a cytoreductive agent used primarily in **polycythemia vera**, not secondary polycythemia.
- This patient's erythrocytosis is driven by **hypoxia** (physiologic EPO response), not autonomous JAK2-driven proliferation.
- Treating the underlying hypoxemia and using phlebotomy is more appropriate than cytoreductive therapy.
*Allogeneic stem cell transplantation*
- Stem cell transplantation is reserved for advanced myeloproliferative neoplasms or myelofibrosis with poor prognosis.
- This is **secondary erythrocytosis**, not a malignant hematologic disorder, and does not warrant such aggressive treatment.
- This option is completely inappropriate for this clinical scenario.
Myeloproliferative neoplasms US Medical PG Question 2: A 47-year-old woman presents for a routine wellness checkup. She complains of general fatigue and lethargy for the past 6 months. She does not have a significant past medical history and is currently not taking any medications. The patient reports that she drinks “socially” approx. 6 nights a week. She says she also enjoys a “nightcap,” which is 1–2 glasses of wine before bed every night. She denies any history of drug use or smoking. The patient is afebrile, and her vital signs are within normal limits. A physical examination reveals pallor of the mucous membranes. Her laboratory findings are significant for a mean corpuscular volume of 72 fL, leukocyte count of 5,300/mL, hemoglobin of 11.0 g/dL, and platelet count of 420,000/mL.
Which of the following is the most likely cause of this patient’s thrombocytosis?
- A. Iron deficiency anemia (Correct Answer)
- B. Essential thrombocytosis
- C. Aplastic anemia
- D. Chronic alcohol abuse
- E. Chronic myelogenous leukemia (CML)
Myeloproliferative neoplasms Explanation: ***Iron deficiency anemia***
- The patient presents with **microcytic anemia** (MCV 72 fL, Hb 11.0 g/dL) and **pallor**, which are classic signs of iron deficiency.
- **Iron deficiency** is a common cause of **secondary thrombocytosis**, as iron plays a role in platelet production and maturation.
*Essential thrombocytosis*
- This is a **myeloproliferative neoplasm** characterized by significantly elevated platelet counts, usually much higher than 420,000/mL (often > 600,000/mL).
- While it causes thrombocytosis, it typically doesn't present with microcytic anemia unless there's a co-existing iron deficiency, which is the primary finding here.
*Aplastic anemia*
- **Aplastic anemia** would present with **pancytopenia** (low red blood cells, white blood cells, and platelets), which is not consistent with the patient's elevated platelet count and normal leukocyte count.
- The patient's presentation of fatigue and pallor would likely be more severe due to significant anemia, and thrombocytosis would not be present.
*Chronic alcohol abuse*
- **Chronic alcohol abuse** typically causes **macrocytic anemia** (elevated MCV) due to folate deficiency or direct bone marrow toxicity, not microcytic anemia.
- While it can sometimes lead to thrombocytopenia, it is not a direct cause of robust thrombocytosis, especially in the context of microcytic anemia.
*Chronic myelogenous leukemia (CML)*
- CML is a **myeloproliferative neoplasm** characterized by the **Philadelphia chromosome (BCR-ABL1 fusion gene)**, leading to a significant increase in **granulocytes** (leukocytosis) and often thrombocytosis.
- Although thrombocytosis can occur, the primary hallmark is significant leukocytosis, which is not seen here (leukocyte count is normal), and the anemia would typically be normocytic or even macrocytic if folate deficient.
Myeloproliferative neoplasms US Medical PG Question 3: A 55-year-old man comes to the physician for a routine health visit. He feels well except for occasional left-sided abdominal discomfort and left shoulder pain. He has smoked 1 pack of cigarettes daily for 20 years. He does not drink alcohol. His pulse is 85/min and his blood pressure is 130/70 mmHg. Examination shows a soft, nontender abdomen. The spleen is palpated 5 cm below the costal margin. There is no lymphadenopathy present. The remainder of the examination shows no abnormalities. Laboratory studies show:
Hemoglobin 12.2 g/dL
Hematocrit 36 %
Leukocyte count 34,000/mm3
Platelet count 450,000/mm3
Cytogenetic testing of his blood cells is pending. Further evaluation of this patient is most likely to show which of the following findings?
- A. Autoimmune hemolytic anemia
- B. Elevated serum β2 microglobulin
- C. Elevated serum calcium
- D. Decreased basophil count
- E. Low leukocyte alkaline phosphatase score (Correct Answer)
Myeloproliferative neoplasms Explanation: ***Low leukocyte alkaline phosphatase score***
- The patient's presentation with **splenomegaly**, **leukocytosis** (34,000/mm³), and a normal hemoglobin/platelet count, strongly suggests a **myeloproliferative neoplasm**, specifically **chronic myeloid leukemia (CML)**.
- A **low leukocyte alkaline phosphatase (LAP) score** is a classic diagnostic feature of CML, as the neutrophils in CML have decreased LAP activity.
*Autoimmune hemolytic anemia*
- This condition is characterized by **anemia** and signs of **hemolysis**, such as elevated reticulocytes and lactate dehydrogenase, which are not described.
- While anemia is present, the primary issue indicated by the high leukocyte count and splenomegaly is a myeloproliferative disorder, not solely autoimmune hemolysis.
*Elevated serum β2 microglobulin*
- Elevated **β2 microglobulin** is a marker of **lymphocytic proliferation** and is commonly seen in conditions like **multiple myeloma** or **lymphoma**.
- The patient's dominant features of **marked leukocytosis** and **splenomegaly** are more consistent with a myeloid disorder than a lymphoid one.
*Elevated serum calcium*
- **Hypercalcemia** is a common complication of **multiple myeloma** or certain **carcinomas** due to bone destruction or paraneoplastic syndromes.
- The patient's symptoms and lab findings (especially high leukocyte count and splenomegaly) do not point to these conditions.
*Decreased basophil count*
- In conditions like **CML**, an **elevated basophil count** is often observed, which contradicts the option of a decreased basophil count.
- Other myeloproliferative neoplasms can also have varying basophil counts, but a decrease is not a hallmark.
Myeloproliferative neoplasms US Medical PG Question 4: A 73-year-old man is brought to the emergency department because of fever and a productive cough for 2 days. He has had increasing fatigue and dyspnea for the past 2 weeks. During this time he has lost 3 kg (6.6 lb). He received chemotherapy for myelodysplastic syndrome (MDS) 1 year ago. He is currently on supportive treatment and regular blood transfusions. He does not smoke or drink alcohol. The vital signs include: temperature 38.5℃ (101.3℉), pulse 93/min, respiratory rate 18/min, and blood pressure 110/65 mm Hg. He has petechiae distally on the lower extremities and several purpura on the trunk and extremities. Several enlarged lymph nodes are detected in the axillary and cervical regions on both sides. On auscultation of the lungs, crackles are heard in the left lower lobe area. Physical examination of the heart and abdomen shows no abnormalities. The laboratory studies show the following:
Hemoglobin 9 g/dL
Mean corpuscular volume 95 μm3
Leukocyte count 18,000/mm3
Platelet count 40,000/mm3
Prothrombin time 11 sec (INR = 1)
Based on these findings, this patient is most likely to have developed which of the following?
- A. Non-cardiogenic pulmonary edema
- B. Acute myeloid leukemia (Correct Answer)
- C. Disseminated intravascular coagulation
- D. Small cell lung cancer
- E. Burkitt lymphoma
Myeloproliferative neoplasms Explanation: ***Acute myeloid leukemia***
- The patient's history of **myelodysplastic syndrome (MDS)**, combined with the current presentation of **fever**, **fatigue**, **dyspnea**, significant **weight loss**, **generalized lymphadenopathy**, **petechiae**, **purpura**, and dramatically altered blood counts (**leukocytosis**, **thrombocytopenia**, **anemia**), is highly suggestive of progression from MDS to **acute myeloid leukemia (AML)**.
- The elevated **white blood cell count (18,000/mm3)** with anemia and thrombocytopenia, along with systemic symptoms and a history of a pre-leukemic condition, points directly to a clonal proliferation of myeloid blasts, which is the hallmark of AML.
*Non-cardiogenic pulmonary edema*
- While the patient has dyspnea and crackles, **non-cardiogenic pulmonary edema** is a symptom or complication, not an underlying primary diagnosis that explains the constellation of other systemic findings like lymphadenopathy, petechiae, weight loss, and the specific blood count abnormalities.
- Pulmonary edema can be a complication of severe sepsis or acute respiratory distress syndrome, but it does not account for the **malignant hematological picture**.
*Disseminated intravascular coagulation*
- **Disseminated intravascular coagulation (DIC)** is a possibility given the petechiae and purpura, indicating a coagulopathy, and it can be a complication of severe infection or malignancy.
- However, the patient's **PT/INR is normal**, and while his platelet count is low, DIC would typically also involve deranged coagulation times (prolonged PT/INR and aPTT) to a greater extent, and the overarching clinical picture with lymphadenopathy and history of MDS points to an underlying hematological malignancy as the primary issue rather than DIC as the sole diagnosis.
*Small cell lung cancer*
- **Small cell lung cancer** could cause weight loss and fatigue, and potentially paraneoplastic syndromes leading to certain hematological changes, but would not typically present with such pronounced **generalized lymphadenopathy** and the specific **pancytopenia-like picture** (anemia, thrombocytopenia with leukocytosis) without a clear lung mass or primary respiratory symptoms beyond crackles.
- The history of MDS transitioning to leukemia is a much more direct and likely explanation for the presenting signs and symptoms.
*Burkitt lymphoma*
- **Burkitt lymphoma** is a high-grade B-cell lymphoma that can cause lymphadenopathy and systemic symptoms, but it typically presents with distinct patterns of involvement (e.g., jaw mass in endemic form, abdominal involvement in sporadic form) and less commonly transitions directly from MDS.
- While it can manifest with bone marrow involvement, the particular blood count abnormalities and the history of **MDS progressing to acute leukemia** makes AML a far more probable diagnosis.
Myeloproliferative neoplasms US Medical PG Question 5: A 35-year-old male presents to his physician with the complaint of fatigue and weakness for six months. His physician orders a CBC which demonstrates anemia and thrombocytopenia. During the subsequent work up, a bone marrow biopsy is performed which ultimately leads to the diagnosis of acute promyelocytic leukemia. Which of the following translocations and fusion genes would be present in this patient?
- A. t(15;17) - PML/RARalpha (Correct Answer)
- B. t(9;22) - PML/RARalpha
- C. t(9;22) - BCR/Abl1
- D. t(14;18) - PML/RARalpha
- E. t(8;14) - BCR/Abl1
Myeloproliferative neoplasms Explanation: ***t(15;17) - PML/RARalpha***
- **Acute Promyelocytic Leukemia (APL)** is uniquely characterized by the **t(15;17) translocation**, which fuses the **PML (promyelocytic leukemia)** gene on chromosome 15 with the **RARalpha (retinoic acid receptor alpha)** gene on chromosome 17.
- This specific genetic alteration is crucial for diagnosis and dictates treatment with **all-trans retinoic acid (ATRA)**, which targets the aberrant RARalpha fusion protein.
*t(9;22) - PML/RARalpha*
- The **t(9;22) translocation** is associated with **Chronic Myeloid Leukemia (CML)**, forming the **BCR-ABL1 fusion gene**, not PML/RARalpha.
- This option incorrectly pairs the translocation with a fusion gene specific to APL.
*t(9;22) - BCR/Abl1*
- While **t(9;22)** and the **BCR/Abl1 fusion gene** are correctly paired, this is the hallmark of **Chronic Myeloid Leukemia (CML)**, not acute promyelocytic leukemia.
- CML typically presents with a different clinical picture and bone marrow findings than APL, primarily **leukocytosis with a left shift** and **basophilia**.
*t(14;18) - PML/RARalpha*
- The **t(14;18) translocation** is characteristic of **follicular lymphoma**, not any form of acute leukemia.
- It results in the overexpression of the **BCL-2 gene**, promoting cell survival, and is not associated with the PML/RARalpha fusion.
*t(8;14) - BCR/Abl1*
- The **t(8;14) translocation** is associated with **Burkitt lymphoma**, leading to the translocation of the **MYC oncogene** close to immunoglobulin heavy chain enhancers.
- This option also incorrectly pairs the translocation with the **BCR/Abl1 fusion gene**, which is characteristic of CML, and is not relevant to APL.
Myeloproliferative neoplasms US Medical PG Question 6: A 70-year-old woman presents with a 2-week history of severe fatigue. Over the past month, she has unintentionally lost 2 kg (4.4 lb). Three years ago, she was diagnosed with myelodysplastic syndrome. Currently, she takes no medications other than aspirin for occasional knee pain. She does not smoke or drink alcohol. Her vital signs are within the normal range. On physical examination, her conjunctivae are pale. Petechiae are present on the distal lower extremities and on the soft and hard palates. Palpation reveals bilateral painless cervical lymphadenopathy. Examination of the lungs, heart, and abdomen shows no abnormalities. Laboratory studies show:
Hemoglobin 9 g/dL
Mean corpuscular volume 90 μm3
Leukocyte count 3000/mm3
Platelet count 20,000/mm3
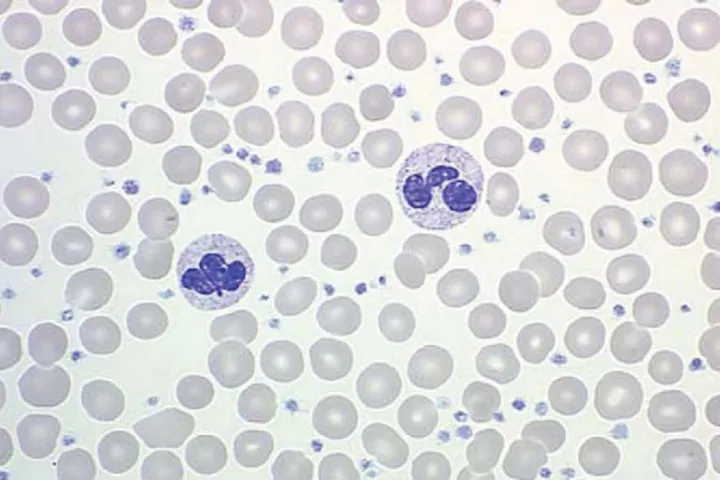
A Giemsa-stained peripheral blood smear is shown in the image. Which of the following best explains these findings?
- A. Primary myelofibrosis
- B. Chronic myelogenous leukemia
- C. Acute myeloid leukemia (Correct Answer)
- D. Hairy cell leukemia
- E. Aplastic anemia
Myeloproliferative neoplasms Explanation: ***Acute myeloid leukemia***
- The patient's history of **myelodysplastic syndrome (MDS)** combined with new symptoms of **fatigue**, unintentional **weight loss**, signs of **pancytopenia** (pallor, petechiae), and the presence of **circulating blasts** (seen as myeloblasts with prominent nucleoli on the Giemsa-stained peripheral smear) strongly indicates transformation to **acute myeloid leukemia (AML)**.
- MDS frequently progresses to AML, characterized by **>20% blasts** in the bone marrow or peripheral blood. The symptoms reflect bone marrow failure due to the proliferation of these immature myeloid cells.
*Primary myelofibrosis*
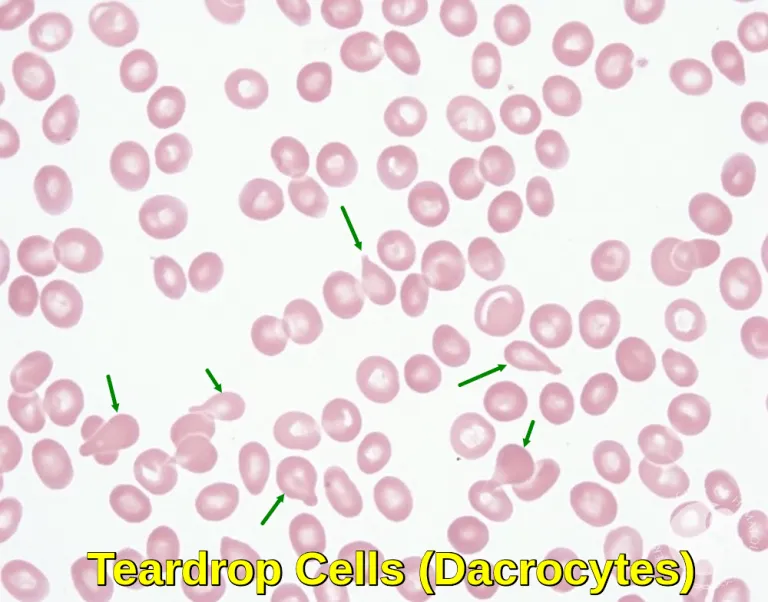
- While **fatigue**, **weight loss**, and **anemia** can occur, primary myelofibrosis typically presents with **splenomegaly**, **leukoerythroblastosis** (immature myeloid and erythroid precursors in peripheral blood), and **teardrop cells** on the smear, none of which are explicitly mentioned or visible as prominent features.
- The defining feature is **bone marrow fibrosis**, which is not suggested by the presence of a high number of circulating blasts.
*Chronic myelogenous leukemia*
- This condition is characterized by a marked **leukocytosis** (often >100,000/mm³) with a full spectrum of mature and immature granulocytes, and the presence of the **Philadelphia chromosome (BCR-ABL1 fusion gene)**.
- The patient's **leukocyte count is low (3000/mm³)**, and the predominant cells on the smear are blasts, not a range of maturing myeloid cells.
*Hairy cell leukemia*
- Patients with hairy cell leukemia typically present with **fatigue**, **weakness**, and often **splenomegaly**, but their peripheral blood smear is characterized by **lymphocytes with cytoplasmic projections** (hairy cells).
- The patient's smear shows **myeloid blasts**, not hairy cells, and her cell counts (e.g., severe thrombocytopenia) are not typical of classic hairy cell leukemia.
*Aplastic anemia*
- Aplastic anemia presents with **pancytopenia** and symptoms of bone marrow failure (fatigue, bleeding, infections), but it is characterized by a **hypocellular bone marrow** with a **paucity of hematopoietic cells** and a **lack of circulating blasts** on the peripheral smear.
- The presence of **circulating blasts** observed in the image **rules out aplastic anemia**.
Myeloproliferative neoplasms US Medical PG Question 7: A 56-year-old man presents to his general practitioner with frequent episodes of facial flushing for the past 2 weeks. He says the episodes are associated with mild headaches and a sensation of fullness in his head and neck. Additionally, he has developed recurrent, often severe, itching after taking a hot shower. The patient denies any smoking history but says he drinks alcohol socially. His blood pressure is 160/90 mm Hg, and his temperature is 37.0°C (98.6°F). On physical examination, his face and neck appear red. Cardiac examination reveals a regular rate and rhythm. Lungs are clear to auscultation bilaterally. The spleen is noted to be palpable just below the costal margin. A complete blood count shows a hemoglobin level of 19.5 g/dL, a total leukocyte count of 12,000/mm3, and a platelet count of 450,000/mm3. Which of the following sets of abnormalities is most likely present in this patient?
- A. ↑ Blood viscosity, ↓ blood flow with an M-spike of immunoglobulin G
- B. ↑ Blood viscosity, ↓ blood flow, ↓ erythropoietin (Correct Answer)
- C. ↑ Blood viscosity, ↓ blood flow with blast cells
- D. ↑ Blood viscosity, ↓ blood flow with an M-spike of immunoglobulin M
- E. ↓ Blood viscosity, ↑ blood flow, ↓ erythropoietin, ↑ ferritin
Myeloproliferative neoplasms Explanation: ***↑ Blood viscosity, ↓ blood flow, ↓ erythropoietin***
- The patient's symptoms (facial flushing, headaches, fullness in head/neck, **post-shower pruritus**) and lab findings (hemoglobin **19.5 g/dL**, elevated WBC and platelets) are classic for **polycythemia vera (PV)**.
- In polycythemia vera, the increased red blood cell mass leads to **increased blood viscosity** and thus **decreased blood flow**, causing hyperviscosity symptoms.
- The excess RBC production is driven by autonomous proliferation (typically due to **JAK2 V617F mutation**), which occurs independently of erythropoietin. The elevated RBC mass suppresses **erythropoietin** levels through negative feedback from the kidneys.
- Post-shower pruritus (aquagenic pruritus) is pathognomonic for PV and results from basophil and mast cell degranulation triggered by temperature changes.
*↑ Blood viscosity, ↓ blood flow with an M-spike of immunoglobulin G*
- While increased blood viscosity and decreased blood flow occur in polycythemia vera, an **M-spike of immunoglobulin G** is characteristic of **multiple myeloma**, not polycythemia vera.
- Multiple myeloma presents with anemia (not erythrocytosis), bone pain, hypercalcemia, and renal dysfunction.
*↑ Blood viscosity, ↓ blood flow with blast cells*
- The presence of **blast cells** in peripheral blood would suggest **acute leukemia**, which is not supported by this clinical picture.
- Polycythemia vera is a chronic myeloproliferative neoplasm; blast cells are generally absent in the peripheral blood unless there is transformation to acute leukemia (rare complication).
*↑ Blood viscosity, ↓ blood flow with an M-spike of immunoglobulin M*
- An **M-spike of immunoglobulin M** is a hallmark of **Waldenström macroglobulinemia**, a lymphoplasmacytic lymphoma.
- While Waldenström can cause hyperviscosity syndrome, it presents with anemia, not erythrocytosis, along with lymphadenopathy and hepatosplenomegaly from lymphoid infiltration.
*↓ Blood viscosity, ↑ blood flow, ↓ erythropoietin, ↑ ferritin*
- **Decreased blood viscosity** and **increased blood flow** would occur in anemia, which is the opposite of this patient's presentation with significantly elevated hemoglobin.
- While erythropoietin is indeed decreased in polycythemia vera, **↑ ferritin** is not a primary feature and would be more suggestive of iron overload (hemochromatosis) or an acute phase response to inflammation.
Myeloproliferative neoplasms US Medical PG Question 8: A 67-year-old man comes to the physician because of a 2-month history of generalized fatigue. On examination, he appears pale. He also has multiple pinpoint, red, nonblanching spots on his extremities. His spleen is significantly enlarged. Laboratory studies show a hemoglobin concentration of 8.3 g/dL, a leukocyte count of 81,000/mm3, and a platelet count of 35,600/mm3. A peripheral blood smear shows immature cells with large, prominent nucleoli and pink, elongated, needle-shaped cytoplasmic inclusions. Which of the following is the most likely diagnosis?
- A. Acute lymphoblastic leukemia
- B. Myelodysplastic syndrome
- C. Hairy cell leukemia
- D. Acute myelogenous leukemia (Correct Answer)
- E. Chronic myelogenous leukemia
Myeloproliferative neoplasms Explanation: ***Acute myelogenous leukemia***
- The presence of immature cells with **large, prominent nucleoli** and **pink, elongated, needle-shaped cytoplasmic inclusions** (**Auer rods**) on peripheral blood smear is pathognomonic for **acute myeloid leukemia (AML)**.
- The pancytopenia (anemia, thrombocytopenia) and extreme leukocytosis, along with generalized fatigue and pale appearance, are consistent with the presentation of AML.
*Acute lymphoblastic leukemia*
- Characterized by the proliferation of **lymphoblasts** (immature lymphocytes) in the bone marrow and peripheral blood, which typically lack Auer rods.
- While it can present with fatigue, pallor, and cytopenias, the specific morphologic features of the blast cells are different.
*Myelodysplastic syndrome*
- Involves ineffective hematopoiesis leading to **cytopenias** and dysplastic features in mature blood cells, but typically features less aggressive proliferation of immature cells than acute leukemias and **lacks Auer rods**.
- While it can progress to AML, the current description points to actively proliferating immature cells.
*Hairy cell leukemia*
- Characterized by **B lymphocytes with cytoplasmic projections** ("hairy cells") and is typically associated with **massive splenomegaly** and **pancytopenia**, but the characteristic Auer rods are absent.
- The cell morphology described (large nucleoli, needle-shaped inclusions) is inconsistent with hairy cells.
*Chronic myelogenous leukemia*
- Characterized by the **Philadelphia chromosome (BCR-ABL1 fusion gene)** and a marked increase in mature and immature myeloid cells, including granulocytes at various stages of maturation, but typically **lacks Auer rods** and usually has a higher proportion of mature rather than acutely immature cells.
- While it presents with leukocytosis and splenomegaly, the prominent immature cells with nucleoli and Auer rods are not features of CML.
Myeloproliferative neoplasms US Medical PG Question 9: A 13-year-old girl is brought to the pediatrician due to a 4-month history of heavy vaginal bleeding during menstrual periods. She endorses episodes of bleeding gums after brushing her teeth and experienced prolonged bleeding after tonsillectomy 6 years ago. Her mother states that she bled significantly during childbirth and that the girl’s older brother has similar symptoms including easy bruising. Vitals were stable and physical exam was not revealing. Laboratory studies show:
Platelet count: 72,000/mm^3
Bleeding time: 14 min
Prothrombin time: 12 secs (INR = 1)
Partial thromboplastin time: 40 secs
Blood smear demonstrates increased megakaryocytes and enlarged platelets. Platelets do not aggregate to ristocetin. Which of the following is the most likely diagnosis?
- A. Glanzmann thrombasthenia
- B. Idiopathic thrombocytopenic purpura (ITP)
- C. Bernard-Soulier syndrome (Correct Answer)
- D. Aspirin or NSAID use
- E. von Willebrand disease (vWD)
Myeloproliferative neoplasms Explanation: ***Bernard-Soulier syndrome***
- The patient presents with **thrombocytopenia** (platelet count 72,000/mm^3), **prolonged bleeding time** (14 min), and **enlarged platelets** and **megakaryocytes** on blood smear.
- The key diagnostic feature is the **failure of platelets to aggregate to ristocetin**, indicating a defect in the GPIb-IX-V receptor complex which mediates platelet adhesion to von Willebrand factor. This clinical picture in a patient with a family history of bleeding is classic for Bernard-Soulier syndrome.
*Glanzmann thrombasthenia*
- This condition is characterized by a defect in the **GPIIb/IIIa receptor**, which is crucial for platelet aggregation; however, patients with Glanzmann thrombasthenia typically have a **normal platelet count** and **normal platelet morphology**.
- Platelets in Glanzmann thrombasthenia would fail to aggregate to ADP, collagen, and epinephrine, but aggregation to **ristocetin** would generally be normal (unless very high concentrations are used), making it less likely given the specific finding of failed ristocetin aggregation and thrombocytopenia.
*Idiopathic thrombocytopenic purpura (ITP)*
- ITP causes isolated **thrombocytopenia** (low platelet count) and **increased megakaryocytes** in the bone marrow, but platelets are typically of **normal size** or may be **larger than normal** but not giant platelets.
- While ITP would cause a prolonged bleeding time, it would not typically show **enlarged platelets** on blood smear, nor would the platelets specifically fail to aggregate to ristocetin due to a receptor defect.
*Aspirin or NSAID use*
- Aspirin and NSAIDs inhibit **cyclooxygenase**, leading to impaired platelet aggregation and a **prolonged bleeding time**, but they do not cause **thrombocytopenia** or changes in **platelet morphology** like enlarged platelets or increased megakaryocytes.
- Platelet aggregation to ristocetin would be normal in the context of aspirin or NSAID use, as the GP Ib-IX-V and GP IIb/IIIa complexes are unaffected.
*von Willebrand disease (vWD)*
- vWD involves a deficiency or defect in **von Willebrand factor**, leading to impaired platelet adhesion and sometimes reduced factor VIII levels, which causes a **prolonged bleeding time**; however, platelet count and morphology are typically **normal**.
- While vWD can show decreased ristocetin-induced platelet aggregation, it does **not present with thrombocytopenia** or **enlarged platelets/megakaryocytes** as seen in this patient.
Myeloproliferative neoplasms US Medical PG Question 10: A 67-year-old woman comes to the physician because of a 3-week history of fatigue and worsening back and abdominal pain. During this period, she has also had excessive night sweats and a 4.6-kg (10-lb) weight loss. She has had swelling of the neck for 3 days. She does not smoke or drink alcohol. Vital signs are within normal limits. Physical examination shows a 4-cm, supraclavicular, nontender, enlarged and fixed lymph node. The spleen is palpated 2 cm below the left costal margin. Laboratory studies show:
Hemoglobin 10.4 g/dL
Mean corpuscular volume 87 μm3
Leukocyte count 5,200/mm3
Platelet count 190,000/mm3
Serum
Lactate dehydrogenase 310 U/L
A CT scan of the thorax and abdomen shows massively enlarged paraaortic, axillary, mediastinal, and cervical lymph nodes. Histopathologic examination of an excised cervical lymph node shows lymphocytes with a high proliferative index that stain positive for CD20. Which of the following is the most likely diagnosis?
- A. Hairy cell leukemia
- B. Adult T-cell lymphoma
- C. Diffuse large B-cell lymphoma (Correct Answer)
- D. Marginal zone lymphoma
- E. Follicular lymphoma
Myeloproliferative neoplasms Explanation: ***Diffuse large B-cell lymphoma***
- The patient presents with **B symptoms** (fever, night sweats, weight loss), rapid progression, generalized **lymphadenopathy** (cervical, supraclavicular, paraaortic, axillary, mediastinal), **splenomegaly**, and elevated **LDH**.
- **Histopathologic examination** showing lymphocytes with a **high proliferative index** and positive **CD20 staining** confirms a B-cell lymphoma with aggressive features, highly characteristic of DLBCL.
*Hairy cell leukemia*
- This condition typically presents with **splenomegaly** and **pancytopenia**, but **lymphadenopathy** is rare and often absent.
- The characteristic "hairy cells" are identified by specific markers (CD103, CD123, CD25), and a **high proliferative index** is not a feature.
*Adult T-cell lymphoma*
- This lymphoma is associated with **HTLV-1 infection** and often presents with hypercalcemia, skin lesions, and generalized lymphadenopathy, but it is a **T-cell lymphoma**.
- The **CD20 positivity** in the histology rules out a T-cell lineage lymphoma.
*Marginal zone lymphoma*
- This is an **indolent B-cell lymphoma** that typically progresses slowly and is often associated with chronic inflammation or autoimmune diseases.
- The patient's aggressive symptoms, rapid progression, significant **B symptoms**, and **high proliferative index** are not consistent with indolent lymphoma.
*Follicular lymphoma*
- This is also an **indolent B-cell lymphoma** characterized by a follicular growth pattern and usually presents with painless lymphadenopathy.
- The rapid onset of symptoms, significant **B symptoms**, and elevated **LDH** indicate an aggressive lymphoma, which is not typical of follicular lymphoma.
More Myeloproliferative neoplasms US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.
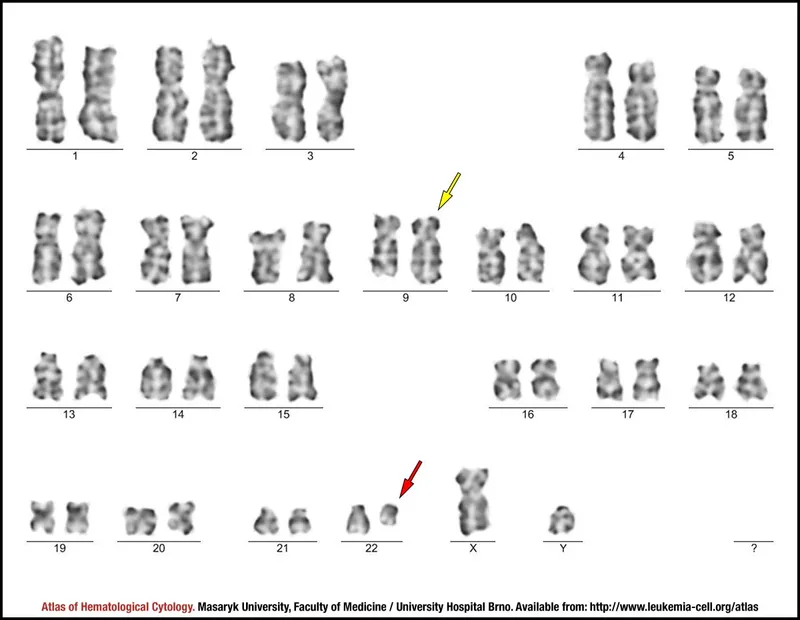 translocation)
translocation)